Abstract
Preexposure prophylaxis (PrEP) with 1% tenofovir (TFV) vaginal gel has failed in clinical trials. To improve TFV efficacy in vaginal gel, we formulated tenofovir disoproxil fumarate nanoparticles in a thermosensitive (TMS) gel (TDF-NP-TMS gel). TDF-NPs were fabricated using poly(lactic-co-glycolic acid) (PLGA) polymer and an ion-pairing agent by oil-in-water emulsification. The efficacy of TDF-NP-TMS gel was tested in humanized bone marrow-liver-thymus (hu-BLT) mice. Hu-BLT mice in the treatment group (Rx; n = 15) were administered TDF-NP-TMS gel intravaginally, having TDF at 0.1%, 0.5%, and 1% (wt/vol) concentrations, whereas the control (Ctr; n = 8) group received a blank TMS gel. All Rx mice (0.1% [n = 4], 0.5% [n = 6], and 1% [n = 5]) were vaginally challenged with two transmitted/founder (T/F) HIV-1 strains (2.5 × 105 50% tissue culture infectious doses). Rx mice were challenged at 4 h (0.1%), 24 h (0.5%), and 7 days (1%) posttreatment (p.t.) and Ctr mice were challenged at 4 h p.t. Blood was drawn weekly for 4 weeks postinoculation (p.i.) for plasma viral load (pVL) using reverse transcription-quantitative PCR. Ctr mice had positive pVL within 2 weeks p.i. Rx mice challenged at 4 h and 24 h showed 100% protection and no detectable pVL throughout the 4 weeks of follow-up (P = 0.009; Mantel-Cox test). Mice challenged at 7 days were HIV-1 positive at 14 days p.i. Further, HIV-1 viral RNA (vRNA) in vaginal and spleen tissues of Rx group mice with negative pVL were examined using an in situ hybridization (ISH) technique. The detection of vRNA was negative in all Rx mice studied. The present studies elucidate TDF-NP-TMS gel as a long-acting, coitus-independent HIV-1 vaginal protection modality.
INTRODUCTION
Presently, a total of 36.9 million people worldwide are living with HIV-1 (1). Topical preexposure prophylaxis (PrEP) currently is a promising preventative strategy (2). The basic idea is to protect the vagina (and/or rectum) from HIV-1 infection by applying gel containing antiretroviral drug(s) around the time of sexual intercourse. This topical preparation is considered a microbicide, inhibiting infection by blocking viral transmission at the mucosal surface. To date, tenofovir (TFV) is the only drug administered locally as a 1% vaginal gel shown to be effective at preventing heterosexual contraction of HIV-1 (3). TFV tissue concentrations indicate a direct relationship between levels of TFV in genitals and protection (4–7). The minimum amount of TFV in cervicovaginal fluid levels when associated with gel that shows protection against HIV-1 infection has been reported to be >1,000 ng/ml (4). This level is greater than 10 times that seen in patients receiving oral TDF and emtricitabine (4). In female macaques given 1% TFV gel, the intracellular half-life for the active metabolite, tenofovir diphosphate, is significantly shorter (averaging 25 h) in vaginal lymphocytes than peripheral PBMCs (averaging 49 h) (7). A coitally independent strategy using 1% TFV gel has not shown efficacy in several clinical trials (8, 9). Based on the dramatic negative results of the Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial, it is important to consider female attitudes and opinions for a vaginal gel-based prevention delivery system. A safe and effective female-controlled, discrete gel-based delivery system has the potential to prevent millions of HIV-1 infections worldwide annually.
When designing female-controlled preventative delivery systems, the gel-based system should have features important for the female user. Namely, the delivery system should be (i) easy to administer; (ii) adherent to the mucosal surface once applied vaginally; (iii) low seepage; and (iv) free of side effects or cytotoxicity to the mucosal surfaces of the female genital tract (10). All of these factors, if not optimized, could diminish gel effectiveness or lead to gel aversion. Finally, a long-acting preparation would be highly desirable if it offered long-term protection from HIV-1 (11).
Our laboratory has been developing a nanotechnology-based gel delivery system (11–16). Our gel delivery system incorporates a thermosensitive (TMS) gel that is liquid at room temperature and a semisolid at body temperature. Tenofovir disoproxil fumarate (TDF) plus emtricitabine (Truvada; Gilead Sciences) is the only FDA-approved oral PrEP. TDF is a TFV prodrug with higher permeability and significantly lower 50% effective concentrations (EC50s) against HIV-1 than those of TFV (17). The TDF-loaded vaginal ring has shown significantly better vaginal delivery than the tenofovir ring (18). Incorporation of TDF into nanoparticles (TDF-NPs) was investigated for improved antiviral protection. The TMS gel allows for easy administration, and once in contact with vaginal tissue, it gelates instantaneously and becomes a pliable semisolid at body temperature. We now report the results of TDF-NPs in a TMS gel (TDF-NP-TMS)-based preventative strategy using TDF-NPs in a humanized BLT (hu-BLT) mouse model of HIV-1 vaginal transmission.
MATERIALS AND METHODS
Poly(lactic-co-glycolic acid) (PLGA; 75:25 lactide-to-glycolide ratio; Mw, 4,000 to 15,000), poly(vinyl alcohol) (PVA) (MW, 13,000 to 23,000), sodium deoxycholate, and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pluronic F127 (PF-127) and Pluronic F68 (PF-68) were purchased from D-BASF (Edinburgh, United Kingdom), whereas TDF (99% purity) was from Gilead Sciences Inc. (Foster City, CA, USA). Dimethyl sulfoxide (DMSO) and acetonitrile were purchased from Fisher (Fair Lawn, NJ, USA). Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS), l-glutamine, trypsin, and penicillin-streptomycin (penstrep) solution were purchased from HyClone (Logan, UT, USA). All reagents were used as received without further purification.
NP preparation and characterization.
TDF-loaded PLGA polymer-based nanoparticles (NPs), i.e., TDF-NPs, were fabricated by the oil-in-water (O/W) emulsion-solvent evaporation method as described previously (11). To improve the encapsulation of hydrophilic TDF in nanoparticles, an ion-pairing agent (sodium deoxycholate) was used that results in hydrophobic an ion pair complex with TDF, resulting in enhanced TDF loading in the PLGA NPs. The size, polydispersity index (PDI), and zeta potential of the TDF-NPs were characterized by using the ZetaPlus zeta potential analyzer (Brookhaven Instruments Corp., Holtsville, NY, USA) (n = 7). To evaluate the encapsulation efficiency (EE) of the TDF in TDF-NPs (with or without the ion-pairing agent), TDF-NPs were dissolved in DMSO to release the trapped drug and the EE determined by high-performance liquid chromatography. The EE was determined using the equation % EE = (ANP/Ainitial) × 100, where Ainitial is the initial amount of drug added to the emulsion and ANP is amount of drug entrapped in the NP.
The topography of the TDF-NPs was evaluated by scanning electron microscopy (SEM) using a Hitachi S-4700 field-emission SEM (New York, NY, USA).
The TMS gel was prepared by following the method we described previously, with a few modifications (13). Briefly, a 20:1 ratio of Pluronic F127 to Pluronic F68 was dissolved in PBS, pH 7.4. The 0.1%, 0.5%, and 1% TDF-NP-TMS gels were obtained by dissolving the calculated amount of TDF-NPs that corresponds to 0.1, 0.5, and 1 g of TDF drug, respectively, in 100 ml of the TMS gel.
Cytotoxicity assay at the cell level.
HeLa (cervical epithelium) and H9 (lymphocyte) cell lines purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) were used to evaluate TDF-NP cytotoxicity at the cellular level. Both cell lines were maintained in DMEM with 10% FBS, 1× l-glutamine, and 1× penstrep solution. Medium was one-half exchanged with fresh media every 2 to 3 days. In these in vitro experiments, 1.0 × 104 HeLa and H9 cells were placed into 96-well plates overnight. Blank NPs, TDF solution, or TDF-NPs (10 μg/ml) were added to the cells in triplicate wells and incubated at 37°C, 5% CO2 over the course of either 24, 48, or 96 h. The cytotoxicity assay was based on percent viability analysis determined by a CellTiter-Glo kit purchased from Promega (Madison, WI, USA). Medium was one-half exchanged with fresh medium every 2 to 3 days. At prespecified intervals (24, 48, and 96 h), the cell viability was estimated by using the CellTiter Glo protocol. The cell viability results were measured using a baseline subtraction method from the luminometer. The data presented were determined based on an untreated control as 100% viability. Comparison of the results to control cell results was assessed using analysis of variance (ANOVA). These experiments were repeated on three independent occasions, and results are presented as means ± standard deviations (SD). These results are available in the supplemental material (see Fig. S1).
Cell viability assay at endocervical tissue level.
To determine vaginal cell viability at the tissue level, a cell viability assay was performed on endocervical tissue cultures. Briefly, endocervical tissue (EpiVaginal cultures; Mattek Corp., Ashland, MA) was placed into 6-well plates using cell culture inserts. After incubation for 1 h in prewarmed media (37°C, 5% CO2) containing the drug in NPs or solution, the medium was replaced with fresh medium, rinsed with ultrapure water, and transferred to a 24-well plate where the tissue samples were incubated on 300 μl of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution and 900 μl of medium. These plates were then incubated for 3 h (37°C, 5% CO2). Further, viability was determined based on optical densities read at 570 nm and 650 nm, respectively. Results were reported as percent viability based on the untreated tissue as 100% viability. All data represent means ± SD from three independent experiments.
Generation of hu-BLT mice.
Hu-BLT mice were generated by following the previously published protocols (19, 20). Briefly, 6- to 8-week-old NOD.Cg-PrkdcscidIL2rgtm1Wjl/Szj (NOD/SCID/IL2rgnull, or NSG) mice (The Jackson Laboratory) were purchased and maintained under pathogen-free conditions at the University of Nebraska-Lincoln Life Sciences Annex. Human fetal livers and thymus tissues were procured from Advanced Bioscience Resources (Alameda, CA, USA). On the day of surgery, mice received 12 cGy/g of mouse body weight from an RS200 X-ray irradiator (Rad Source Technologies). The irradiated mice were transplanted with two pieces of human fetal liver and one piece of thymic tissue fragment under the left kidney capsules, followed by injection of 1.5 × 105 to 2.3 × 105 fetal liver-derived CD34+ human stem cells (HSCs) intravenously (i.v.). These mice were allowed to grow for another 12 to 16 weeks to regenerate a human immune system. Those hu-BLT mice that showed a ratio of human leukocytes to total leukocytes greater than 50% in the peripheral blood were considered for challenge with HIV-1 infection.
Ethics statement.
All experiments in NSG mice adhered to the NIH Guide for the Care and Use of Laboratory Animals (Institutional Animal Care and Use Committee [protocol 1059], University of Nebraska-Lincoln) (21). The UNL IACUC committee approved protocol 1059.
HIV-1 vaginal challenge in hu-BLT mouse.
Female hu-BLT mice received 2 mg intraperitoneally (i.p.) of methoxyprogesterone to thin the female reproductive tract 5 days before experiments commenced. Mice had a ball roller (oral gavage apparatus) delivered to the vagina several times to simulate coitus, followed by delivery of the respective TMS gel formulations. Mice received 30 μl of TDF-NP-TMS gel, respectively, containing 0.1% (n = 4) or 0.5% (n = 6) TDF-NPs (i.e., 1 and 5 μg/ml TDF) intravaginally. After 4 and 24 h, the treatment (Rx) mice received 2.5 × 105 50% tissue culture infective doses (TCID50) in 20 μl from two HIV transmission/founder (T/F) viruses (WITO.c/2474 and SUMA.c/2821) from acutely infected patients. Control (Ctr) mice (n = 8) received blank TMS gel and were infected with the same T/F viruses after 4 h of gel administration. An additional cohort of humanized mice (n = 5) received 1% TDF-NP-TMS gel and was challenged after 7 days of gel administration.
Plasma viral load (pVL).
Plasma viral RNA was extracted from plasma using a QIAamp viral RNA minikit (Qiagen, Valencia, CA). vRNA was extracted from the pellet with proteinase K (2.5 μg/μl; Life Technology) and the high pure viral RNA kit (Roche). vRNA (100 μl) was eluted in 50 μl, from which 20 μl was reverse transcribed using MultiScribe reverse transcriptase (Life Technology) in a 50-μl gene-specific reaction mixture. Fourteen microliters of cDNA was added to TaqMan gene expression master mix (Life Technology), along with forward (5′-GCCTCAATAAAGCTTGCCTTGA-3′) and reverse (5′-GGGCGCCACTGCTAGAGA-3′) primers and a probe (5′-6-carboxyfluorescein-CCAGAGTCACACAACAGACGGGCACA-black hole quencher 1-3′) targeting the gag region of HIV-1, and the solutions were subjected to 45 cycles of quantitative PCR (qPCR) analyses. Fluorescence signals were detected with an Applied Biosystems 7500 sequence detector. Data were captured and analyzed with Sequence Detector software (Life Technology). Viral copy numbers were calculated by plotting threshold cycle (CT) values obtained from samples against a standard curve generated with in vitro-transcribed RNA representing known viral copy numbers (22). The limit of detection of the assay was 800 copies per ml of plasma.
HIV-1 vRNA detection in tissues using ISH.
In situ hybridization (ISH) was conducted according to previously published methods (22, 23). In brief, animal vaginal and spleen tissues were collected after euthanasia and fixed in 4% paraformaldehyde. Approximately 6-μm tissue sections of the vaginal and spleen tissues were cut and adhered to a SuperFrost plus slide, fixed, and air dried. The sections were then rehydrated, permeabilized, and acetylated prior to hybridization to 35S-labeled HIV riboprobes. After washing and digestion with RNase, sections were coated with nuclear track emulsion, exposed for 7 days, developed, and counterstained with hematoxylin and eosin (H&E) stain.
TFV cervicovaginal lavage concentrations over time.
TDF-NPs in TMS gel was administered to NSG mice (not humanized), and cervicovaginal lavage was performed in parallel at 24 h after vaginal instillation of TDF-NPs-TMS gel (1% TDF-NPs) and compared it to the hydroxyethyl cellulose (HEC) gel containing 1% TFV. Cervicovaginal lavage (90 μl) was subjected to heat inactivation (65°C), protein precipitation with 70 μl of acetonitrile, and centrifugation at 13,000 rpm for 10 min. Aliquot samples (10 μl) were analyzed on an ABSCI 5000 liquid chromatograph-tandem mass spectrometer (LC/MS-MS). The TFV isotope (13C adenine moiety) was used as the internal standard. The LC/MS-MS was run in the positive ion mode as previously described (24). Interday and intraday variation was ≤15%, and the limit of detection was 10 ng/ml.
RESULTS
TDF nanoparticle characterization.
As represented in Table S1 in the supplemental material, the TDF-NPs obtained by the O/W emulsion method has a mean (± SD) particle size of TDF-NPs of 148.6 ± 2.8 nm, and the surface charge was −26.7 ± 1.08 mV. The polydispersity index reveals that the TDF-NPs obtained had a very narrow size distribution, i.e., they were quite uniform in size. The EE analysis of TDF in TDF-NPs (in the absence of ion-pairing agents) was 16.1%. However, in the presence of an ion-pairing agent, the mean EE of TDF in TDF-NPs increased to 52.9%. We have already reported characteristics of our TMS gel formulation (13). Further, we optimized the TMS gel fabrication process to incorporate TDF-NPs to obtain uniform TDF-NP-TMS gels having different concentrations of TDF (0.1%, 0.5%, and 1%, wt/vol). Figure 1 shows an electron microscope image of the nanoparticles illustrating the uniformity of the size and surface morphology of the TDF-NPs (25).
FIG 1.

Electron microscopy image of TDF-NPs.
TDF NP cytotoxicity at in vitro level.
An in vitro cytotoxicity examination of HeLa and H9 cell lines validates that TDF-NPs were noncytotoxic during all studied time points (see Fig. S1 in the supplemental material). Interestingly, TDF-NPs as well as TDF solution showed a trend of inducing cell viability. As expected, as a positive control, 1% Triton X-100 treatment results in extreme cytotoxicity.
TDF-NPs designed to be utilized in the vaginal gel required cytotoxicity assessment at the endocervical tissue level compared to results with TDF solution and the untreated control (Fig. 2). The results demonstrate that TDF-NPs and TDF solution were noncytotoxic compared to control, untreated endocervical tissue at 24 h. Both TDF-NPs and TDF solution demonstrated significant cytotoxicity at 48 h, which resolved by the 96-h determination. Again, as expected, 1% Triton treatment as a positive control results in extreme cytotoxicity.
FIG 2.
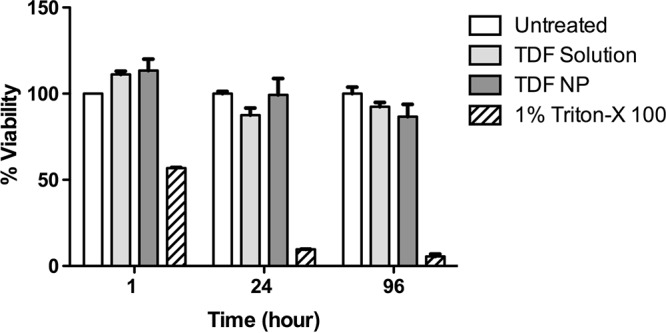
Endocervical tissue viability. Vaginal tissue explants (n = 3) were subjected to TDF solution, TDF-NPs, or 1% Triton X-100, and untreated tissue served as positive and negative controls. Tissue viability was determined using MTT assay methodology at 1, 24, and 96 h of incubation in triplicate for each experiment.
Hu-BLT PrEP experiments.
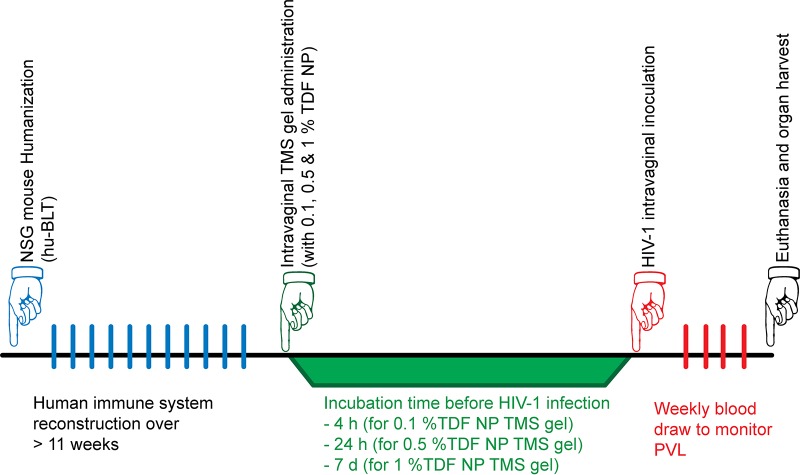
The prevention experimental study design using hu-BLT mice is illustrated in Fig. 3. The Ctr hu-BLT mice (n = 8) that received blank TMS gel intravaginally were challenged with the same dose and strains of HIV-1 after 4 h, and all contracted HIV-1 within 7 to 14 days (Fig. 4). However, treated hu-BLT mice that received 0.1% TDF-NPs (n = 4) that were challenged with HIV-1 4 h later were all protected from HIV-1 infection. Similarly, all of the Hu-BLT mice (n = 6) that received 0.5% TDF-NPs prior to viral exposure and were challenged at 24 h with HIV-1 were also protected from HIV-1 infection (Fig. 4). However, hu-BLT mice that received 1% TDF-NP-TMS gel and were challenged 7 days later with HIV-1 were found to be infected at 14 days postinfection. Taking these results in total, TDF-NPs in TMS gel given at 4 h (0.1%) and 24 h (0.5%) demonstrated significant (P = 0.005; Mantel-Cox test) protection from HIV-1 vaginal transmission when delivered in the TMS vaginal gel.
FIG 3.
Schematic presentation of the HIV-1 prevention experimental design. To generate humanized NSG mice, the mice underwent a humanization procedure and were allowed to reconstitute their human immune system for a minimum of 12 weeks. We randomly divided them into control (Ctr) and treatment (Rx) groups. The Rx group received TMS gel containing 0.1% or 0.5% (wt/vol) TDF-NPs intravaginally for 4 h, 24 h, or 7 days. After the respective treatment incubation time, all of the Ctr and Rx mice were inoculated with HIV-1 (two strains; 2.4 × 105 TCID50) intravaginally. At weekly intervals, blood was withdrawn to monitor for pVL using qRT-PCR. At euthanasia, FRT and spleen were harvested and fixed in 4% paraformaldehyde for detection of viral RNA (vRNA) using ISH.
FIG 4.
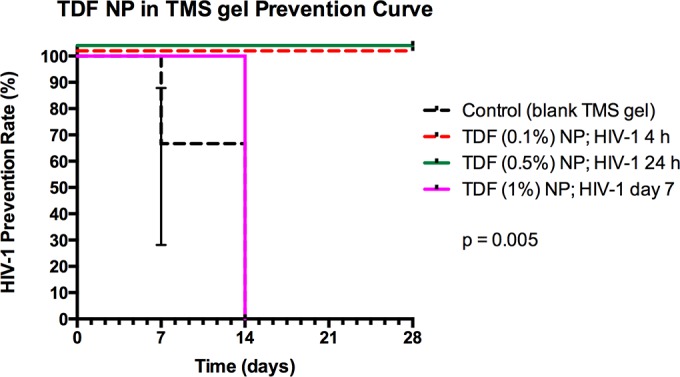
TDF-NPs in TMS gel prevention curve. Control humanized mice (n = 8) received blank TMS gel, and 100% of them were infected within 14 days p.i. All humanized mice receiving 0.1% TDF-NPs and challenged 4 h later (n = 4) or receiving 0.5% TDF NPs and challenged 24 h later (n = 6) were protected from HIV-1 infection (P = 0.005).
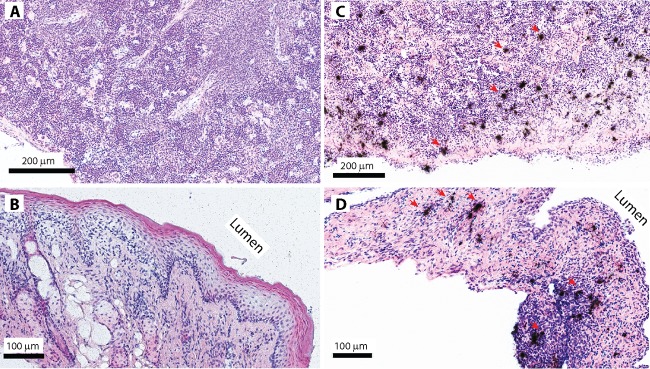
To determine the efficacy of TDF-NP-TMS gel in vivo at the tissue level, the presence of HIV-1 vRNA was evaluated in the female reproductive tract (FRT) and spleen tissues using ISH, and results are shown in Fig. 5. Interestingly, those hu-BLT mice that had undetectable pVL (0.1% and 0.5% TDF-NPs) also showed the absence of HIV-1 vRNA both in vaginal and spleen tissues. These results substantiate that compared to control mice, 0.5% TDF-NPs were able to protect female hu-BLT mice from HIV-1 challenge over a prolonged time frame using an intravaginal route of transmission.
FIG 5.
HIV-1 vRNA detection in p24 in female reproductive tract and spleen tissues. Shown are representative images of HIV-1 vRNA detection in vaginal and spleen tissues of hu-BLT mice using in situ hybridization. The clusters of black silver grains overlay SIV vRNA-positive cells after the autoradiography of 35S-labeled HIV-specific riboprobes. HIV-1 vRNA was not detected in the spleen (A) and vaginal tissues (B) of animals that were protected from vaginal HIV-1 challenge with TDF-NPs in TMS gel. In contrast, HIV-1 vRNA was readily detected in the spleen (C) and vaginal tissues (D) of control animals (red arrows).
TFV CVL samples.
Finally, TFV concentrations in cervicovaginal lavage (CVL) samples were characterized in female NSG mice in parallel to understand the intravaginal TFV levels at the time of HIV-1 inoculation. The lower limit of detection was 10 ng/ml. TFV drug levels (means ± SD) were 28.3 ± 15.8 ng/ml when 1% TFV in HEC gel was administered to the mice and CVL collected at 24 h. TFV levels averaged 49.3 ± 66.6 ng/ml when TDF-NPs were administered and CVL collected at 24 h. This difference is not significant. Further study of TFV drug levels as well as active metabolite levels at the time of HIV infection would be necessary to demonstrate a concentration-effect response.
DISCUSSION
TFV is the most widely investigated antiretroviral drug used for HIV-1 prevention. However, clinical trial results employing 1% tenofovir vaginal gel have been discouraging (9, 26). Hydrophilicity and low cellular permeability can be reasons for the poor performance of TFV vaginal gel. Indeed, <5% of TFV permeated through HEC-1A cells using a transwell experimental design, corroborating low permeability (6). Therefore, it is essential to develop a strategy that will improve TFV cellular permeability when used for prevention of HIV-1. TDF has greater bioavailability and offers higher cellular permeability, as evidenced by a 100-fold lower 50% infectious dose (IC50) against HIV-1BaL than against TFV (7). TDF is an ester prodrug that hydrolyzes in water and/or in the presence of cellular esterases to TFV. We focused on developing a TDF-loaded PLGA nanoparticle formulation in a TMS gel for improved delivery of TFV. However, TDF has considerable water solubility (13.4 mg/ml at 25°C), and it is well known that hydrophilic drugs are not easy to encapsulate into nanoparticles. There have been few attempts to encapsulate TFV or TDF in PLGA nanoparticles (27, 28). None of these investigations were able to achieve greater than 10% encapsulation of TFV or TDF in the nanoparticles. To achieve successful translation, nanoparticles should be able to achieve high encapsulation of TDF so that maximum drug is utilized. We therefore focused on an ion-pairing approach to increase the encapsulation efficiency of TDF in the nanoparticles (12).
The use of a thermosensitive vaginal gel incorporating TDF-NPs is a novel formulation for a female-controlled delivery system that has many advantages. As the gel is liquid at room temperature, it is easy to administer and forms a firm semisolid gel upon contact with body temperature surfaces; therefore, it is likely to be less prone to have vaginal seepage after application than the hydroxyethyl cellulose gel that is commonly used in vaginal preparations. Employing the thermosensitive properties of this gel will provide less vaginal seepage and increased compliance with use of the preparation. The TMS gel may also keep TDF nanoparticles in contact with the epithelial surface of the FRT over a longer time frame (15).
Rheology has been used to study the thermogelation properties of our gel in the presence of simulated cervicovaginal fluid (11–14). In the presence of simulated vaginal fluids, the optimized formulation of TMS gel was able to retain thermogelling behavior at approximately 32 to 34°C. We believe that this will increase acceptance and adherence for women. Additionally, this gel is a female-controlled, discrete prevention method not currently available. When TMS gel without TDF nanoformulation was used in the control mice, it offered no protection from HIV-1 challenge; thus, it is a delivery vehicle that allows for greater contact between the antiretroviral therapy and the cells on the epithelial surface (15).
The TDF-NPs with an ion-pairing agent, docusate sodium, required a cytotoxicity evaluation. Hence, we evaluated the cytotoxicity of TDF solution and TDF-NPs against various cell lines using a CellTiter-Glo assay (see Fig. S1 in the supplemental material). The TDF solution and TDF-NPs caused minimal toxicity to HeLa and H9 cells. There was no difference in cytotoxicity profile of TDF-NPs and TDF solution, indicating that docusate sodium did not adversely affect the cytotoxicity potential.
It is known that nucleoside reverse transcriptase inhibitors, notably stavudine, have been associated with mitochondrial toxicity (30). Other investigators that have fabricated nucleoside reverse transcriptase inhibitor-based nanoformulations containing zidovudine or didanosine triphosphate (at 15 μg/ml) have demonstrated reduced mitochondrial toxicity similar to these results (31). We carried out ex vivo cytotoxicity studies using three-dimensional human vaginal endocervical tissue (EpiVaginal; MatTek Corp). We evaluated the toxicity potential of TDF-NPs, TDF solution, and untreated and 1% Triton X-100-treated solutions (controls) for 1, 24, 48, and 96 h per the manufacturer's protocol (Fig. 2). As expected, the vaginal irritant Triton X-100 showed considerable cytotoxicity to vaginal endocervical tissues. TDF-NPs and solution demonstrated cytotoxicity at 48 h, which resolved at 96 h. These results could be due to the exchange of fresh media.
The hu-BLT mouse model of HIV-1 infection has gained attention for evaluation of microbicides. It has already been demonstrated that 1% TFV gel applied 4 h before and after HIV-1 challenge (same design as that of the CAPRISA 004 trial) offered partial protection in hu-BLT mice, indicating a good correlation with CAPRISA 004 results (32). Additionally, mice rapidly metabolize TFV compared to humans; therefore, they may not completely mimic humans for microbicide discovery. We elected not to include a mouse cohort that received TFV solution in TMS gel, as the control mice became infected after gel administration. All humanized BLT mice that received 1% TDF-NPs and were challenged 7 days after gel administration became infected (Fig. 4). This may be related to the TFV vaginal tissue levels, the active metabolite in tissue, or the volume of administered TMS gel not remaining in the vaginal tract for this length of time. Nevertheless, the hu-BLT mouse model offers an economical means of screening microbicides before moving to macaques and humans.
Previous data from humans have demonstrated that 1% TFV gel produces a protective effect when CVL levels were >1,000 ng/ml (4). Donnell et al. investigated TFV plasma levels in serodiscordant couples receiving an oral tenofovir-emtricitabine combination (33). The majority of HIV-negative individuals in the serodiscordant relationship had TFV plasma concentrations of >40 ng/ml, consistent with daily drug dosing, whereas individuals who contracted HIV did not have that threshold level of TFV. In our experiments with NSG mice, the CVL TFV levels from the NP formulation were 1.75 times higher than those for mice receiving the 1% TFV in HEC gel at 24 h after administration. More study of the concentration-response relationship is necessary to determine the amount of TFV and/or active metabolite required for HIV prevention. Veselinovic et al. investigated mucosal tenofovir tissue levels in humanized mice (34). These investigators used oral TDF at 61.5 mg/kg daily by oral gavage for 5 days before harvesting plasma and tissue for tenofovir determination. The half-life of oral TDF in these mice averaged 9 h. Our NP formulation (0.5%) instilled into the female mouse vagina was 5 mg/ml, or 150 μg in 30 μl, which could be administered into the female mouse vagina. We did not investigate TFV drug levels or obtain the levels of the active metabolite, tenofovir diphosphate, in the FRT tissue but did get cervicovaginal lavage fluid from NSG mice in parallel. The diphosphate metabolite was below detectable limits, as in the previous humanized mouse study (32). Further study in macaques is necessary to develop this microbicide.
Our nanoparticles, fabricated using an FDA-approved PLGA polymer, are different from the particles made by wet-milling nanofabrication methods that others have published for treatment as well as prevention (35–38). Roy et al. used their wet-milled nanoparticles containing atazanavir and ritonavir for treatment of HIV-1 in NSG mice reconstituted with human peripheral blood leukocytes (PBLs) (35). Jackson et al. utilized Elan nanofabrication technology to fabricate rilpivirine nanoparticles (36). In this study, HIV-negative human volunteers received increasing single doses of rilpivirine-LA and plasma, and genital levels were measured in females and plasma and rectal levels were assessed in men. The results of these experiments showed that rilpivirine-LA injected intramuscularly would be able to prevent HIV-1 challenge in both men and women, but protective concentrations in plasma or reproductive tract tissues are not known. Andrews et al. investigated the protective effect of increasing single or multiple doses of cabotegravir (GSK1265744) in nonhuman primates against multiple low doses of HIV challenge (37). Results demonstrated correlation between protection and plasma drug levels, and a potential every-3-month dosing schedule in humans would be useful to reduce adherence difficulties that are problematic for preexposure prophylaxis.
TFV 1% vaginal gel and combinations of TDF-plus-emtricitabine tablets (Gilead Science) are currently the only prevention regimens that have shown efficacy in human trials. Additional prevention modalities are needed. Additionally, the prevention options should be controlled by the end-user. Each woman has different circumstances and needs. Therefore, vaginal and oral ingestion options are needed. We present a new preventive option that integrates a thermosensitive gel along with TDF nanoparticles that has shown sustained release properties in these humanized BLT mice when instilled locally. Further pharmacokinetic and efficacy studies in other animal models are needed to extend these results to humans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lee Winchester at the University of Nebraska Medical Center College of Pharmacy for determining tenofovir CVL drug levels by liquid chromatography-mass spectrometry. We thank Gilead Sciences Inc. for donating TDF powder.
These experiments were supported by a Nebraska Center for Virology (NCV) intramural award and R01AI117740-01 (to C.J.D.), LB692 Clinical and Translational Science Research Grant (to A.A.D. and C.J.D.), and the Creighton University President's Research Award (to A.S.). NCV is supported by an NIH Institutional Development Award (IDeA; P20GM1034267).
The funding agencies did not have any role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00450-16.
REFERENCES
- 1.UNAIDS global report. 2013. UNAIDS report on the global AIDS epidemic 2013. UNAIDS, Geneva, Switzerland: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf Accessed 19 February 2014. [Google Scholar]
- 2.Baeten J, Celum C. 2013. Systemic and topical drugs for prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med 64:219–232. doi: 10.1146/annurev-med-050911-163701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Masoor LE, Kharsany AB, Sibeko S, Misana KP, Omar Z, Gengiah TB, Maarschalk S, Arulappan M, Moltshwa M, Morris L, Taylor D, CAPRISA 004 Trial Group . 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdool Karim SS, Kashuba AD, Werner L, Abdool Karim Q. 2011. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller MJ, Madan RP, Torres NM, Fazzari MJ, Cho S, Kalyoussef S, Shust G, Mesquita PM, Louissaint N, Chen J, Cohen HW, Diament EC, Lee AC, Soto-Torres L, Hendrix CW, Herold BC. 2011. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik A, Lipscomb J, Hanson DL, Smith J, Novembre FJ, Garcia-Lerma JG, Heneine W. 2012. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol 86:718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duwal S, Schutte C, von Kleist M. 2012. Pharmacokinetics and pharmacodynamics of the reverse transcriptase inhibitor tenofovir and prophylactic efficacy against HIV-1 infection. PLoS One 7:e40382. doi: 10.1371/journal.pone.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celum C, Baeten J. 2012. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evidence and evolving questions. Curr Opin Infect Dis 25:51–57. doi: 10.1097/QCO.0b013e32834ef5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D, FEM-PrEP Study Group . 2012. Pre-exposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham BK, Gulick R. 2012. Next-generation oral preexposure prophylaxis: beyond tenofovir. Curr Opin HIV AIDS 7:600–606. doi: 10.1097/COH.0b013e328358b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Date AA, Destache CJ. 2013. Potential of nanotechnology to improve prophylaxis for HIV/AIDS. Biomaterials 34:6202–6228. doi: 10.1016/j.biomaterials.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Date AA, Shibata A, Goede M, Sanford B, La Bruzzo K, Belshan M, Destache CJ. 2012. Development and evaluation of a thermosensitive vaginal gel containing raltegravir + efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res 96:430–436. doi: 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Date AA, Shibata A, McMullen E, La Bruzzo K, Bruck P, Belshan M, Zhao Y, Destache CJ. 2015. Thermosensitive gel containing cellulose acetate phthalate-efavirenz nanoparticles for prevention of HIV-1 infection. J Biomed Nanotech 11:416–427. doi: 10.1166/jbn.2015.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect Dis 9:198. doi: 10.1166/jbn.2015.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovarova M, Council OD, Date AA, Long JM, Nochiii T, Belshan M, Shibata A, Vincent H, Baker CE, Thayer WO, Kraus G, Lachaud-Durand S, Williams P, Destache CJ, Garcia JV. Nanoformulations of rilpivirine for topical pericoital and systemic coitus-independent administration efficiently prevent HIV transmission. PLoS Pathog 11:e1005075. doi: 10.1166/jbn.2015.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata A, McMullen E, Pham A, Belshan M, Sanford B, Zhou Y, Goede M, Date AA, Destache CJ. Polymeric nanoparticles containing combination antiretroviral drugs for HIV type 1 treatment. AIDS Res Hum Retrovir 29:746–754. doi: 10.1166/jbn.2015.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesquita PM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, Kiser PF, Herold BC. 2012. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 67:1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss JA, Malone Am Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau CP, Srinivasan P, Smith JM, Baum MM. 2012. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. AIDS 26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roncarolo MG, Carballido JM. 2001. Construction of human-SCID chimeric mice. Curr Protoc Immunol Chapter 4:Unit 4.8. [DOI] [PubMed] [Google Scholar]
- 20.Wang L-X, Kang G, Kumar P, Lu W, Li Y, Zhou Y, Li Q, Wood C. 2014. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc Natl Acad Sci U S A 111:3146–3151. doi: 10.1073/pnas.1318175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 22.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Miller CJ, Haase AT. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152. [DOI] [PubMed] [Google Scholar]
- 23.Abdulhaqq SA, Martinez MI, Kang G, Foulkes AS, Rodriguez IV, Nichols SM, Hunter M, Sariol CA, Ruiz LA, Ross BN, Yin X, Speicher DW, Haase AT, Marx PA, Li Q, Kraiselburd EN, Montaner LJ. 2014. Serial cervicovaginal exposures with replication-deficient SIVsm induce higher dendritic cell (pDC) and CD4+ T-cell infiltrates not associated with prevention but a more severe SIVmax251 infection of rhesus macaques. J Acquir Immune Defic Syndr 65:405–413. doi: 10.1097/QAI.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delahunty T, Bushman L, Robbins B, Fletcher CV. 2009. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci 877:1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal S, Zhou Y, Shibata A, Destache CJ. 2015. Confocal fluorescence microscopy: an ultra-sensitive tool used to evaluate intracellular antiretroviral nano-drug delivery in HeLa cells. AIP Adv 5:084803. doi: 10.1063/1.4926584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celum C, Baeten JM. 2012. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis 25:1–7. doi: 10.1097/QCO.0b013e32834f14fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng J, Zhang T, Agrahari V, Ezoulin MJ, Youan BB. 2014. Comparative biophysical properties of tenofovir-loaded, thiolated and nonthiolated chitosan nanoparticles intended for HIV prevention. Nanomedicine (Lond) 9:1595–1612. doi: 10.2217/nnm.13.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. 2015. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retrovir 31:107–114. doi: 10.1089/aid.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose T, Latawiec D, Mondal PP, Mandal S. 2014. Overview of nano-drugs characteristics for clinical application: the journey from the entry to the exit point. J Nanoparticle Res 16:1–25. doi: 10.1007/s11051-014-2527-7. [DOI] [Google Scholar]
- 30.Galluzzi L, Pinti M, Troiano L, Prada N, Nasi M, Ferraresi R, Salomoni P, Mussini C, Cossarizza A. 2005. Changes in mitochondrial RNA production in cells treated with nucleoside analogues. Antivir Ther 10:191–195. [PubMed] [Google Scholar]
- 31.Vinogradov SV, Poluektova LY, Makarov E, Gerson T, Senanayake MT. 2010. Nano-NRTIs: efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicity. Antivir Chem Chemother 21:1–14. doi: 10.3851/IMP1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, Welch BD, Kay MS, Payne DA, Gallay P, Appella E, Estes JS, Lu M, Garcia JV. 2011. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, Celum C. 2014. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 66:340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veselinovic M, Yang K-H, LeCureux J, Sykes C, Remling-Mulder L, Kashuba AD, Akkina R. 2014. HIV pre-exposure prophylaxis: mucosal tissue drug distribution of RT inhibitor tenofovir and entry inhibitor maraviroc in a humanized mouse model. Virology 464-465:253–263. doi: 10.1016/j.virol.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy U, McMillan J, Alnouti Y, Gautum N, Smith N, Balkundi S, Dash P, Gorantla S, Martinez-Skinner A, Meza J, Kanmogne G, Swindells S, Cohen SM, Mosley RL, Poluektova L, Gendelman HE. 2012. Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1-infected human peripheral blood lymphocyte-reconstituted mice. J Infect Dis 206:1577–1588. doi: 10.1093/infdis/jis395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson AG, Else LJ, Mesquita PMM, Egan D, Back DJ, Karolia Z, Ringner-Nackter L, Higgs CJ, Herold BC, Gazzard BG, Boffito M. 2014. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther 96:214–323. doi: 10.1038/clpt.2014.74. [DOI] [PubMed] [Google Scholar]
- 37.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, Russell-Lodrigue K, Bohm RP, Cheng-Mayer C, Hong Z, Markowitz M, Ho DD. 2014. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 343:1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spreen WR, Margolis DA, Pottage JC Jr. 2013. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV and AIDS 8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.