Abstract
Several population pharmacokinetic models describe the dose-exposure relationship of tobramycin in pediatric patients. Before the implementation of these models in clinical practice for dosage adjustment, their predictive performance should be externally evaluated. This study tested the predictive performance of all published population pharmacokinetic models of tobramycin developed for pediatric patients with an independent patient cohort. A literature search was conducted to identify suitable models for testing. Demographic and pharmacokinetic data were collected retrospectively from the medical records of pediatric patients who had received intravenous tobramycin. Tobramycin exposure was predicted from each model. Predictive performance was assessed by visual comparison of predictions to observations, by calculation of bias and imprecision, and through the use of simulation-based diagnostics. Eight population pharmacokinetic models were identified. A total of 269 concentration-time points from 41 pediatric patients with cystic fibrosis were collected for external evaluation. Three models consistently performed best in all evaluations and had mean errors ranging from −0.4 to 1.8 mg/liter, relative mean errors ranging from 4.9 to 29.4%, and root mean square errors ranging from 47.8 to 66.9%. Simulation-based diagnostics supported these findings. Models that allowed a two-compartment disposition generally had better predictive performance than those that used a one-compartment disposition model. Several published models of the pharmacokinetics of tobramycin showed reasonable low levels of bias, although all models seemed to have some problems with imprecision. This suggests that knowledge of typical pharmacokinetic behavior and patient covariate values alone without feedback concentration measurements from individual patients is not sufficient to make precise predictions.
INTRODUCTION
Tobramycin is an aminoglycoside antibiotic and one of the most effective antibiotics for the treatment of severe Gram-negative bacterial infections (1). Tobramycin is particularly effective against Pseudomonas aeruginosa and is commonly used in patients with cystic fibrosis (2). Tobramycin exhibits concentration-dependent bactericidal action, whereby higher drug concentrations are correlated with a greater magnitude and a higher speed of bacterial eradication (3, 4). Treatment success and a decreased incidence of antibiotic resistance have been correlated with a ratio of the peak (maximum) tobramycin concentration (Cmax) to the MIC of >7 to 10 (5, 6) and a ratio of the area under the concentration-time curve (AUC) to the MIC of >80 (7, 8). A prolonged high level of exposure to tobramycin and, in particular, sustained, elevated trough (minimum) serum concentrations (Cmin) have been associated with significant adverse effects (9, 10), such as nephrotoxicity, which is typically reversible, and ototoxicity, which can be permanent and result in hearing loss (4). Consequently, tobramycin dosing aims to achieve a sufficiently high Cmax and AUC for effective bactericidal activity while producing a sufficiently low Cmin to minimize the potential for toxicity (11) and the emergence of resistant microorganisms (12).
Furthermore, tobramycin has a narrow therapeutic window and displays a high level of pharmacokinetic variability (11). This variability is particularly increased in pediatric patients, if great changes in maturation, body size, and renal function over a short time frame are not accounted for (13). As a result, if treatment for more than 48 h is required (14), some guidelines recommend computerized therapeutic drug monitoring with Bayesian forecasting software programs (15, 16). Such methods are increasingly being used in practice (17). Bayesian forecasting methods use a priori pharmacokinetic parameters from a suitable population model and current concentration observations to estimate each patient's exposure (15, 18, 19). A suitable population model that characterizes average exposure after drug administration, along with pharmacokinetic variability and the influence of patient covariate factors, such as the patient's age, weight, and creatinine clearance (CLCR), on pharmacokinetic parameters, is required (20–22). Before a population pharmacokinetic model can be confidently used in clinical practice in a Bayesian forecasting computer program, it must be adequately validated (23). Model validation seeks to quantify the reproducibility and accuracy of a model (24). The most robust form of validation is external validation, which involves the collection of data from a new patient cohort receiving the drug and assessment of the predictive performance of the model (23, 24).
This study aimed to identify and compare population pharmacokinetic models of intravenous tobramycin previously published in the literature and externally validate these models by applying them to a new patient cohort to assess their predictive performance. The overarching goal of the study was to find suitable models to aid computerized therapeutic drug monitoring.
MATERIALS AND METHODS
Literature search.
A search of the literature in the databases PubMed, Medline, and EMBASE was performed, and published models of the population pharmacokinetics of tobramycin were identified. The search was conducted from February to March 2016 using the terminology “tobramycin,” “pharmacokinetics,” and “intravenous” and/or “population pharmacokinetics,” “nonlinear mixed effects modeling,” and “NONMEM.” A manual search for population models was also conducted by inspecting the bibliographies of relevant journal articles. No restriction was placed on the study publication date; the study language was limited to English. Population models were included in this study if they had been developed with data from humans and included data from pediatric patients, the pharmacokinetic analyses had involved a compartmental approach, and the majority of concentration-time points modeled (>50%) were based on measurements for tobramycin, as opposed to other aminoglycosides. Population models were excluded from the external validation if not enough information was provided for the model to be fully reproducible in terms of typical parameter values or covariate relationships or the residual unexplained variability (RUV) reported was above 50%.
Patients and validation data collected.
Pharmacokinetic and demographic data were collected retrospectively from the medical records of pediatric patients with cystic fibrosis who received treatment with intravenous tobramycin at the Lady Cilento Children's Hospital in Brisbane, Australia. This study was approved by the Ethics Committees of the University of Queensland (approval number 2012001415) and Lady Cilento Children's Hospital (approval number HREC/12/QHC75). No patient data in the external validation data set had previously been included in the development of any of the models. The pharmacokinetic data recorded included the tobramycin dose administered, the time of dosing, the infusion time and rate, tobramycin plasma concentrations, and the times at which serum samples were taken for measurement of the tobramycin concentration. Data were included if at least one intravenous dose of tobramycin and the respective concentration-time measurement were available. The demographic data recorded included the following: patient age, sex, weight, and height; the indication for tobramycin treatment; and serum creatinine (SCR) concentrations. Missing SCR measurements were replaced by an expected mean SCR concentration, based on the age and gender of the patient, as described by Ceriotti et al. (25). SCR measurements recorded as <30 μmol/liter, which is below the limit of quantification (LOQ), were replaced by a value of 29 μmol/liter. Data were excluded from the analysis if there was uncertainty about the time of dosing or the time of drug concentration measurement and if dosing information was absent. An occasion was defined as a single tobramycin dosing interval. Tobramycin concentrations were measured using fluorescence polarization immunoassays, which had a lower LOQ of 0.2 mg/liter. The within- and between-assay coefficients of variation were below 10%.
Assessment of predictive performance.
Each selected population pharmacokinetic model was separately implemented in NONMEM (version 7.3) (26) as described in the original article, and the output was visually explored by the use of graphics produced by the Rstudio program (version 0.99.484). Parameter values and covariate relationships for each model were set to those determined in the publications, and predictions were generated on the basis of the doses, sampling times, and covariate values recorded in the validation data set. If the models did not report between-subject variability (BSV) for the pharmacokinetic parameters or RUV in the model, a BSV value of 30% for clearance was supplemented and an additive error of 0.05 mg/liter and a proportional error of 20% were supplemented for RUV. For models that included renal function as a covariate, creatinine clearance (CLCR) was estimated from patient demographics in the validation data set according to the exact method described in each published study.
The predictive performance of each model was assessed visually by comparing plots of the population predicted concentration (Cpred) and the observed concentration (Cobs) to look for bias (a systematic upward or downward deviation from the line of unity in these plots) and imprecision (a high degree of scatter of data points around the line of unity) and numerically through calculation of the mean error (ME; in milligrams per hour; equation 1), the mean relative error (MRE; in percent; equation 2), and the relative root mean squared error (RMSE; in percent; equation 3) (27).
| (1) |
| (2) |
| (3) |
Predicted performance was also assessed by the use of simulation-based diagnostics. Visual predictive check (VPC) (28) plots were generated on the basis of 1,000 Monte Carlo simulations by applying the literature models and by using the validation data set design. The 10th and 90th percentiles of the simulated and validation data were plotted versus time after dosing, and the plots were compared visually for model deviations from the validation data. Plots of the normalized prediction distribution error (npde) versus time after dosing were also generated on the basis of 1,000 Monte Carlo simulations of the validation data set's patient dosage history and covariate values. The mean, variance, skewness, and kurtosis of the npde values were calculated and examined statistically (29, 30).
RESULTS
Literature search.
Figure S1 in the supplemental material shows the steps involved in the literature search. Seven studies describing a total of eight population pharmacokinetic models of tobramycin were deemed appropriate for external validation. The models were numbered from 1 to 8 and are referred to as M1 to M8. Table 1 provides a summary of the key information about the published population pharmacokinetic models of tobramycin that were examined. Two models were developed with data from both adults and pediatric patients (age, ≤18 years), and six models were developed only with data from pediatric populations. The largest model was developed with data from 732 adults and pediatric patients with and without cystic fibrosis and 5,605 samples in which the tobramycin concentration was determined, while the smallest model was developed with data from 35 cystic fibrosis patients providing 318 concentration samples in a mainly pediatric population (0.5 to 17.9 years). All models were based on intravenous administration of tobramycin with first-order linear elimination. Five models were developed solely with data from cystic fibrosis patients, one was developed with data from patients with and without cystic fibrosis, and the remaining model was developed with data from general population patients, neonates, and patients with severe infections or in an intensive care unit (ICU). While some models included data from patients with moderate renal impairment, the average renal function in each study population was normal to mildly reduced.
TABLE 1.
Summary of published models of the population pharmacokinetics of tobramycin for external validationa
| Model no. (reference) | No. of patients (total no. of samples) | Age (yr) | Wt (kg) | CLCR | Structural model | Parameter values and covariate relationships included | BSV (%) (BOV [%]) | RUV |
|---|---|---|---|---|---|---|---|---|
| Adults and pediatric patients | ||||||||
| M1 (32) | 136 CF patients (NR) | 5–50b | 15–82b | 30–148 ml/minb | 1 CMT | kel (h−1) = 0.01 + 0.00294 × CLCR | NR | NR |
| CL (liters/h) = V × kel | ||||||||
| V (liters) = 0.335 × wt | ||||||||
| M2 (36) | 732 CF and general patients (5,605) | 0.01–85.0b | 3.3–120.0b | 34–145 ml/minb | 2 CMT | CL (female) (liters/h/70 kg) = 8.08 × (LBW/70)0.952 × (1 + [−0.021 × (age − 18)]) × (mean SCR/SCR)0.222 | CL = 25.9 (12.7) | Prop = 20.4% |
| CL (male) (liters/h/70 kg) = 9.39 × (LBW/70)0.952 × (1 + [−0.021 × (age − 18)]) × (mean SCR/SCR)0.222 | V1 = 15.2 | |||||||
| V1 (female) (liters/70 kg) = 20.1 × (LBW/70) | Q = 41.8 | |||||||
| V1 (male) (liters/70 kg) = 25.1 × (LBW/70) | V2 = 58.5 | |||||||
| V2 (liters/70 kg) = 9.98 × (LBW/70) | ||||||||
| Q (liters/h/70 kg) = 1.5 × (LBW/70)0.952 | ||||||||
| M3 (33) | 44 CF patients (329) | 13.4 (0.75–20)c | 39 ± 14.6 | NR | 1 CMT | CL (liters/h) = 0.103 × wt0.75 | NR | NR |
| V (liters) = 0.267 × wt | ||||||||
| Pediatric patients | ||||||||
| M4 (32) | 85 CF patients (NR) | 10.0 (5–15)c | 34.0 (15–78)c | 80 ml/min (30–128 ml/min)c | 1 CMT | kel (h−1) = 0.01 + 0.00281 × CLCR | NR | NR |
| CL (liters/h) = V × kel | ||||||||
| V (liters) = 0.363 × wt | ||||||||
| M5 (31) | 140 neonates with severe infections (365) | 0.012 ± 0.008 (0.003–0.044)d | 2.75 ± 0.76 (0.8–4.25)d | 0.76 ± 0.19 mg/dl (0.04–1.20 mg/dl)e | 1 CMT | CL (liters/h) = 0.0508 × wtf | CL = 25.8 | Prop = 19.2% |
| V (liters) = 0.533 × wt | V = 21.9 | |||||||
| M6 (11) | 35 CF patients (318) | 9.5 (0.5–17.9)c | 34 (6.0–72.6)c | 106 ml/min (23–195 ml/min)c | 2 CMT | CL (liters/h/70 kg) = 6.37 × (wt/70)0.75 | CL = 11.7 (6.5) | Prop = 19.0% |
| V1 (liters/70 kg) = 18.7 × (wt/70) | V1 = 11.7 | |||||||
| Q (liters/h) = 0.39 | V2 = 42.0 | |||||||
| V2 (liters) = 1.32 | ||||||||
| M7 (3) | 257 CF patients (2,015) | 8.1 (4.6–13.9)g | 23.9 (16.3–42.6)g | 0.5 mg/dl (0.4–0.6 mg/dl)h | 1 CMT | CL (liters/h) = 5.59 × (wt/70)0.75 | CL = 22.8 | Prop = 19.1% |
| V (liters) = 18.90 × (wt/70) | V = 11.2 | Add = 1.4 mg/liter | ||||||
| M8 (41) | 614 ICU patients (1,273) | 0.008 (0–18)c | 2.01 (0.485–85)c | 0.81 mg/dl (0.13–1.17 mg/dl)i | 2 CMT | CL (liters/h) = 0.28 × (wt/4)BDE | CL = 39.7 | Prop = 29.8% |
| BDE = 2.23 × wt−0.065 | Add = 0.05 mg/liter | |||||||
| V1 (liters) = 1.9 × (wt/4)0.735 | ||||||||
| Q (liters/h) = CL | ||||||||
| V2 (liters) = V1 |
Add, additive residual unexplained error; BDE, body weight-dependent exponent; BOV, between-occasion variability; BSA, body surface area; BSV, between-subject variability; CF, cystic fibrosis; CL, total body clearance; CLCR, creatinine clearance (milliliters per minute); CMT, distribution compartment; CV, coefficient of variation; GFR, glomerular filtration rate calculated, as described by Manjunath et al. (44); ICU, intensive care unit; kel, elimination rate constant; LBW, lean body weight (in kilograms); NR, not reported; Prop, proportional residual unexplained error; Q, typical intercompartmental clearance; RUV, residual unexplained variability; V, apparent volume of distribution; V1, typical volume of the central compartment; V2, typical volume of the peripheral compartment.
The median or mean only was reported separately for pediatric patients and adults and was not reported for the data combined. Values represent the range of values obtained.
Data represent the mean (range).
Data represent the mean ± standard deviation (range).
Data represent the mean ± standard deviation (range) serum creatinine concentration.
CL (liters per hour) = 0.0508 × 0.843 if the birth weight is ≤2.5 kg.
Data represent the mean (interquartile range).
Data represent the mean (interquartile range) serum creatinine concentration.
Data represent the mean (range) serum creatinine concentration.
The disposition of tobramycin was described by a one-compartment distribution in five models (62.5%) and a two-compartment distribution in three models. However, a trend toward the reporting of two-compartment models in more recent publications was noted. As a significant predictor of variability in tobramycin clearance, 75% of the models included a measure of body size (total body weight or lean body weight) and 37.5% of the models included a measure of renal function (CLCR or SCR). All models included a measure of patient size as a significant predictor of variability in the tobramycin volume of distribution (V), with 12.5% using lean body weight and all others using total body weight. Other covariates reported to be significant in some studies included the patient's sex and age and low birth weight. Typical values for tobramycin clearance in the studies ranged from 0.06 to 4.42 liters/h, with the lowest value being reported in studies performed with neonates. In studies based on a one-compartment model, the typical volume of distribution varied across different subpopulations, ranging from 1.47 to 12.34 liters, with the lowest values being reported in studies performed with neonates. In models based on a two-compartment model, the typical volume of the central compartment ranged from 1.15 to 2.83 liters, with lower values being reported for neonates. Most models (62.5%) estimated the BSV (coefficient of variation [CV], in percent) associated with tobramycin clearance, with values ranging from 11.7 to 39.7%. The highest BSV in clearance was reported in a study conducted with ICU patients. The between-occasion variability (BOV) (CV) in clearance was reported in only two studies and ranged from 6.5 to 12.7%. RUV (CV) was modeled using a proportional or combined proportional/additive error model in 37.5% and 25% of models, respectively, with the values ranging from 19.0 to 29.8% for the proportional component and 0.05 to 1.4 mg/liter for the additive component. Further information can be found in Table 1. The majority (87.5%) of the models were internally validated, most commonly using a log-likelihood ratio test, goodness-of-fit plots, visual predictive checks, and/or a nonparametric bootstrap. One study employed external validation by using data from a new patient population (31). Six of the eight models were developed using NONMEM software; the others used MW/Pharm (32) and WinNonMix (33) software to develop the population pharmacokinetic model.
Patient and validation data collected.
The data used for external evaluation were collected from 41 patients. A summary of the key demographic and pharmacokinetic features of the patients providing data for this data set is provided in Table 2. All children in this population had cystic fibrosis. The median age, weight, and height of the population were 2.2 years (interquartile range [IQR], 1.1, 4.9 years), 12.4 kg (IQR, 8.6, 21.0 kg), and 89.0 cm (IQR, 71.0, 112.0 cm), respectively. The majority of patients for whom data were collected had normal renal function with a median CLCR of 90.6 ml/min/1.73 m2 (IQR, 71.7, 112.0 ml/min/1.73 m2), calculated using the modified Schwartz formula (34). Heights were not recorded for two children, and their height was estimated using average age-associated values from WHO growth charts (35). For five patients, SCR was measured greater than 3 months before or after tobramycin administration, and in these instances, the expected mean SCR was used (25). In total, data from 269 concentration-time points were available for external validation. No concentration measurements were below the assay LOQ for tobramycin. Figure 1 displays these concentration measurements versus time after dosing. Ninety-eight percent of the tobramycin doses were administered as an intravenous infusion over 30 min, with the other 2% of the doses being given over 45 to 60 min. In general, concentration measurements were obtained at about 2 and 6 h after the dose was administered.
TABLE 2.
Key demographic and pharmacokinetic features of the patients providing data for the external validation data seta
| Characteristic | Value(s) |
|---|---|
| Median (IQR) age (yr) | 2.2 (1.1, 4.9) |
| No. of patients aged (yr): | |
| <2 | 20 |
| 2–12 | 19 |
| >12 | 2 |
| No. of males/no. of females | 24/17 |
| Median (IQR) total body wt (kg) | 12.4 (8.6, 21.0) |
| Median (IQR) ht (cm) | 89.0 (71.0, 112.0) |
| Median (IQR) serum creatinine concn (μmol/liter) | 29 (<30, 51) |
| Median (IQR) creatinine clearance (ml/min/1.73 m2)b | 90.6 (71.7, 112.0) |
| No. of observations | 269 |
| Median (IQR) no. of sampling occasions/patient | 6.6 (3, 11) |
| Median (IQR) dose (mg/kg) | 11.3 (9.9, 12.5) |
| Median (IQR) time of sampling after dosing (h) | 5.1 (2.1, 6) |
| Median (IQR) tobramycin concn (mg/liter) | 6.1 (2.8, 15.2) |
Data are for 41 patients.
Calculated using the modified Schwartz formula (34).
FIG 1.
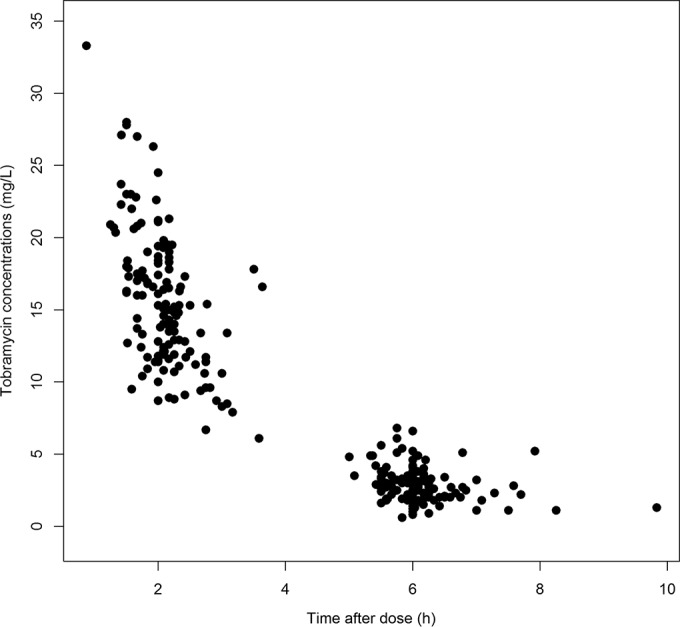
Tobramycin concentration versus time after dosing obtained with data available for external validation of the identified models.
Assessment of predictive performance.
Figure 2 displays the observed versus the population predicted concentrations when each selected population pharmacokinetic model was implemented in NONMEM. Models 2, 6, and 7 exhibited the smallest bias and imprecision in graphic comparisons of the predicted versus the observed concentrations. Graphical results were closely mirrored in numerical estimates of the model predictive performance presented in Table 3. Models 2, 6, and 7 were consistently ranked as the top three models with the lowest values of ME, MRE, and RMSE. Models 3 and 5 consistently obtained the highest values for these metrics. ME was found to be similar among the models ranked as the top three, with the smallest ME, which was for model 3, being −0.4 mg/liter (95% confidence interval [CI], −0.7, 0.01 mg/liter) for model 6. The overall highest ME was 12.1 mg/liter (95% CI, 11.4, 12.7 mg/liter). MREs were 4.9%, 19.2%, and 29.4% for the models ranked as the top three. Overall, the lowest MRE was 4.9% (95% CI, −0.8, 10.6%), which was for model 6, and the highest MRE was 274.1% (95% CI, 240.4, 307.7%), which was for model 3. Overall RMSE values ranged from 47.8% for model 6 to 392.4% for model 3.
FIG 2.
Observed drug concentrations versus population predicted concentrations for eight models of the population pharmacokinetics models of tobramycin. The M numbers represent the models described in Table 1.
TABLE 3.
Predictive performance of published pharmacokinetic models of tobramycin, including ME, MRE, and RMSE, and results of simulation-based npde diagnosticsa
| Model no. (reference) | Bias and imprecision |
npde diagnostics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ME (mg/liter)b | MRE (%)b | RMSE (%) | Mean | Variance | Skewness | Kurtosis |
P value |
|||
| Wilcoxon signed-rank test | Fisher test for variance | Shapiro-Wilks test | ||||||||
| Adult and pediatric patients | ||||||||||
| M1 (32) | 3.0 (2.5,3.5) | 75.3 (62.5, 88.2) | 131.1 | −0.3 | 1.5 | 0.5 | −0.1 | 0.0 | 0.0 | 0.0 |
| M2 (36) | 1.0 (0.5,1.4) | 19.2 (12.7, 25.7) | 57.6 | −0.1 | 0.7 | −0.1 | 1.2 | 0.2 | 0.0 | 0.0 |
| Pediatric patients | ||||||||||
| M3 (33) | 12.1 (11.4, 12.7) | 274.1 (240.4, 307.7) | 392.4 | −1.1 | 1.1 | −0.2 | −0.6 | 0.0 | 0.5 | 0.0 |
| M4 (32) | 2.4 (2.0, 2.9) | 71.5 (58.6, 84.4) | 129.1 | −0.3 | 1.6 | 0.4 | −0.3 | 0.0 | 0.0 | 0.0 |
| M5 (31) | 6.0 (5.3, 6.6) | 222.0 (187.2, 256.8) | 365.9 | −0.9 | 2.3 | 0.2 | −0.7 | 0.0 | 0.0 | 0.0 |
| M6 (11) | −0.4 (−0.7, 0.01) | 4.9 (−0.8, 10.6) | 47.8 | 0.0 | 0.8 | 0.0 | 1.6 | 0.5 | 0.0 | 0.0 |
| M7 (3) | 1.8 (1.4, 2.3) | 29.4 (22.2, 36.6) | 66.9 | −0.1 | 0.7 | −0.1 | 1.2 | 0.2 | 0.0 | 0.0 |
| M8 (41) | −4.0 (−4.6, −3.4) | −20.2 (−27.9, −12.5) | 67.4 | 0.2 | 2.7 | 0.0 | −1.3 | 0.0 | 0.0 | 0.0 |
Presented are summary statistics for the distribution of the npde’s: mean, variance, skewness, and kurtosis. The expected value of these four variables for N(0, 1) are, respectively, 0, 1, 0, and 0. Further results for (i) a Wilcoxon signed-rank test, to test whether the mean is significantly different from 0, (ii) a Fisher test for variance, to test whether the variance is significantly different from 1, and (iii) a Shapiro-Wilks test, to test whether the distribution is significantly different from a normal distribution, are presented (30).
Data in parentheses are 95% confidence intervals.
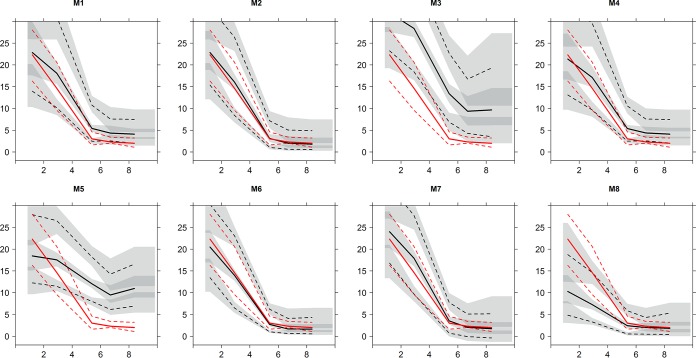
Table 3, Fig. S2 in the supplemental material, and Fig. 3 illustrate the results from the simulation-based diagnostics. Models 2, 6, and 7 showed visually better VPC graphics than the other models and an improved npde-versus-time after dosing statistics compared to those of the other models. Several models, in particular, models with a one-compartment distribution (four out of five models), showed weaknesses in model fit when the simulation was examined.
FIG 3.
Visual predictive checks performed using the identified models (M1 to M8; Table 1) and the validation data set. Here, the 95th and the 5th percentiles (broken lines) and the 50th percentile (continuous lines) of the validation data concentrations (red) and the model-predicted concentrations (black) on the x axis are plotted versus time after dosing (hours) on the y axis. The shaded areas present the binning and the 95% prediction interval.
DISCUSSION
This study is the first to review models of the population pharmacokinetics of tobramycin in pediatric patients currently published in the literature and to systemically externally evaluate the predictive performance of these models. Population pharmacokinetic models 2, 6, and 7 stood out as having the best predictive performance across all the different tests. These models displayed visually favorable observed concentration-versus-population predicted concentration plots, with model 6 appearing to be the best. The same models were distinguished in the numerical predictive performance assessment, ranking in the top three for at least two of the three criteria: ME, MRE, and RMSE. They also stood out as being the best using simulation-based diagnostics.
Model 2 (36), which was developed using the largest data set and which included the greatest number of covariate influences on pharmacokinetic parameters, was consistently among the top three models for all assessments. This model's distinguished performance likely suggests that large population studies which include a number of covariate influences will likely have a better predictive ability with future patients. A trend toward an improved predictive performance was noted for models with a two-compartment disposition rather than a one-compartment disposition, with the two best models (M2 and M6) being two-compartment models and the two least favorable models (M3 and M5) being one-compartment models. A multiple-compartment distribution likely better characterizes the tissue accumulation reported for tobramycin (37). Distinguishing between distribution and elimination processes is, however, highly dependent on the sampling scheme (6, 38). It can be seen that model 3, a one-compartment model developed with data from a pediatric cystic fibrosis population, struggles to predict peak concentrations (Fig. 3; see also Fig. S2 in the supplemental material). All models incorporated the influence of body size on either CL or V; this makes sense, as tobramycin distributes into extracellular fluid, which tends to increase with increasing patient size (39). Models 2, 3, 6, 7, and 8 used a nonlinear function to describe the relationship between drug clearance and patient size; while three of these were the best-performing models, the others were the worst-performing models. Only one of the top three models included the influence of a renal function descriptor on tobramycin clearance, which is surprising, as tobramycin is largely renally eliminated (40). However, most studies were based on patients with normal to moderately impaired renal function, and hence, the influence of renal function on drug clearance may not be significant enough to be detected in this circumstance. Similarly, most patients in the validation data set had normal to only moderately impaired renal function, so the inclusion or the lack of inclusion of renal function in the population model may be of little importance in this circumstance.
Some models may have performed poorly, as they were not externally validated with data from a population similar to that with which they were developed. The validation data set did not include any ICU patients and included only 3 infants between the ages of 5 and 15 weeks. Model 8 (41) was developed with data from ICU patients who were predominately less than 1 month old and reported the large pharmacokinetic variability commonly seen in this population (39, 42). Similarly, model 5 (31) was developed using data from neonates, which may exhibit pharmacokinetics different from those exhibited by older pediatric patients (31, 41). This model was the only one originally externally validated with data from a patient population very similar to the population with which it was developed, but it did not perform so well in this study. The underprediction of particular peak tobramycin concentrations (Fig. 3; see also Fig. S2 in the supplemental material) seen with these models might be due to the larger volume of distribution (on a per-kilogram basis) often seen in individuals and neonates in ICUs than in other populations.
For all models tested, RMSE was >30%. This suggests that knowledge of typical pharmacokinetic behavior and patient covariate values alone without feedback concentration measurements from individual patients is not sufficient to make precise predictions. This could improve by incorporation of the model in Bayesian forecasting software, which would allow feedback from subsequent measurements and the further individualization of the parameters (43).
There were limitations associated with this study. One weakness of the analysis is the limited size and heterogeneity of the external data set used for validation. Data came only from cystic fibrosis patients, and few patients were neonates or had renal impairment. As with any study involving retrospective data collection, uncertainty is associated with data records (21). Interpretation of the published models was complicated in some instances where exact methodologies were not clearly described and details were absent. Three of the eight models did not report any RUV or BSV values. Substitute values were added for application of the models, but this may have altered the ability of models 1, 3, and 4 to accurately predict tobramycin concentrations. Furthermore, some patients contributed more data to the validation data set than others, and all observations were treated independently in the analysis, which can result in bias toward results for patients that contributed larger amounts of data. Of note, for 56% of patients, at least one SCR measurement was recorded as <30 μmol/liter, which is below the LOQ of the assay used to measure this compound. In order to be able to partly utilize this information, these measurements were fixed to 29 μmol/liter, as no other data were available. This, however, may have influenced the predictive performance of the models that included renal function as a covariate.
This study externally evaluated eight published models of the population pharmacokinetics of tobramycin with newly collected clinical data. Several of the published models were found to have reasonably low levels of bias but a greater degree of imprecision. This suggests that knowledge of typical pharmacokinetic behavior and patient covariate values alone without feedback concentration measurements from individual patients is not sufficient to make precise predictions.
Supplementary Material
ACKNOWLEDGMENTS
The Australian Centre of Pharmacometrics is acknowledged for the NONMEM software license. We thank Claire Wainwright (Lady Cilento Children's Hospital, Brisbane, Australia) for her support in the ethics application and enabling data collection.
No external funding supported this study.
S.H. and C.E.S. were involved in the original development of model 2. S.H. was involved in the original development of model 6. C.E.S. has previously worked with the developers of model 7.
C.B. collected the data, performed the analysis, and wrote the manuscript; S.H. applied for ethics approval, aided in data collection and analysis of the data, and revised the manuscript; C.E.S. aided in data collection and analysis of the data and revised the manuscript; and S.U. supported the ethics application and data collection and identified patients.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02654-15.
REFERENCES
- 1.Agence Française de Sécurité Sanitaire des Produits de Sant. 2012. Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect 42:301–308. doi: 10.1016/j.medmal.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey BW, Pepe M, Quan J, Otto K, Montgomery BA, Williams-Warren J, Vasiliev M, Borowitz D, Bowman MC, Marshall BC, Marshall S, Smith AL. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin CM, Zobell JT, Stockmann C, McCrory BE, Wisdom M, Young DC, Olson J, Ampofo K, Spigarelli MG. 2014. Pharmacokinetic and pharmacodynamic optimisation of intravenous tobramycin dosing among children with cystic fibrosis. J Pharmacokinet Pharmacodyn 41:71–79. doi: 10.1007/s10928-013-9348-7. [DOI] [PubMed] [Google Scholar]
- 4.Young DC, Zobell JT, Stockmann C, Waters CD, Ampofo K, Sherwin CM, Spigarelli MG. 2013. Optimization of anti-pseudomonal antibiotics for cystic fibrosis pulmonary exacerbations. V. Aminoglycosides. Pediatr Pulmonol 48:1047–1061. doi: 10.1002/ppul.22813. [DOI] [PubMed] [Google Scholar]
- 5.Moore RD, Smith CR, Lietman PS. 1984. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med 77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 6.Conil JM, Georges B, Ruiz S, Rival T, Seguin T, Cougot P, Fourcade O, Houin G, Saivin S. 2011. Tobramycin disposition in ICU patients receiving a once daily regimen: population approach and dosage simulations. Br J Clin Pharmacol 71:61–71. doi: 10.1111/j.1365-2125.2010.03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother 43:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouton JW, Jacobs N, Tiddens H, Horrevorts AM. 2005. Pharmacodynamics of tobramycin in patients with cystic fibrosis. Diagn Microbiol Infect Dis 52:123–127. doi: 10.1016/j.diagmicrobio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother 43:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlgren JG, Anderson ET, Hewitt WL. 1975. Gentamicin blood levels: a guide to nephrotoxicity. Antimicrob Agents Chemother 8:58–62. doi: 10.1128/AAC.8.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennig S, Norris R, Kirkpatrick CM. 2008. Target concentration intervention is needed for tobramycin dosing in paediatric patients with cystic fibrosis—a population pharmacokinetic study. Br J Clin Pharmacol 65:502–510. doi: 10.1111/j.1365-2125.2007.03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig WA. 2001. Does the dose matter? Clin Infect Dis 33(Suppl 3):S233–S237. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood I. 2014. Dosing in children: a critical review of the pharmacokinetic allometric scaling and modelling approaches in paediatric drug development and clinical settings. Clin Pharmacokinet 53:327–346. doi: 10.1007/s40262-014-0134-5. [DOI] [PubMed] [Google Scholar]
- 14.Antibiotic Expert Group. 2014. Therapeutic guidelines: antibiotic. Version 15 Therapeutic Guidelines Ltd, Melbourne, Australia: Accessed 20 February 2015. [Google Scholar]
- 15.Burton ME, Brater DC, Chen PS, Day RB, Huber PJ, Vasko MR. 1985. A Bayesian feedback method of aminoglycoside dosing. Clin Pharmacol Ther 37:349–357. doi: 10.1038/clpt.1985.51. [DOI] [PubMed] [Google Scholar]
- 16.Durieux P, Trinquart L, Colombet I, Nies J, Walton R, Rajeswaran A, Rege-Walther M, Harvey E, Burnand B. 2008. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database Syst Rev 11:CD002894. doi: 10.1002/14651858.CD002894. [DOI] [PubMed] [Google Scholar]
- 17.Paviour S, Hennig S, Staatz CE. 2016. Usage and monitoring of intravenous tobramycin in cystic fibrosis in Australia and the United Kingdom. J Pharm Pract Res 46:15–21. [Google Scholar]
- 18.Burton ME, Ash CL, Hill DP Jr, Handy T, Shepherd MD, Vasko MR. 1991. A controlled trial of the cost benefit of computerized Bayesian aminoglycoside administration. Clin Pharmacol Ther 49:685–694. doi: 10.1038/clpt.1991.86. [DOI] [PubMed] [Google Scholar]
- 19.Hennig S, Holthouse F, Staatz CE. 2015. Comparing dosage adjustment methods for once-daily tobramycin in paediatric and adolescent patients with cystic fibrosis. Clin Pharmacokinet 54:409–421. doi: 10.1007/s40262-014-0211-9. [DOI] [PubMed] [Google Scholar]
- 20.Duffull SB, Wright DF, Winter HR. 2011. Interpreting population pharmacokinetic-pharmacodynamic analyses—a clinical viewpoint. Br J Clin Pharmacol 71:807–814. doi: 10.1111/j.1365-2125.2010.03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffull SB, Wright DF. 2015. What do we learn from repeated population analyses? Br J Clin Pharmacol 79:40–47. doi: 10.1111/bcp.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennig S, Karlsson MO. 2014. Concordance between criteria for covariate model building. J Pharmacokinet Pharmacodyn 41:109–125. doi: 10.1007/s10928-014-9350-8. [DOI] [PubMed] [Google Scholar]
- 23.Ette EI, Williams PJ (ed). 2007. Pharmacometrics: the science of quantitative pharmacology. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 24.Sherwin CM, Kiang TK, Spigarelli MG, Ensom MH. 2012. Fundamentals of population pharmacokinetic modelling: validation methods. Clin Pharmacokinet 51:573–590. doi: 10.1007/BF03261932. [DOI] [PubMed] [Google Scholar]
- 25.Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, Panteghini M. 2008. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 54:559–566. doi: 10.1373/clinchem.2007.099648. [DOI] [PubMed] [Google Scholar]
- 26.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. 2009. NONMEM user's guides. (1989-2009), v7 Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 27.Sheiner LB, Beal SL. 1981. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson MO, Holford NHG. 2008. A tutorial on visual predictive checks, abstr 1434 Abstr Annu Meet Population Approach Group Europe, Marseille, France http://www.page-meeting.org/?abstract=1434. [Google Scholar]
- 29.Brendel K, Comets E, Laffont C, Laveille C, Mentre F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comets E, Brendel K, Mentre F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Falcao AC, Buelga DS, Mendez ME, Garcia MJ, Pardo M. 2001. Population kinetics of tobramycin in neonates. Ther Drug Monit 23:202–208. doi: 10.1097/00007691-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Touw DJ, Knox AJ, Smyth A. 2007. Population pharmacokinetics of tobramycin administered thrice daily and once daily in children and adults with cystic fibrosis. J Cyst Fibros 6:327–333. doi: 10.1016/j.jcf.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Massie J, Cranswick N. 2006. Pharmacokinetic profile of once daily intravenous tobramycin in children with cystic fibrosis. J Paediatr Child Health 42:601–605. doi: 10.1111/j.1440-1754.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. 2009. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2015. The WHO child growth standards. World Health Organization, Geneva, Switzerland: http://www.who.int/childgrowth/standards/en/. [Google Scholar]
- 36.Hennig S, Standing JF, Staatz CE, Thomson AH. 2013. Population pharmacokinetics of tobramycin in patients with and without cystic fibrosis. Clin Pharmacokinet 52:289–301. doi: 10.1007/s40262-013-0036-y. [DOI] [PubMed] [Google Scholar]
- 37.Schentag JJ, Lasezkay G, Cumbo TJ, Plaut ME, Jusko WJ. 1978. Accumulation pharmacokinetics of tobramycin. Antimicrob Agents Chemother 13:649–656. doi: 10.1128/AAC.13.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touw DJ, Vinks AA, Neef C. 1997. Pharmacokinetic modelling of intravenous tobramycin in adolescent and adult patients with cystic fibrosis using the nonparametric expectation maximization (NPEM) algorithm. Pharm World Sci 19:142–151. doi: 10.1023/A:1008633526772. [DOI] [PubMed] [Google Scholar]
- 39.Dasta JF, Armstrong DK. 1988. Variability in aminoglycoside pharmacokinetics in critically ill surgical patients. Crit Care Med 16:327–330. doi: 10.1097/00003246-198804000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Naber KG, Westenfelder SR, Madsen PO. 1973. Pharmacokinetics of the aminoglycoside antibiotic tobramycin in humans. Antimicrob Agents Chemother 3:469–473. doi: 10.1128/AAC.3.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Cock RF, Allegaert K, Brussee JM, Sherwin CM, Mulla H, de Hoog M, van den Anker JN, Danhof M, Knibbe CA. 2014. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res 31:2643–2654. doi: 10.1007/s11095-014-1361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dager WE. 1994. Aminoglycoside pharmacokinetics: volume of distribution in specific adult patient subgroups. Ann Pharmacother 28:944–951. [DOI] [PubMed] [Google Scholar]
- 43.Zhao CY, Jiao Z, Mao JJ, Qiu XY. 17 November 2015. External evaluation of published population pharmacokinetic models of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol doi: 10.1111/bcp.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manjunath G, Sarnak MJ, Levey AS. 2001. Estimating the glomerular filtration rate. Dos and don'ts for assessing kidney function. Postgrad Med 110:55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.