Abstract
Although curcumin can increase the effectiveness of drugs against malaria, combination therapies using the molecule have never been investigated in Chagas disease (ChD). Therefore, we evaluated the efficacy of curcumin as a complementary strategy to benznidazole (Bz)-based chemotherapy in mice acutely infected with Trypanosoma cruzi. Eighty-four 12-week-old Swiss mice were equally randomized into seven groups: uninfected (NI), T. cruzi infected and untreated (INF), infected and treated with 100 mg/kg of body weight Bz (B100), 50 mg/kg Bz (B50), 100 mg/kg curcumin (C100), 100 mg/kg Bz plus 100 mg/kg curcumin (B100 plus C100), and 50 mg/kg Bz plus 100 mg/kg curcumin (B50 plus C100). After microscopic identification of blood trypomastigotes (4 days after inoculation), both drugs were administered by gavage once a day for 20 days. Curcumin showed limited antiparasitic, anti-inflammatory, and antioxidant effects when administered alone. When curcumin and Bz were combined, there was a drastic reduction in parasitemia, parasite load, mortality, anti-T. cruzi IgG reactivity, circulating levels of cytokines (gamma interferon [IFN-γ], interleukin 4 [IL-4], and MIP1-α), myocardial inflammation, and morphological and oxidative cardiac injury; these results exceeded the isolated effects of Bz. The combination of Bz and curcumin was also effective at mitigating liver toxicity triggered by Bz, increasing the parasitological cure rate, and preventing infection recrudescence in noncured animals, even when the animals were treated with 50% of the recommended therapeutic dose of Bz. By limiting the toxic effects of Bz and enhancing its antiparasitic efficiency, the combination of the drug with curcumin may be a relevant therapeutic strategy that is possibly better tolerated in ChD treatment than Bz-based monotherapy.
INTRODUCTION
Chagas disease (ChD) is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi (1, 2). Estimates indicate that about 13 million people are infected in Latin America and the Caribbean (3). In North America, there are about 1 million reported cases, and in Western Europe, the number of reported cases is over 40,000 (2; http://www.dndi.org/diseases-projects/chagas/). Chagas cardiomyopathy (ChC) develops in 30 to 40% of infected patients and is the most severe clinical form of the disease and the most common cause of nonischemic cardiomyopathy worldwide (4). There is evidence that ChC is the result of oxidative and immune-mediated myocardial damage, which occurs due to the progressive deterioration of host endogenous antioxidant defenses and the establishment of an excessive Th1 immune response against T. cruzi (5–7).
In the absence of more effective drugs, conventional chemotherapy based on nitrocompounds, such as benznidazole (Bz) and nifurtimox (discontinued in countries where the disease is endemic), remains the main etiological treatment strategy for ChD (7–9). However, these drugs are highly toxic and do not guarantee parasitological cure after the spread and colonization of the parasite in multiple organs and tissues of the vertebrate host (2, 10). It is widely recognized that reactive oxygen (ROS) and nitrogen (RNS) species produced during Bz metabolism by the NADPH-cytochrome P450 system (especially OH˙ and NO) are involved in the trypanocidal effect of the drug. However, these reactive species also cause the oxidation of lipids, proteins, and nucleic acids, culminating in the injury or death of host cells (7, 10, 11).
Considering the high toxicity and limited efficacy of currently available chemotherapy, it is of the utmost importance to develop new methods and therapeutic regimens that are less hazardous and more efficient for ChD treatment (8, 9). In the last decade, Curcuma longa has emerged as a potentially useful natural resource in the treatment of parasitic diseases, such as malaria (12–14), schistosomiasis (15), leishmaniasis (16), and ChD (17). Curcumin [(1E,6E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the main bioactive compound extracted from C. longa rhizomes (16, 18). Previous studies have shown potent immunomodulatory, anti-inflammatory, and antioxidant properties of the molecule (12, 15–17). The immunomodulatory activity of curcumin has been associated with a reduction in the synthesis of proinflammatory cytokines, such as interleukin 1 (IL-1), IL-2, IL-6, tumor necrosis factor alpha (TNF-α), and IFN-γ (13, 18), and modulation of the activities of antigen-presenting cells and lymphocytes (15, 19–21). In addition, the high antioxidant potential of curcumin, which can overcome the action of anti-inflammatory steroids such as hydrocortisone (22), has been systematically reported (13, 18, 23). Taken together, these biological properties make curcumin a candidate for the development of drugs with antiparasitic potential (12, 14, 20).
To date, only one study has evaluated the effect of curcumin on ChD (17). In this study, curcumin significantly reduced the parasitemia, parasitism, and mortality of mice infected with T. cruzi. Apparently, these effects were mediated by the inhibition of nuclear translocation of sterol regulatory element-binding protein 1 (SREBP-1) and SREBP-2 and a consequent reduction in the expression of low-density lipoprotein receptor (LDLr), a molecule used by the parasite in cell invasion (24), in host cells. As the trypanocidal activity of curcumin has been little explored, the therapeutic potential of the molecule in ChD treatment remains uncertain.
Since ChC evolves into a worse prognosis than noninflammatory cardiomyopathies and the main difference between these pathologies is the presence of inflammatory infiltrate in the cardiac tissue of chagasic patients (1, 2), it was previously shown that anti-inflammatory and antioxidant interventions can mitigate the severity of ChC (4, 8, 11, 25). Considering that combined therapy is indicated as a rational approach to improve treatment efficiency while decreasing toxicity and the likelihood of the development of resistance (9), we investigated the efficacy of curcumin as a complementary strategy to Bz-based chemotherapy in the treatment of acute experimental ChD. With a positive effect, although limited, of the combination of Bz and curcumin in reducing parasitemia, cell parasitism, oxidative stress, inflammation, and cardiac injury, this strategy is relevant, as it allows a reduction in the therapeutic dose of Bz while maintaining satisfactory host protection.
MATERIALS AND METHODS
Animals and infection.
Groups of 12 female 8-week-old Swiss mice (weight, 30.17 ± 3.85 g) were used. The animals were maintained under conditions of controlled temperature (21 ± 2°C), humidity (60 to 70%), and photoperiod (12-h/12-h light/dark cycles). The animals had free access to food and water. The infected animals were inoculated intraperitoneally with 2,000 T. cruzi strain Y blood trypomastigotes. The parasites were obtained from the blood of previously infected animals (26). The study was approved by the Ethics Committee of Animal Use of the Federal University of Alfenas (CEUA/UNIFAL) (protocol 580/2014).
Therapeutic regimen and experimental groups.
To evaluate the isolated and combined effects of the reference drug (Bz) and curcumin, the animals were treated for 20 consecutive days (26). Bz (Pernambuco State Pharmaceutical Laboratory [LAFEPE], Recife, Pernambuco, Brazil) and curcumin (Sigma-Aldrich, St. Louis, MO, USA) were suspended in an aqueous solution of 1% carboxymethylcellulose and administered orally by gavage once a day. The experiments were performed using the therapeutic dose of Bz for mice, 100 mg/kg of body weight (26), and 50 mg/kg. Curcumin was administered at a fixed dose of 100 mg/kg, which has a trypanocidal effect on the Brazil strain of T. cruzi (17). The treatments were administered immediately after identification by microscopy of blood trypomastigotes, approximately 4 days after inoculation. The groups and treatment regimens were defined as follows: uninfected (NI); T. cruzi infected and untreated (INF); and infected and treated with 100 mg/kg Bz (B100), 50 mg/kg Bz (B50), 100 mg/kg curcumin (C100), 100 mg/kg Bz plus 100 mg/kg curcumin (B100 plus C100), or 50 mg/kg Bz plus 100 mg/kg curcumin (B50 plus C100). The results were obtained from two independent experiments.
Parasitemia and mortality.
Parasitemia was evaluated daily. The number of parasites was determined according to the technique described by Brener (27) in 5 μl of peripheral blood collected from the tail. The parasitemia curve of all infected animals was plotted. The mortality rate was recorded daily (26).
Hepatic toxicity.
Hepatic toxicity in each treatment and combination therapy was assessed by measuring the levels in serum of the enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Enzymatic assays were performed by spectrophotometry using commercial enzymatic kits and the instructions provided by the manufacturer (Human in Vitro Diagnostics, Belo Horizonte, MG, Brazil).
Infection recrudescence.
After 20 days of treatment, the animals showing negative parasitemia were submitted to three successive experiments to evaluate infection recrudescence (28). In assay 1, parasitemia was assessed daily for 20 days after treatment to determine the natural recrudescence of the infection. In assay 2, animals with negative parasitemia in this period were treated with the immunosuppressant cyclophosphamide (Genuxal; Baxter Oncology GmbH, Westphalia, Germany). The drug was dissolved in sterile phosphate buffer (pH 7.2) and administered at 50 mg/kg in three cycles of four consecutive days with 3 days between cycles. Parasitemia was measured daily and 5 days following the end of the immunosuppression protocol. In assay 3, animals that remained without detectable parasitemia were evaluated by hemoculture. For this, 400 μl of blood was collected from the orbital plexus and divided equally into two tubes containing 3 ml of sterile liver infusion tryptose (LIT) culture medium. The tubes were incubated at 28°C for 90 days and examined monthly for parasite detection.
Parasite load and parasitological cure.
The parasite load was estimated by the quantification of T. cruzi DNA in cardiac tissue. Animals that were positive in any test of infection recrudescence and those in which parasite DNA was detected were considered not cured (26). Extraction of total genomic DNA from cardiac tissue was performed using a commercial kit (Genomic DNA purification kit; Promega) according to the method of Caldas et al. (29). DNA concentrations were adjusted to 25 ng/μl (GeneQuant; Pharmacia Biotech, Piscataway, NJ, USA).
PCR was performed in a 10-μl volume containing 50 ng of genomic DNA, 5 μl of SYBR Green PCR Mastermix (Applied Biosystems, Carlsbad, CA, USA), and either 0.35 μM T. cruzi 195-bp-repeat DNA-specific primers or 0.50 μM murine-specific TNF-α primers. The primers for T. cruzi repetitive DNA (TCZ-F, 5′-GCTCTTGCCCACAMGGGTGC-3′, where M is A or C, and TCZ-R, 5′-CCAAGCAGCGGATAGTTCAGG-3′) amplify a 182-bp fragment. The primers for murine TNF-α (TNF-5241, 5′-TCCCTCTCATCAGTTCTATGGCCCA-3′, and TNF-5411, 5′-CAGCAAGCATCTATGCACTTAGACCCC-3′) amplify a 170-bp product (30). The PCR assay was performed under previously described analytical conditions (time, temperature, and cycles) (7). Each 96-well reaction plate contained a standard curve and two negative controls with T. cruzi-specific or murine-specific primers without DNA and also tissue DNA from noninfected mice. The mean quantification values for T. cruzi DNA were normalized by the data obtained with murine-specific (TNF-α) primers as follows: normalized value = (mean T. cruzi DNA/mean TNF-α DNA) × 1,000, where “1,000” corresponds to the expected value for TNF-α from 30 mg of cardiac tissue. The efficiencies of amplification were determined with StepOne Software v2.0 by the following calculation: efficiency (E) = 10−1/slope (31).
Immunoglobulin assay.
Blood samples were collected from the orbital venous sinus (0.5 ml). Anti-T. cruzi specific antibodies were detected by enzyme-linked immunosorbent assay (ELISA) (26). Polystyrene microplates (96 wells) were coated with T. cruzi antigens and incubated with plasma from each animal. Anti-mouse immunoglobulin G (IgG), IgG1, IgG2a, and IgG2b peroxidase conjugate antibodies were used (Bethyl Laboratories, Montgomery, TX, USA). The optical density (OD) was determined at 490 nm (Anthos Zenyth 200; Biochrom, Cambridge, United Kingdom). The mean absorbance for 10 negative-control samples plus 2 standard deviations (SD) was used as the cutoff point to discriminate positive and negative results.
Cytokine assay.
Cytokine plasma levels were measured by the sandwich ELISA method using a commercial kit according to the manufacturer's instructions (PeproTech, Rocky Hill, NJ). The cytokines IFN-γ, IL-2, and IL-4 and the chemokine MIP-1α were investigated. The reaction was developed with a streptavidin-peroxidase-conjugated antibody (Vector Laboratories, Burlingame, CA, USA), followed by incubation with the chromogen 3,3′,5,5′ tetramethylbenzidine (Promega, Madison, WI, USA). The ODs of the samples were detected at 450 nm (Anthos Zenyth 200; Biochrom, Cambridge, United Kingdom), and cytokine concentrations were calculated by extrapolating the OD obtained from a standard curve for each recombinant cytokine.
Histopathology and stereology.
Heart fragments from each animal were fixed in 4% paraformaldehyde (1 M; pH 7.2). The samples were embedded in glycolmethacrylate resin (Leica Microsystems, Wetzlar, Germany). Histological sections (3 μm thick) were obtained with a rotary microtome and glass knives. The sections were evaluated in semiseries (using one out of every 20 sections) and were stained with hematoxylin and eosin (32). For each histological section, six microscope images were captured at ×400 magnification (Axioscope A1; Zeiss, Germany). The histopathological analysis was performed by evaluating the organization and size of cardiomyocytes and the distribution of cardiac stroma, tissue cellularity, inflammatory infiltrate, T. cruzi amastigote nests, and tissue necrosis.
The intensity of myocardial inflammation was assessed by the stereological method (32). For this, a quadratic test system with a standardized test area (At) (At = 0.0423 mm2) was applied to the histological images. The number of cell nuclei in the test area (QAinf) was determined by image analysis using Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD, USA). The number of cell nuclei was determined by the following ratio: QAinf = Σinf/At, where Σinf is the number of interstitial cell nuclei counted within the test system (26).
Oxidative tissue damage.
The oxidative damage to lipids and proteins was assessed by the quantification of malondialdehyde (MDA) and protein carbonyls (PCN), respectively. Briefly, heart and liver fragments (100 mg) were homogenized in phosphate buffer (pH 7.2) and centrifuged at 10,000 × g at 4°C for 10 min. To determine MDA levels, the supernatant was incubated with thiobarbituric acid solution (15% trichloroacetic acid, 0.375% thiobarbituric acid, and 0.25 N HCl) for 15 min. The formation of thiobarbituric acid-reactive substances was monitored at 535 nm (Power Wave X; Bio-Tek Instruments, Winooski, VT, USA) (33). The protein carbonyl content was measured biochemically in the cardiac and hepatic tissue pellets by adding 0.5 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH). The reaction involved derivatization of the carbonyl group with DNPH, leading to the formation of a stable 2,4-dinitrophenyl (DNP) hydrazone product, which indicates the content of protein carbonyl in the samples. The OD was measured at 370 nm (34).
Statistical analysis.
Data are reported as the mean and SD. The normality in the data distribution was verified by the D'Agostino-Pearson test. Parametric data were compared between groups by one-way analysis of variance (ANOVA), followed by the Student-Newman-Keuls post hoc test. Nonparametric variables were compared using the Kruskal-Wallis test. The confidence level of all tests was set at 95% (P < 0.05).
RESULTS
Untreated infected animals had high parasitemia and mortality compared to the other groups (P < 0.05) (Table 1). Treatment with doses of Bz alone or combined with curcumin reduced parasitemia compared to the infected, untreated group (P < 0.05). The B100-plus-C100 and B50-plus-C100 groups showed lower parasitemia than animals treated with only Bz (P < 0.05). Among the treated animals, mortality was observed only in the C100 group (n = 3; 25.0%).
TABLE 1.
Parasitemia and survival in mice infected with T. cruzi and treated with benznidazole and curcumina
| Group | No. of parasites × 103 (0.1 ml blood) (mean ± SD)b | No. of mice with PCc (%) | Survival | Mortality (%) |
|---|---|---|---|---|
| NI | NDd | ND | 12/12 | 0 |
| INF | 39.50 ± 26.62A | 0/12 (0) | 5/12 | 58.33 |
| B100 | 2.75 ± 0.60B | 12/12 (100) | 12/12 | 0 |
| B50 | 4.93 ± 1.56C | 12/12 (100) | 12/12 | 0 |
| C100 | 22.41 ± 10.19D | 9/12 (75) | 9/12 | 25.00 |
| B100 + C100 | 1.38 ± 0.36E | 12/12 (100) | 12/12 | 0 |
| B50 + C100 | 1.45 ± 0.47E | 12/12 (100) | 12/12 | 0 |
The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg).
Different letters (A to E) indicate a statistical difference among the groups (P < 0.05), and groups that have a common letter do not differ statistically (P > 0.05).
PC, parasitemia clearance (absence of blood trypomastigotes) 24 h after the last dose of treatment (day 21).
ND, not detected.
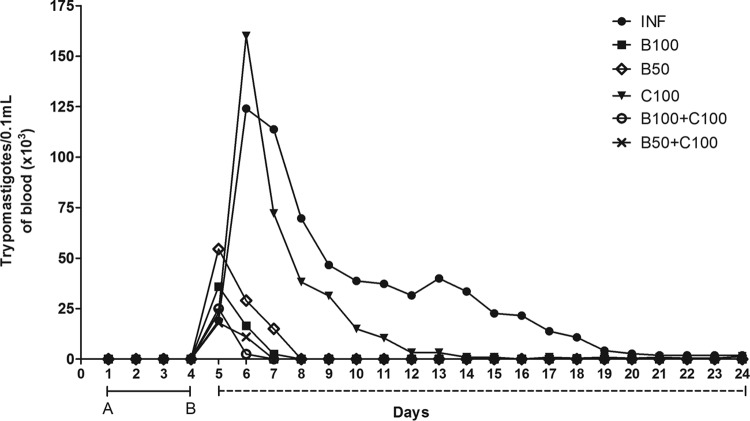
Animals in the INF and C100 groups showed a slower decrease in blood parasite numbers (Fig. 1). These groups also showed a higher peak of parasitemia than the groups treated with Bz alone (B100 and B50) or Bz combined with curcumin (B100 plus C100 and B50 plus C100), in which complete parasite clearance was identified from the ninth day of treatment.
FIG 1.
Time-dependent evolution of mean parasitemia in mice infected with T. cruzi and treated with benznidazole and curcumin. The solid line between A and B represents the days until detection of blood trypomastigotes. The dashed line represents the days of treatment. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg).
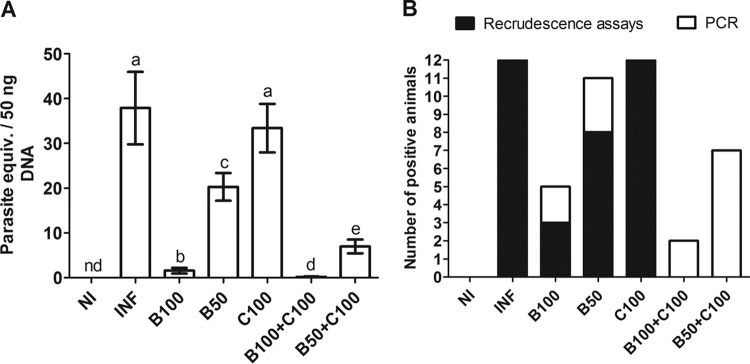
All infected, untreated animals presented patent parasitemia, and no cases of complete parasitemia clearance were observed at the end of the treatment period. At that time, positive parasitemia was observed in 25.0% (n = 3) of the animals in the C100 group (Fig. 2 and Table 1). Complete parasite clearance was identified in the other groups. Infection recrudescence occurred in 75.0% (n = 9) of the animals in the C100 group. In the B50 and B100 groups, the recrudescence rates were 66.67% (n = 8) and 25.0% (n = 3), respectively. Recrudescence was not identified in the B100-plus-C100 and B50-plus-C100 groups (Fig. 2).
FIG 2.
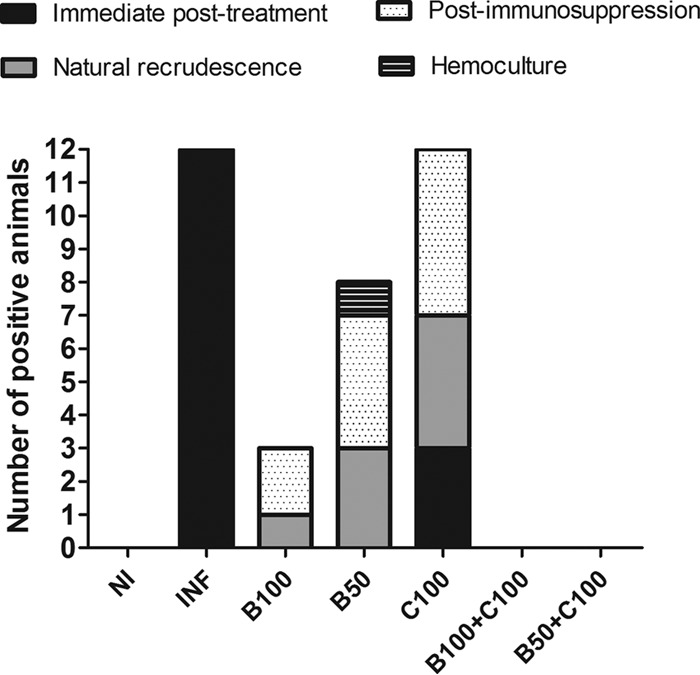
Infection recrudescence in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg).
PCR analysis demonstrated a high parasitic load in the INF group, which was similar to that in the C100 group (P > 0.05). The parasite load was dramatically reduced in animals treated with Bz (P < 0.05). This reduction was even greater in the B100-plus-C100 and B50-plus-C100 groups compared to the B50 and B100 groups (P < 0.05) (Fig. 3A). PCR also revealed parasitological cure rates of 58.33% (n = 7) and 8.33% (n = 1) in the B50 and B100 groups, respectively. In the B50-plus-C100 and B100-plus-C100 groups, the cure rates were increased to 41.67% (n = 5) and 83.33% (n = 10), respectively (Fig. 3B).
FIG 3.
Parasite load in cardiac tissue (means ± SD) (A) and parasitological cure (B) evaluated by quantitative PCR (qPCR) in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). The recrudescence assays were based on examination of fresh blood (before and after immunosuppression with cyclophosphamide) and hemoculture (see Materials and Methods for details). Different letters above the bars (a to e) indicate statistical differences among the groups (P < 0.05), and groups that have a common letter do not differ statistically (P > 0.05). nd, not detected.
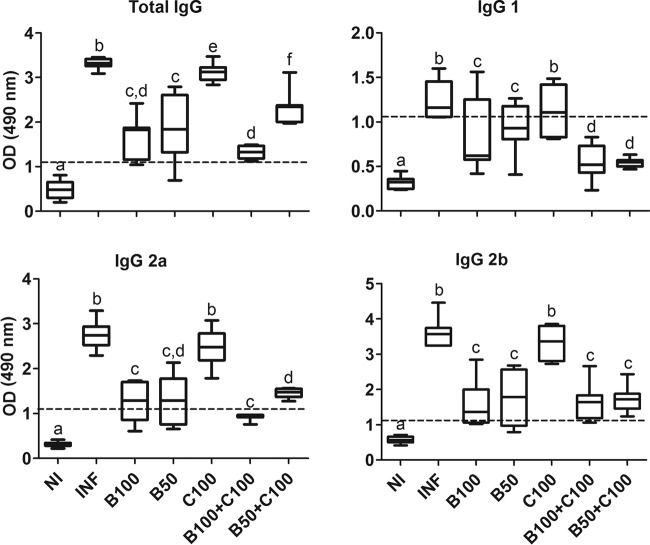
The plasma reactivity of anti-T. cruzi IgG was reduced in all groups treated with Bz alone or combined with curcumin compared to the INF and C100 groups (P < 0.05). The B100-plus-C100 and B50-plus-C100 groups showed negative reactivity to IgG1 and IgG2a, similar to the uninfected group (P > 0.05). When administered alone, curcumin showed results similar to those for the infected, untreated group (P > 0.05) (Fig. 4).
FIG 4.
Plasma reactivity of anti-T. cruzi IgG in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). The dashed lines represent the discriminant values of reactivity calculated by dividing the individual absorbance by the cutoff values (mean ± 2 SD) from uninfected blood samples (negative control). The boxes represent the interquartile ranges; the horizontal lines are medians, and the whiskers show the upper and lower quartiles. Different letters above the bars (a to f) indicate statistical differences among the groups (P < 0.05), and groups that have common letters do not differ statistically (P > 0.05).
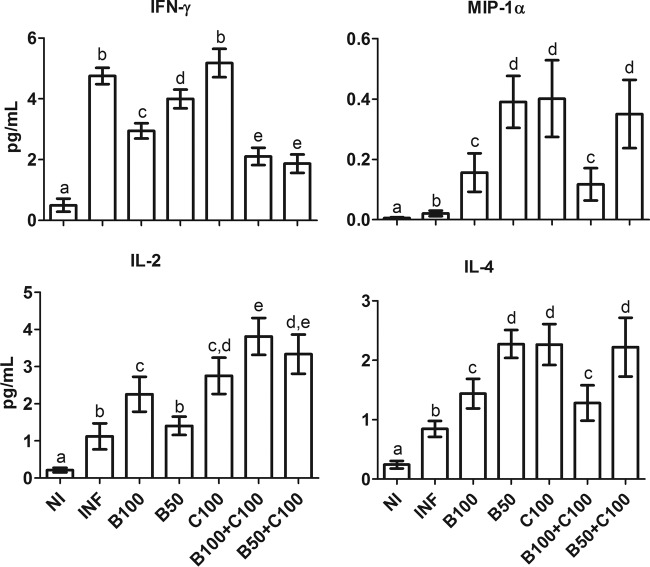
All the investigated cytokines (IFN-γ, MIP-1α, IL-2, and IL-4) were increased in the INF group compared to the NI group (P < 0.05) (Fig. 5). IFN-γ levels were similar in the INF and C100 groups (P > 0.05) but higher than for the other treatment groups (P < 0.05). Treatment with Bz, especially when combined with curcumin, reduced IFN-γ levels compared to the other infected groups (P < 0.05). Independent of curcumin coadministration, animals treated with the lowest dose of Bz (50 mg/kg) showed increased levels of MIP-1α and IL-4 (P < 0.05), which were reduced in the groups receiving the highest dose of Bz (100 mg/kg) (P < 0.05). IL-2 levels were high in the animals treated with curcumin compared to the NI and INF groups (P < 0.05).
FIG 5.
Plasma cytokine levels (means ± SD) in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). Different letters above the bars (a to e) indicate statistical differences among the groups (P < 0.05), and groups that have common letters do not differ statistically (P > 0.05).
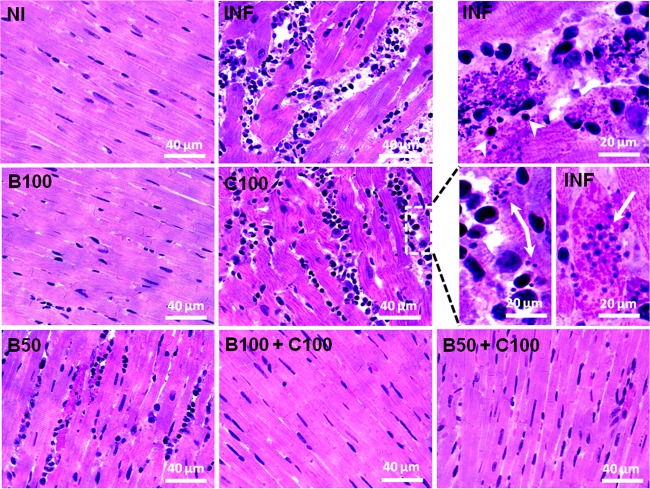
As shown in Fig. 6, infected, untreated animals showed intense myocardial inflammatory infiltrate, connective tissue expansion, cardiomyocyte hypertrophy, and necrosis. All the animals treated with Bz alone, and especially those receiving Bz combined with curcumin, showed attenuation of the cardiac inflammatory process. Cardiomyocyte hypertrophy was observed only in the C100 group.
FIG 6.
Representative photomicrographs of the myocardia of mice infected with T. cruzi and treated with benznidazole and curcumin (hematoxylin and eosin staining). The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). The arrows indicate T. cruzi amastigote nests. (Top right) Necrotic cardiomyocytes surrounded by inflammatory cells (the arrowheads indicate lymphocytes).
Stereological analysis indicated intense cardiac inflammation in the INF and C100 groups compared to the other groups (P < 0.05) (Fig. 7). The myocardial cellularity in the B100 and B100-plus-C100 groups was similar to that in the NI group (P > 0.05). In animals treated with half the therapeutic dose of Bz, a greater reduction in the inflammatory infiltrate was found when the drug was combined with curcumin (P < 0.05).
FIG 7.
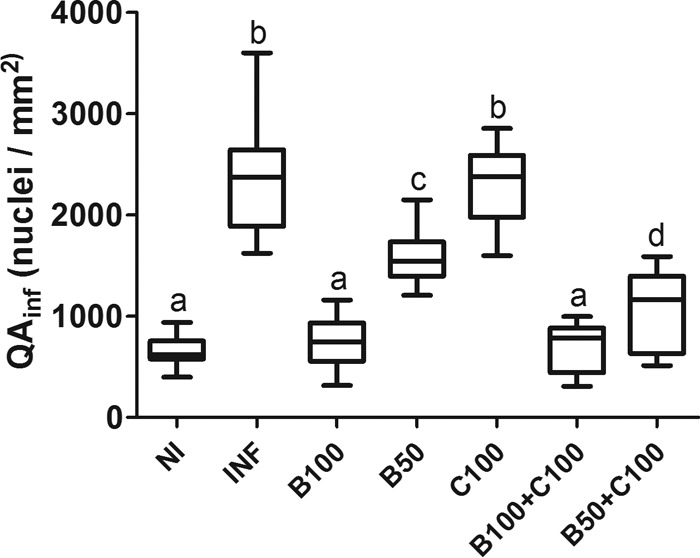
Number and density of interstitial cells (QAinf) in the myocardia of mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). The boxes represent the interquartile ranges. The horizontal lines are medians, and the whiskers are upper and lower quartiles. Different letters above the bars (a to d) indicate statistical differences among the groups (P < 0.05), and groups that have common letters do not differ statistically (P > 0.05).
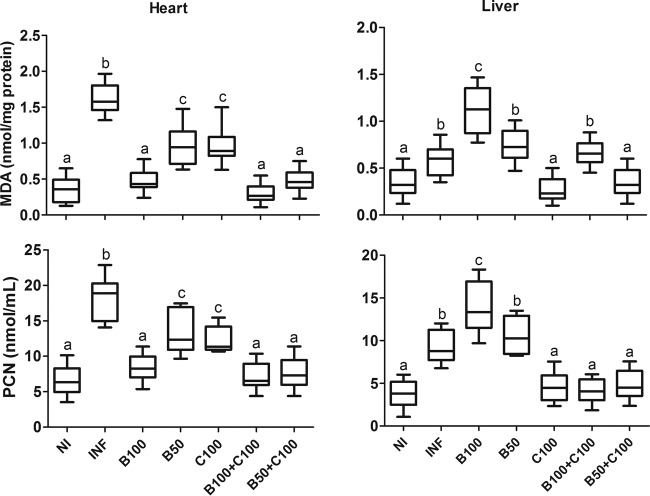
High cardiac and hepatic MDA and PCN levels were identified in the INF group compared to the NI group (P < 0.05). These parameters were reduced in the heart and increased in liver tissue from animals treated with the highest dose of Bz (P < 0.05). The animals treated with curcumin showed reduced tissue MDA and PCN levels compared to the INF group. In general, the B100-plus-C100, B50-plus-C100, and NI groups presented similar tissue MDA and PCN levels (Fig. 8).
FIG 8.
Cardiac and hepatic levels of MDA and PCN in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). The boxes represent the interquartile ranges; the horizontal lines are medians, and the whiskers are upper and lower quartiles. Different letters above the bars (a to c) indicate statistical differences among the groups (P < 0.05), and groups that have common letters do not differ statistically (P > 0.05).
In general, plasma AST and ALT levels were increased in all the groups receiving Bz, especially B100 (P < 0.05) (Fig. 9). The B100-plus-C100 and B50-plus-C100 groups had lower AST and ALT levels than the B100 and B50 groups, respectively (P < 0.05). The levels of these enzymes were similar in the INF and C100 groups (P > 0.05).
FIG 9.
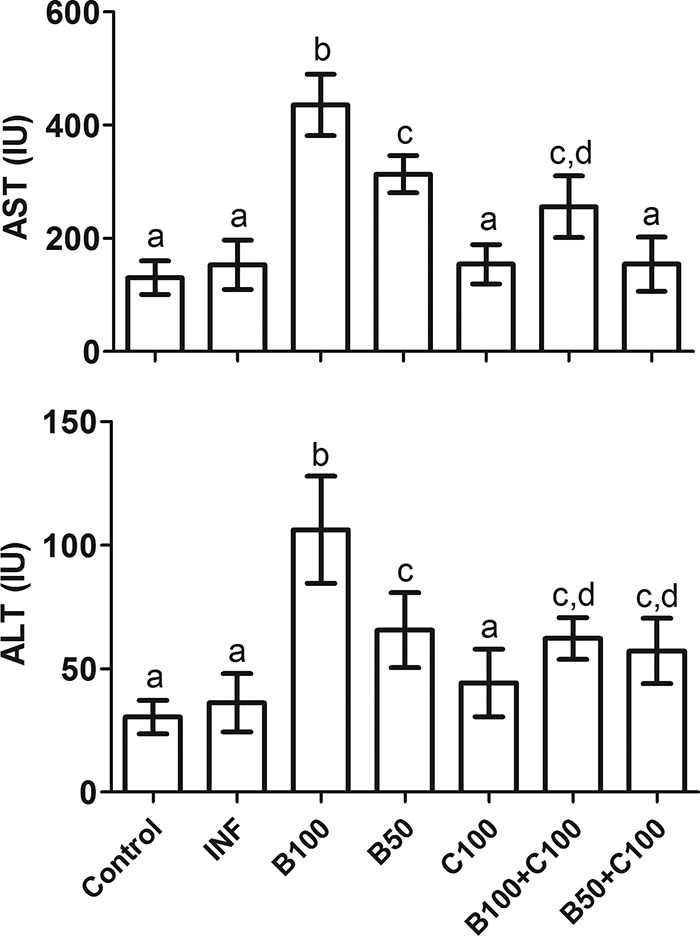
Plasma levels of transaminases (means ± SD) in mice infected with T. cruzi and treated with benznidazole and curcumin. The treated animals received benznidazole (100 or 50 mg/kg) and curcumin (100 mg/kg). Different letters above the bars (a to d) indicate statistical differences among the groups (P < 0.05), and groups that have common letters do not differ statistically (P > 0.05).
DISCUSSION
Considering the need for new therapeutic approaches for ChD treatment, our findings indicate that curcumin administered alone was effective in reducing parasitemia and mortality, but not the parasitic load, in animals infected with T. cruzi. Although monotherapy with Bz showed a satisfactory effect, greater attenuation of parasitemia, the parasite load, and mortality and a higher cure rate were found in the groups receiving Bz combined with curcumin. However, the presence of blood trypomastigotes or parasite DNA in cardiac tissue from some animals treated with this combination was still detected after immunosuppression, hemocultures, or PCR. Previous studies showed that although early treatment with Bz is effective at eliminating parasitemia, parasitological cure is not always achieved due to the presence of T. cruzi reservoirs resistant to chemotherapy, which are distributed throughout multiple organs and tissues (35, 36). Thus, there is evidence that the efficiency of etiological treatment is directly influenced by multiple factors, especially the variable profiles of resistance to infection observed in different mouse strains (28, 37), parasite virulence, and the different sensitivities of T. cruzi strains to Bz (9, 38). All these factors represent major challenges to the rational development of effective drugs or therapeutic regimens for the treatment of ChD.
Surprisingly, we found that even when curcumin was associated with half the therapeutic dose of Bz (50 mg/kg), there was no infection recrudescence after treatment, as occurred in the groups receiving only Bz. This finding indicates a potential relevance of the combination of Bz and curcumin, since infection recrudescence has been associated with a worse prognosis in ChC due to accumulation of cardiac lesions (39, 40). Although combination therapies using curcumin have never been investigated in ChD, previous studies have demonstrated that the molecule can increase the effectiveness of chemotherapy used in the treatment of malaria. Thus, curcumin combined with artemisinin or α,β-arteether was effective in reducing parasitemia in mice infected with Plasmodium berghei, increasing the survival rate to 95% and preventing infection recrudescence (14, 41). Currently, the mechanism of action of curcumin is not completely understood. However, it is believed that the antiparasitic effects are associated with the action of the molecule in potentiating innate and adaptive immunological responses, especially by modulating the expression of cytokines and the effector activity of macrophages (15, 19), T and B cells, neutrophils (21), and natural killer and dendritic cells (20).
In the present study, treatment with Bz, especially when combined with curcumin, caused a remarkable reduction in serum reactivity for both IgG1 and IgG2a isotypes. Although the production of IgG2a is predominant in the humoral response against T. cruzi, similar lytic activities of IgG2a and IgG1 have been previously reported; these antibodies have been shown to be the major effector molecules that control parasitemia (37, 42). However, high IgG1 levels are not always beneficial to the host, since IgG1 can block the activities of different isotypes of anti-T. cruzi antibodies through cross-linking with irrelevant antigens in the defense against the parasite, thus deflecting humoral immunity toward a nonspecific and ineffective response (37, 43). According to previous investigations (26, 44), the attenuation of the humoral response observed in this study is consistent with the best parasitological control and reduction of the parasite load induced by the treatment. Traditionally, IgG analysis has been used as a marker of parasitological cure in ChD, as it has a high correlation with the detection of T. cruzi antigens by PCR-based techniques (26, 45). In fact, our findings indicate a possible relationship between parasitological cure and IgG, since lower antibody reactivity was found in animals that showed no infection recrudescence, followed by the groups with a low recrudescence rate.
Although curcumin alone did not influence the IFN-γ results, when used in combination with Bz, it was able to drastically reduce the levels of the cytokine. This finding is consistent with the antiparasitic activity of this combination, since it is natural that a reduction in parasitemia and the parasite load is accompanied by a reduction in IFN-γ levels, as the inflammatory process associated with the infection undergoes resolution (46, 47). In contrast, IL-4 levels were higher in all the treatment groups. IL-4 has recognized importance in the polarization of the immune response to a Th2 profile (48, 49). Although a Th2 response increases host susceptibility to T. cruzi infection, increased levels of Th2 cytokines also operate in the homeostatic control of the immune system to regulate the intensity of the Th1 cellular response (essential against T. cruzi), thereby preventing the development of an exacerbated inflammatory process that may be harmful to the host (7, 35, 50). There is evidence that animals deficient in IL-4 and infected with T. cruzi develop severe myocarditis, extensive cardiac fibrosis, and higher mortality than wild-type animals expressing the cytokine (50, 51). Thus, IL-4 favors the integrity of the cardiac muscle by adjusting the balance between parasitism and the intensity of the innate and acquired resistance mechanisms of the host against parasitic infection (49, 52).
As for IL-4, circulating MIP-1α levels were higher in all the treatment groups. Since both groups receiving the highest dose of Bz showed the lowest MIP-1α values, it is possible that this finding is associated with the lower parasite load observed in these groups, a condition that requires less cell recruitment (48). There is evidence that MIP-1α favors the control of the parasite load, since the chemokine is directly involved in the recruitment of macrophages and neutrophils to infected organs (53). Furthermore, it was previously shown that MIP-1α inhibition increases the tissue parasite load in T. cruzi-infected mice (53). Interestingly, the combination of Bz and curcumin led to high circulating IL-2 levels. IL-2 acts as an important growth factor for CD8+ T cells and activates their cytotoxic effector functions (54). As a typical Th1 cytokine, IL-2 promotes cellular and humoral immunity to T. cruzi, contributing to reduced parasitemia and mortality in infected mice (55, 56). Thus, by stimulating IL-2 production, the combination of Bz and curcumin could enhance host defenses against the parasite.
Considering the structure of the heart, Bz alone and combined with curcumin was also effective in attenuating cardiac damage, especially inflammation, tissue necrosis, and connective tissue expansion. It is possible that the attenuation of inflammation and pathological myocardial remodeling are associated with modulation of the immune system. This proposition is reinforced by the reduction in IFN-γ and IL-2 levels and the concomitant increase in IL-4, indicating that the preservation of cardiac structure may be dependent on controlling the intensity of the inflammatory process, which results from a proper balance between Th1 and Th2 cytokine levels (35, 50, 51). The anti-inflammatory activity of curcumin has been linked to the presence of hydroxyl and phenol chemical groups in the molecule, which are essential for inhibiting both cyclooxygenase and lipoxygenase metabolic pathways and reducing the biosynthesis of prostaglandin and leukotriene, which are potent proinflammatory mediators (57, 58). Thus, curcumin may have potential applicability in minimizing cardiac deterioration secondary to an exaggerated inflammatory process (4, 7).
The combination of Bz and curcumin was also beneficial in reducing oxidative tissue damage. It has been demonstrated that inflammatory cells recruited to infected organs activate molecular mechanisms against T. cruzi (the respiratory burst) (5, 6), which causes cumulative reactive damage, especially in cardiac tissue (7, 10). Thus, antioxidant drugs have shown promising results in attenuating cardiac oxidative damage and ChC progression (25, 59). The antioxidant properties of curcumin are associated with its direct free-radical scavenger action and ability to increase the synthesis and activity of antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione S-transferase (18, 23). However, the modulatory effect of curcumin on the endogenous antioxidant system is complex and poorly understood, requiring further investigation.
Confirming recent evidence described by our research group (10), Bz treatment increased circulating levels of ALT and AST, indicating morphofunctional liver damage. According to Ozer et al. (60), these enzymes are considered highly sensitive and specific markers of hepatotoxicity in animals and humans, reflecting the intensity of morphological and functional lesions in hepatocytes. Thus, the findings observed here indicate that curcumin reduced Bz toxicity, an effect potentially mediated by the attenuation of inflammation and liver oxidative damage caused by both infection and chemotherapy. This hypothesis is reinforced by reduced lipid and protein oxidation in liver tissue in curcumin-treated mice, which is consistent with the action of antioxidant drugs used to treat intoxication by inducers of oxidative stress, including Bz (7, 10). Thus, our findings indicate that by limiting the toxic effects of Bz, the combination of the drug with curcumin may be a better-tolerated therapeutic strategy than the use of Bz alone.
Taken together, our findings show that curcumin has a limited antiparasitic effect, but when combined with Bz, it can potentiate the therapeutic efficacy of reference chemotherapy. Apparently, this effect is mediated by a greater reduction in parasitemia and the parasite load, as well as increased survival and cure rate in mice infected with T. cruzi. Furthermore, the combination of curcumin and Bz drastically reduced cardiac inflammation and oxidative damage and at the same time attenuated hepatic toxicity induced by Bz. Using this association, it was possible to prevent infection recrudescence after completion of the treatment, even when the animals received half the recommended therapeutic dose of Bz. As the treatment was started immediately after confirming the infection, our results are limited to the acute phase of experimental Chagas disease. Thus, further studies are required to determine the relevance of curcumin as a complementary strategy to Bz-based chemotherapy in the treatment of the established, chronic form of the disease, which is currently the most common clinical presentation in both areas of endemicity and areas of nonendemicity.
ACKNOWLEDGMENT
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) (449903/2014-1) and by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil).
REFERENCES
- 1.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. 2007. Pathogenesis of chronic Chagas' heart disease. Circulation 115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 2.Rassi A Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Dumonteil E, Woc-Colburn L, Serpa JA, Bezek S, Edwards MS, Hallmark CJ, Musselwhite LW, Flink BJ, Bottazzi ME. 2012. Chagas disease: “the new HIV/AIDS of the Americas”. PLoS Negl Trop Dis 6:e1498. doi: 10.1371/journal.pntd.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melo L, Caldas IS, Azevedo MA, Gonçalves KR, Da Silva Do Nascimento AF, Figueiredo VP, De Figueiredo DL, De Lima WG, Torres RM, Bahia MT, Talvani A. 2011. Low doses of simvastatin therapy ameliorate cardiac inflammatory remodeling in Trypanosoma cruzi-infected dogs. Am J Trop Med Hyg 84:325–331. doi: 10.4269/ajtmh.2011.10-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Bhatia V, Wen JJ, Wu Y, Huang MH, Garg NJ. 2009. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic Biol Med 47:1414–1421. doi: 10.1016/j.freeradbiomed.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Wen J-J, Garg NJ. 2009. Oxidative stress in Chagas disease. Interdiscip Perspect Infect Dis 2009:190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos EC, Novaes RD, Cupertino MC, Bastos DS, Klein RC, Silva EA, Fietto JL, Talvani A, Bahia MT, Oliveira LL. 2015. Chemotherapy with benznidazole and suramin: applicability of their concomitant treatment in mice infected with a virulent strain of Trypanosoma cruzi. Antimicrob Agents Chemother 59:5999–6006. doi: 10.1128/AAC.00779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz MJ, Murcia L, Segovia M. 2011. The urgent need to develop new drugs and tools for the treatment of Chagas disease. Expert Rev Anti Infect Ther 9:5–7. doi: 10.1586/eri.10.144. [DOI] [PubMed] [Google Scholar]
- 9.Diniz LDF, Urbina JA, De Andrade IM, Mazzeti AL, Martins TA, Caldas IS, Talvani A, Ribeiro I, Bahia MT. 2013. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis 7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novaes RD, Santos EC, Cupertino MC, Bastos DSS, Oliveira JM, Carvalho TV, Neves MM, Oliveira LL, Talvani A. 2015. Trypanosoma cruzi infection and benznidazole therapy independently stimulate oxidative status and structural pathological remodeling of the liver tissue in mice. Parasitol Res 114:2873–2881. doi: 10.1007/s00436-015-4488-x. [DOI] [PubMed] [Google Scholar]
- 11.Maya JD, Cassels BK, Iturriaga-Vasquez P, Ferreira J, Fáundez M, Galanti N, Ferreira A, Morello A. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol 146:601–620. doi: 10.1016/j.cbpa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Cui L, Miao J, Cui L. 2007. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother 51:488–494. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimche PN, Taramelli D, Vivas L. 2011. The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar J 10(Suppl 1):S10. doi: 10.1186/1475-2875-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vathsala PG, Dende C, Nagaraj VA, Bhattacharya D, Das G, Rangarajan PN, Padmanaba G. 2012. Curcumin-arteether combination therapy of Plasmodium berghei-infected mice prevents recrudescence through immunomodulation. PLoS One 7:e29442. doi: 10.1371/journal.pone.0029442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allam G. 2009. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 214:712–727. doi: 10.1016/j.imbio.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Das R, Roy A, Dutta N, Majumder HK. 2008. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 13:867–882. doi: 10.1007/s10495-008-0224-7. [DOI] [PubMed] [Google Scholar]
- 17.Nagajyothi F, Zhao D, Weiss LM, Tanowitz HB. 2012. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res 110:2491–2499. doi: 10.1007/s00436-011-2790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strimpakos AS, Sharma RA. 2008. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 19.Ranjan D, Chen C, Johnston TD, Jeon H, Nagabhushan M. 2004. Curcumin inhibits mitogen stimulated lymphocyte proliferation NF-kB activation and IL-2 signaling. J Surg Res 121:171–177. doi: 10.1016/j.jss.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK. 2005. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 27:485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 21.Jagetia GC, Aggarwal BB. 2007. “Spicing up” of the immune system by curcumin. J Clin Immunol 27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 22.Ammon HPT, Anazodo MI, Safayhi H, Dhawan BN, Srimal RC. 1992. Curcumin: a potent inhibitor of leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL). Planta Med 58:226. doi: 10.1055/s-2006-961438. [DOI] [PubMed] [Google Scholar]
- 23.Reddy AC, Lokesh BR. 1994. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem Toxicol 32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 24.Nagajyothi F, Weiss LM, Silver DL, Desruisseaux MS, Scherer PE, Herz J, Tanowitz HB. 2011. Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Negl Trop Dis 5:e953. doi: 10.1371/journal.pntd.0000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen JJ, Garg NJ. 2008. Mitochondrial generation of reactive oxygen species is enhanced at the Qo site of the complex III in the myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant. J Bioenerg Biomembr 40:587–598. doi: 10.1007/s10863-008-9184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldas IS, Talvani A, Caldas S, Carneiro CM, De Lana M, Da Matta Guedes PM, Bahia MT. 2008. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res 103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 27.Brener Z. 1962. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–396. [PubMed] [Google Scholar]
- 28.Caldas S, Santos FM, Lana M, Diniz LF, Machado-Coelho GL, Veloso VM, Bahia MT. 2008. Trypanosoma cruzi: acute and long-term infection in the vertebrate host can modify the response to benznidazole. Exp Parasitol 118:315–323. doi: 10.1016/j.exppara.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Caldas S, Caldas IS, Diniz LF, Lima WG, Oliveira RP, Cecílio AB, Ribeiro I, Talvani A, Bahia MT. 2012. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop 123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Cummings KL, Tarleton RL. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol 129:53–59. doi: 10.1016/S0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 31.Stordeur P, Poulin LF, Craciun L, Zhou L, Schandené L, De Lavareille A, Gorielys S, Goldman M. 2002. Cytokine mRNA quantification by real-time PCR. J Immunol Methods 259:55–64. doi: 10.1016/S0022-1759(01)00489-6. [DOI] [PubMed] [Google Scholar]
- 32.Novaes RD, Penitente AR, Gonçalves RV, Talvani A, Peluzio MC, Neves CA, Natali AJ, Maldonado IR. 2013. Trypanosoma cruzi infection induces morphological reorganization of the myocardium parenchyma and stroma and modifies the mechanical properties of atrial and ventricular cardiomyocytes in rats. Cardiovasc Pathol 22:270–279. doi: 10.1016/j.carpath.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Buege JA, Aust SD. 1978. Microsomal lipid peroxidation. Methods Enzymol 52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 34.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. 1990. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira AR, Hecht MM, Guimaro MC, Sousa AO, Nitz N. 2011. Pathogenesis of Chagas' disease: parasite persistence and autoimmunity. Clin Microbiol Rev 24:592–630. doi: 10.1128/CMR.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, Garg NJ, Tanowitz HB. 2012. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol 14:634–643. doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryan MA, Guyach SE, Norris KA. 2010. Specific humoral immunity versus polyclonal B cell activation in Trypanosoma cruzi infection of susceptible and resistant mice. PLoS Negl Trop Dis 4:e733. doi: 10.1371/journal.pntd.0000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caldas S, Caldas IS, Cecílio AB, Diniz LD, Talvani A, Ribeiro I, Bahia MT. 2014. Therapeutic responses to different anti-Trypanosoma cruzi drugs in experimental infection by benznidazole-resistant parasite stock. Parasitology 141:1628–1637. doi: 10.1017/S0031182014000882. [DOI] [PubMed] [Google Scholar]
- 39.Bocchi EA, Bellotti VIP, Kalil J, Higuchi ML, Fiorelli A, Stolf N, Jatene A, Pilleggi F. 1993. Long-term follow up after heart transplantation in Chagas' disease. Transplant Proc 25:1329–1330. [PubMed] [Google Scholar]
- 40.Campos SV, Strabelli TM, Amato Neto V, Silva CP, Bacal F, Bocchi EA, Stolf NA. 2008. Risk factors for Chagas' disease reactivation after heart transplantation. J Heart Lung Transplant 27:597–602. doi: 10.1016/j.healun.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Nandakumar DN, Nagaraj VA, Vathsala PG, Rangarajan P, Padmanaban G. 2006. Curcumin-artemisinin combination therapy for malaria. Antimicrob Agents Chemother 50:1859–1860. doi: 10.1128/AAC.50.5.1859-1860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pyrrho AS, Moraes JLC, Peçanha LMT, Gattas CR. 1998. Trypanosoma cruzi: IgG1 and IgG2b are the main immunoglobulins produced by vaccinated mice. Parasitol Res 84:333–337. doi: 10.1007/s004360050406. [DOI] [PubMed] [Google Scholar]
- 43.Powell MR, Wassom DL. 1993. Host genetics and resistance to acute Trypanosoma cruzi infection in mice. I. Antibody isotype profiles. Parasite Immunol 15:215–221. [DOI] [PubMed] [Google Scholar]
- 44.Guedes PMM, Bahia MT. 2004. Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts. Antimicrob Agents Chemother 48:4286–4292. doi: 10.1128/AAC.48.11.4286-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyland KV, Leon JS, Daniels MD, Giafis N, Woods LM, Bahk TJ, Wang K, Engman DM. 2007. Modulation of autoimmunity by treatment of an infectious disease. Infect Immun 75:3641–3650. doi: 10.1128/IAI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maya JD, Orellana M, Ferreira J, Kemmerling U, López-Muñoz R, Morello A. 2010. Chagas disease: present status of pathogenic mechanisms and chemotherapy. Biol Res 43:323–331. doi: 10.4067/S0716-97602010000300009. [DOI] [PubMed] [Google Scholar]
- 47.Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Talvani A, Ribeiro CS, Aliberti JC, Michailowsky V, Santos PV, Murta SM, Romanha AJ, Almeida IC, Farber J, Lannes-Vieira J, Silva JS, Gazzinelli RT. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect 2:851–866. doi: 10.1016/S1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 49.Golgher D, Gazzinelli RT. 2004. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity 37:399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]
- 50.Soares MB, Silva-Mota KN, Lima RS, Bellintani MC, Pontes-De-Carvalho L, Ribeiro-Dos-Santos R. 2001. Modulation of chagasic cardiomyopathy by interleukin-4: dissociation between inflammation and tissue parasitism. Am J Pathol 159:703–709. doi: 10.1016/S0002-9440(10)61741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutra WO, Gollob KJ. 2008. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis 21:287–292. doi: 10.1097/QCO.0b013e3282f88b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D, Bocchi EA, Teixeira HC, Mady C, Kalil J, Cunha-Neto E. 2001. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun 17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 53.Petray P, Corral R, Meckert P, Laguens R. 2002. Role of macrophage inflammatory protein-1alpha (MIP-1alpha) in macrophage homing in the spleen and heart pathology during experimental infection with Trypanosoma cruzi. Acta Trop 83:205–211. doi: 10.1016/S0001-706X(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 54.Martin D, Tarleton R. 2004. Generation specificity and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol Rev 201:304–317. doi: 10.1111/j.0105-2896.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 55.Choromanski L, Kuhn RE. 1985. Interleukin 2 enhances specific and nonspecific immune responses in experimental Chagas' disease. Infect Immun 50:354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acosta Rodriguez EV, Zuniga EI, Montes CL, Merino MC, Bermejo DA, Amezcua Vesely MC, Motran CC, Gruppi A. 2007. Trypanosoma cruzi infection beats the B-cell compartment favouring parasite establishment: can we strike first? Scand J Immunol 66:137–142. doi: 10.1111/j.1365-3083.2007.01968.x. [DOI] [PubMed] [Google Scholar]
- 57.Iwakami S, Shibuya M, Tseng CF, Hanaoka F, Sankawa U. 1986. Inhibition of arachidonate 5-lipoxygenase by phenolic compounds. Chem Pharm Bull 34:3960–3963. doi: 10.1248/cpb.34.3960. [DOI] [PubMed] [Google Scholar]
- 58.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. 1992. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull 40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 59.Maçao LB, Wilhelm Filho D, Pedrosa RC, Pereira A, Backes P, Torres MA, Fröde TS. 2007. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int J Cardiol 123:43–49. doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 60.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. 2008. The current state of serum biomarkers of hepatotoxicity. Toxicology 245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]