Abstract
The objective of the study was to determine the effects of Candida albicans respiratory tract colonization on Acinetobacter baumannii pneumonia in a rat model. Rats were colonized with C. albicans by instillation of 3 × 106 CFU into their airways, while sterile saline was instilled in the control group. The colonized rats were further divided into two groups: treated with amphotericin B or not. The rats were subsequently infected with A. baumannii (108 CFU by tracheobronchial instillation). A. baumannii lung CFU counts, cytokine lung levels, and rates of A. baumannii pneumonia were compared between groups. In vitro expression of A. baumannii virulence genes was measured by reverse transcription (RT)-PCR after 24-hour incubation with C. albicans or with Mueller-Hinton (MH) broth alone. Rats with Candida colonization developed A. baumannii pneumonia more frequently and had higher A. baumannii CFU burdens and heavier lungs than controls. After A. baumannii infection, lung interleukin 17 (IL-17) concentrations were lower and gamma interferon (IFN-γ) concentrations were higher in Candida-colonized rats than in controls. Candida-colonized rats treated with amphotericin B had a decreased rate of A. baumannii pneumonia and lower IFN-γ levels but higher IL-17 levels than untreated rats. Expression of basC, barB, bauA, ptk, plc2, and pld2 was induced while expression of ompA and abaI was suppressed in A. baumannii cultured in the presence of C. albicans. C. albicans colonization facilitated the development of A. baumannii pneumonia in a rat model. Among Candida-colonized rats, antifungal treatment lowered the incidence of A. baumannii pneumonia. These findings could be due to modification of the host immune response and/or expression of A. baumannii virulence genes by Candida spp.
INTRODUCTION
Candida albicans is a commensal of the human skin and mucosae but also the most common fungal human pathogen (1–3). Recent data are suggestive of clinically significant interactions between C. albicans and bacteria, potentially affecting their virulence (2, 4, 5) and propensity to develop antibacterial resistance (6–9).
Specifically, clinical and laboratory investigations have focused on the interplay between C. albicans and Pseudomonas aeruginosa (10). In animal models and observational studies, C. albicans airway colonization has been associated with increased incidence of P. aeruginosa pneumonia (11, 12). Prior antifungal treatment was found to reduce the risk of P. aeruginosa pulmonary infection (11, 13). However, in burn patients, P. aeruginosa inhibited the growth of Candida spp. on the wound surface (4).
Acinetobacter baumannii is an emerging nosocomial pathogen associated with significant morbidity and multidrug resistance (14–16). A. baumannii can firmly adhere to the hyphae of C. albicans and inhibit their growth via production of the OmpA protein (17). Nevertheless, A. baumannii exhibited enhanced growth in the presence of another yeast, Saccharomyces cerevisiae (18).
These observations indicate complex, possibly both synergistic and antagonistic yeast-bacterial interactions that have not been adequately investigated. Therefore, we aimed to study the relationship between C. albicans respiratory tract colonization and bacterial pneumonia caused by A. baumannii in a rat model, as well as the effect of culture in the presence of C. albicans on the expression of A. baumannii virulence genes.
MATERIALS AND METHODS
Animals.
We used 2.5- to 3-month-old pathogen-free male Wistar rats, weighing 250 to 275 g (11), purchased from the Laboratory Animal Center (Southern Medical University, Guangzhou, China). All experiments were approved by the Ethics of Animal Experiments Committee of Nan Fang Hospital, an affiliate of the Southern Medical University, Guangzhou, China. The rats were housed under standard conditions (12 h light/12 h dark; 22 to 24°C) in the Animal Care Facility Service (Southern Medical University, Guangzhou, China). The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
C. albicans respiratory tract colonization.
Candida colonization was achieved by transglottal instillation of 3 × 106 CFU C. albicans SC5314 on the first day of experiments, as previously described (11). To establish the model, 4 animals were sacrificed at each time point, 4, 24, and 72 h and 7 and 14 days after instillation, and their lungs were removed. The lungs from one animal were homogenized and used for Candida CFU counts, whereas those from the other three were used for histopathologic analyses. We compared lung cytokine levels between Candida-colonized and control rats without A. baumannii infection 48 h after Candida or saline instillation, respectively.
Assessing the effect of C. albicans respiratory tract colonization on A. baumannii pneumonia.
Rats were divided into C. albicans respiratory tract colonization (n = 16) and saline control (n = 18) groups. A suspension of 108 CFU of A. baumannii ATCC 19606 was transglottally instilled in each animal on the second day of the experiment. At this inoculum, A. baumannii pneumonia develops in <50% of immunocompetent rats. The animals were sacrificed on the third day. Lung weights, A. baumannii CFU counts, and lung cytokine concentrations were compared between the two groups. Pneumonia was defined as macroscopic and/or microscopic lung inflammation with a bacterial burden of >104 CFU per lung (11).
Antifungal treatment.
The Candida-colonized rats were divided into those treated with 1 mg/kg of body weight/day of intraperitoneal amphotericin B (treatment group; n = 19) or normal saline intraperitoneal injections (control group; n = 16), as previously described (11), for 3 days after airway colonization with Candida. On the 4th day, the rats were infected with A. baumannii as described above, and on the 5th day, they were sacrificed for lung tissue bacterial CFU and cytokine measurements. In a separate experiment, we compared lung cytokine levels between Candida-colonized rats treated for 3 days with amphotericin B or not and without Acinetobacter infection.
Cytokine measurements.
Levels of interleukin 2 (IL-2), IL-5, IL-6, IL-10, IL-17, and gamma interferon (IFN-γ) were determined using the Milliplex Map Rat Cytokine/Chemokine Magnetic Bead panel (Millipore Corporation, USA) on a Luminex (Austin, TX) 100 IS system according to the manufacturer's instructions to evaluate the host local immune response.
In vitro experiments, RNA isolation, and real-time PCR analysis.
For in vitro experiments, 1 ml of 5 × 108 CFU/ml A. baumannii in Mueller-Hinton (MH) broth suspension was cultured with 1 ml of C. albicans MH broth suspension (3 × 107 cells/ml) (Candida) or 1 ml of MH broth (control). After a 24-hour incubation at 37°C, 1 ml was moved to a 1.5-ml tube and centrifuged for 10 min at 5,000 × g to obtain bacterial pellets; 100 μl of TE buffer (10 mM Tris · Cl, 1 mM EDTA, pH 8.0) with 1 mg/ml lysozyme was added to the precipitate, which was resuspended and incubated for 5 min at room temperature.
Total A. baumannii mRNA was extracted using an RNeasy Mini RNA isolation kit (Qiagen, Shanghai, China). RNA integrity, concentration, and purity were assessed using a Nanodrop spectrophotometer. Samples with a 260/280-nm ratio between 1.9 and 2.1 were used for further analyses. Any potential carryover genomic DNA (gDNA) contamination was removed using gDNA Eraser (TaKaRa, Dalian, China); 1 μg of total RNA was used to synthesize cDNA with the PrimeScript RT reagent kit with gDNA Eraser (Perfect Real Time; TaKaRa, Dalian, China). Quantitative analyses of virulence genes of A. baumannii (ompA, pgaC, lpxA, basC, basD, barB, bauA, ptk, plc2, pld2, and abaI) were performed on a LightCycler 480 real-time PCR system (Roche, Switzerland) using the SYBR Premix Ex Taq II (Tli RNaseH Plus) kit (TaKaRa, Dalian, China).
Using 16S rRNA as the reference gene (19), reverse transcription (RT)-PCR data from at least 3 independent experiments were analyzed by the 2−ΔΔCt method. Changes in the expression of A. baumannii virulence genes in the presence of C. albicans were expressed as a ratio relative to the control group.
Statistical analysis.
Data were analyzed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). All data are presented as means and standard deviations (SD) unless otherwise specified. Normality of distribution was tested with the Kolmogorov-Smirnoff test. We determined statistical significance (P < 0.05) by t test, analysis of variance (ANOVA), or Mann-Whitney U test, where appropriate.
RESULTS
C. albicans respiratory tract colonization model.
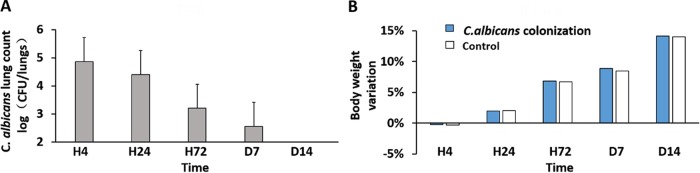
On the basis of the model provided by Roux et al. (11), the respiratory tracts of Wistar rats were colonized with C. albicans. The 14-day survival of the colonized rats was 100%, and there were no differences in activity, fecal shape, and average daily weight gain between animals colonized with C. albicans and controls (airway instillation of normal saline) (Fig. 1). We found no histopathologic evidence of pneumonia in Candida-colonized rats.
FIG 1.
Rat respiratory tract colonization with C. albicans. A total of 3 × 106 CFU of C. albicans was transglottally instilled into rats. At different time points, the rats were sacrificed, and their lungs were homogenized. (A) C. albicans CFU counts at 4 h, 24 h, 72 h, 7 days, and 14 days after instillation. (B) Animal weight variation compared with that of noncolonized rats (control) at each time point. The error bars indicate SD.
Candida colonization of the respiratory tract facilitates the development of subsequent A. baumannii pneumonia.
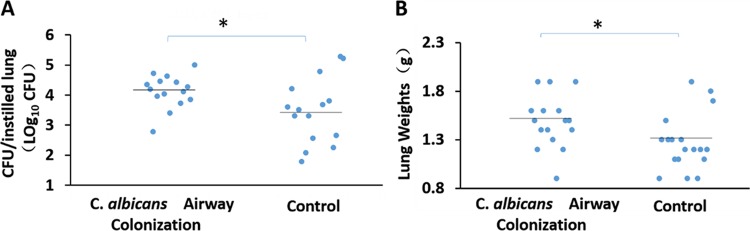
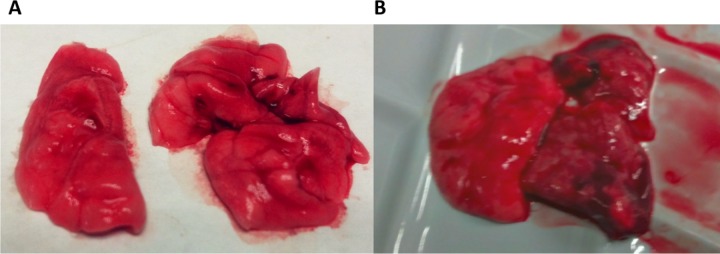
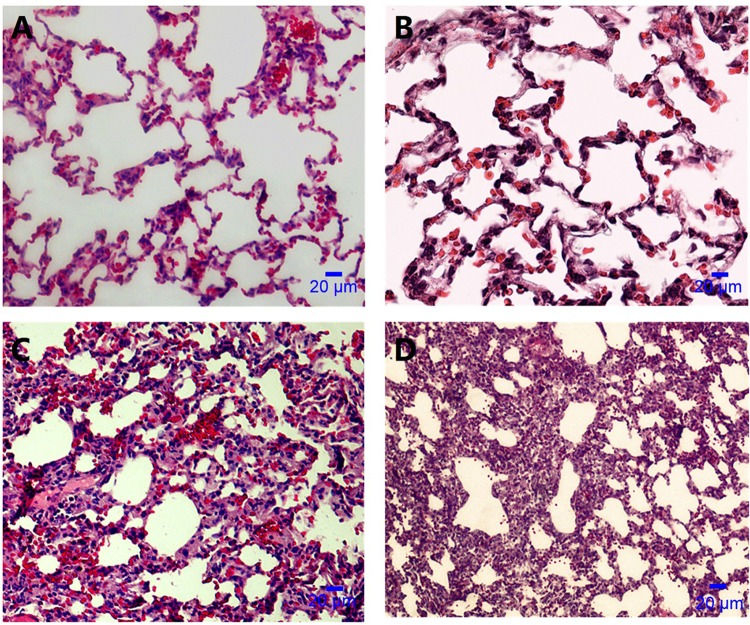
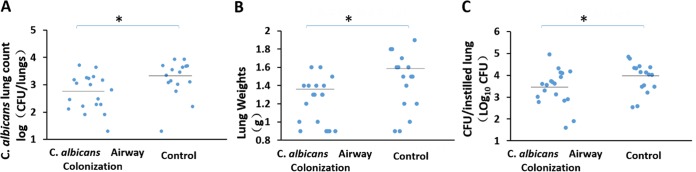
Twenty-four hours after lung instillation of A. baumannii, rats with prior C. albicans respiratory tract colonization had higher A. baumannii lung CFU counts (P = 0.026) and lung weights (P = 0.034) than controls (Fig. 2). Ten of 16 rats with C. albicans airway colonization developed A. baumannii pneumonia as opposed to 4/18 in the control group (P = 0.017). Two lungs infected with A. baumannii are shown in Fig. 3. Consolidation is evident in the lung of a Candida-colonized rat (Fig. 3B). Microscopically, animal lungs colonized with C. albicans prior to A. baumannii infection demonstrated heavier infiltration of inflammatory cells and alveolar damage (Fig. 4D) than lungs with A. baumannii infection in the absence of prior C. albicans colonization (Fig. 4C).
FIG 2.
Fungal colonization facilitates development of subsequent A. baumannii pneumonia. Median A. baumannii CFU counts per lung (log transformed) (A) and lung weights (B) (horizontal lines) are shown. Mann-Whitney test; *, P < 0.05.
FIG 3.
Macroscopic appearance of A. baumannii pneumonia. (A) Normal macroscopic appearance of the lungs from a rat that was not colonized with Candida and did not develop pneumonia after transglottal instillation of 108 CFU of A. baumannii. (B) Marked consolidation of the upper and middle lobes in the right lung of a rat colonized with C. albicans that developed pneumonia after instillation of the same A. baumannii inoculum.
FIG 4.
Microscopic appearance of an A. baumannii-instilled lung by light microscopy (hematoxylin-eosin stain; magnification, ×400). (A) Normal microscopic appearance of lungs after saline instillation. (B) C. albicans airway colonization; lungs appear similar to those in the control group. (C) A. baumannii infection in the absence of prior Candida colonization; inflammatory-cell infiltration and alveolar damage. (D) C. albicans plus A. baumannii infection; heavier infiltration of inflammatory cells and alveolar damage than with A. baumannii infection in the absence of prior C. albicans colonization (panel C).
C. albicans colonization downregulated the effect of A. baumannii on host IL-17 production and upregulated its effect on IFN-γ production.
We measured lung IFN-γ, IL-2, and IL-17 levels in C. albicans-colonized rats and controls in the absence of (48 h after Candida or saline instillation) and 24 h after A. baumannii infection. Levels of IL-17 (41.4 ± 7.0 versus 17.7 ± 4.2 pg/ml; P = 0.01) (Fig. 5A), IL-2 (84.6 ± 8.3 versus 54.3 ± 7.1 pg/ml; P = 0.02) (Fig. 5B), and IFN-γ (130.2 ± 33.9 versus 54.3 ± 10.8 pg/ml; P = 0.005) (Fig. 5C) were higher in rats colonized with Candida than in controls in the absence of bacterial infection.
FIG 5.
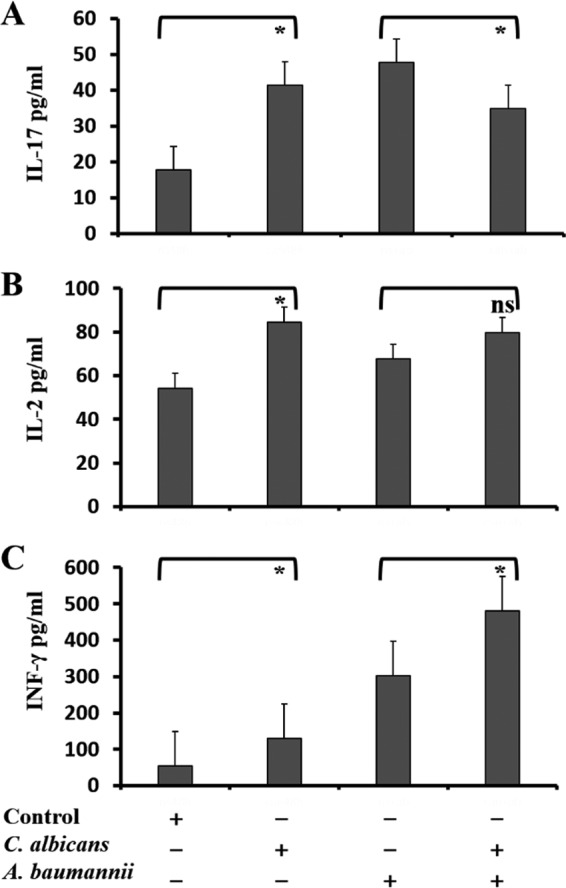
C. albicans respiratory tract colonization modulates lung immune response to A. baumannii infection. Production of IL-17 (A), IL-2 (B), and IFN-γ (C) in rat lungs was measured 48 h after Candida or saline instillation in the absence of A. baumannii instillation (left) or 24 h after A. baumannii instillation (right) in C. albicans-colonized rats versus controls (normal saline instillation). The data are presented as means and SD. Student's t test; *, P < 0.05; ns, not significant.
In the control group, IFN-γ (P = 0.002), IL-17 (P < 0.001), and IL-2 (P = 0.02) concentrations were significantly increased with A. baumannii instillation, whereas in Candida-colonized rats, only IFN-γ levels were increased with bacterial infection (P < 0.001). A. baumannii-infected rats with prior C. albicans airway colonization had significantly lower IL-17 (34.9 ± 6.53 versus 47.8 ± 0.7 pg/ml; P = 0.029) (Fig. 5A) and higher IFN-γ (480.5 ± 11.7 versus 301.8 ± 97.4 pg/ml; P = 0.011) (Fig. 5C) concentrations than A. baumannii-infected controls. There was no difference in IL-2 levels (79.8 ± 19.4 versus 67.8 ± 2.8 pg/ml; P = 0.265) (Fig. 5B).
Effect of antifungal therapy.
In rats with C. albicans airway colonization, we studied the effects of systemic amphotericin B (1 mg/kg daily), starting on the day of Candida instillation and administered for 3 days, on the lung fungal burden, subsequent A. baumannii pneumonia, and host cytokine production.
The pulmonary fungal burden was significantly decreased in amphotericin B-treated rats compared to those that did not receive antifungal treatment (P = 0.015) (Fig. 6A). Fewer rats in the treatment group developed A. baumannii pneumonia (5/19 versus 10/16; P = 0.031). Lung weights (P = 0.027) (Fig. 6B) and lung tissue Acinetobacter CFU counts (P = 0.038) (Fig. 6C) were also significantly lower in Candida-colonized rats that were treated with amphotericin B than in those that were not.
FIG 6.
Effects of antifungal treatment on respiratory tract fungal colonization and A. baumannii pneumonia. (A) C. albicans lung CFU counts were significantly decreased after 3 days of antifungal therapy (amphotericin B). (B and C) Bacterial counts (C) and lung weights (B) were significantly lower in Candida-colonized rats treated with amphotericin B than in those that received no treatment. *, P < 0.05.
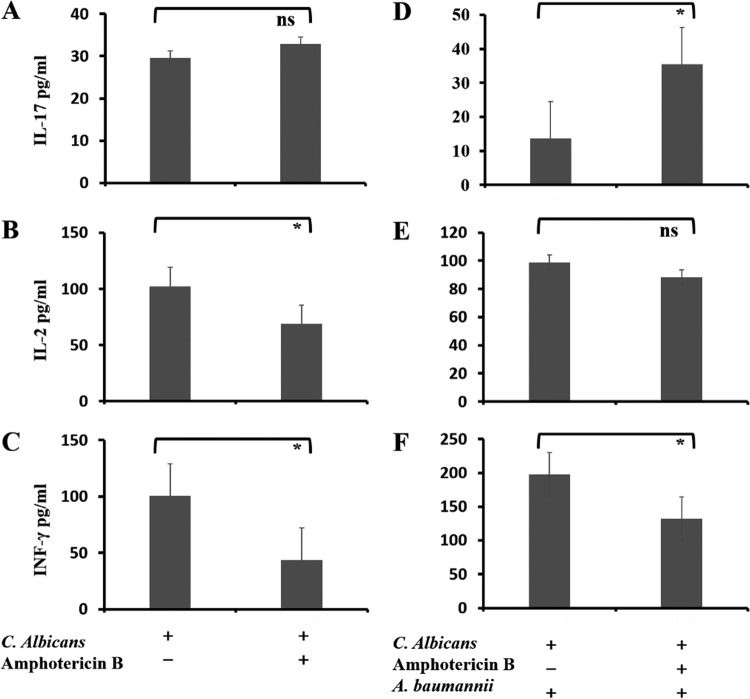
In Candida-colonized rats without A. baumannii infection, IL-2 (Fig. 7B) and IFN-γ (Fig. 7C) lung concentrations were decreased with antifungal treatment (P < 0.05), whereas there was no significant difference in IL-17 levels (Fig. 7A). After A. baumannii instillation, IL-17 levels were higher (P = 0.005) (Fig. 7D) whereas the concentration of IFN-γ was lower (P = 0.032) (Fig. 7F) in Candida-colonized rats that received antifungal treatment than in those that did not. IL-2 levels were similar in the two groups (P = 0.693) (Fig. 7E). We did not find any significant effects of Candida colonization or antifungal treatment on IL-5, IL-6, or IL-10 levels (see Fig. S1 and S2 in the supplemental material).
FIG 7.
Effects of antifungal treatment on cytokine production. Colonized rats received either amphotericin B or saline by intraperitoneal injection for 3 days and were subsequently infected with A. baumannii and sacrificed after 24 h. Concentrations of IL-17 (A and D), IL-2 (B and E), and IFN-γ (C and F) were measured in rat lung homogenates. The data are presented as means and SD. We compared lung cytokine levels between Candida-colonized rats treated for 3 days with amphotericin B (right bars) or not (left bars) without (A to C) or after (D to F) Acinetobacter infection. Student's t test; *, P < 0.05; ns, not significant.
Effect of C. albicans on expression of A. baumannii virulence genes.
The expression of A. baumannii virulence genes was assessed by RT-PCR after 24 h of culture with C. albicans compared to MH broth alone. The primers used in this experiment are listed in Table S1 in the supplemental material. In the presence of C. albicans, the expression of basC, barB, bauA, ptk, plc2, and pld2 was upregulated, whereas the expression of abaI and ompA was suppressed (Table 1).
TABLE 1.
In vitro expression of virulence genes relative to housekeeping gene 16S rRNA in A. baumannii ATCC 19606
| Gene | Relative expression |
P value | |
|---|---|---|---|
| C. albicans | Controls | ||
| lpxA | 0.47 ± 0.23 | 1 | 0.300 |
| basC | 8.49 ± 4.43 | 1 | 0.006 |
| pgaC | 1.01 ± 0.11 | 1 | 0.935 |
| ompA | 0.55 ± 0.06 | 1 | 0.006 |
| basD | 1.98 ± 0.87 | 1 | 0.066 |
| barB | 2.30 ± 0.64 | 1 | 0.010 |
| bauA | 2.60 ± 0.56 | 1 | 0.003 |
| ptk | 1.24 ± 0.15 | 1 | 0.024 |
| plc2 | 1.31 ± 0.20 | 1 | 0.023 |
| pld2 | 2.60 ± 0.77 | 1 | 0.004 |
| abaI | 0.47 ± 0.22 | 1 | 0.016 |
DISCUSSION
We studied a simple, reproducible rat model of C. albicans respiratory tract colonization previously described by Roux et al. (11) and extended their findings for the evaluation of C. albicans-A. baumannii (an emerging opportunistic pathogen) coinfection. After instillation, Candida organisms were retrieved from animal lungs with slow clearance over time, 14-day survival was 100%, and there were no differences in weight or health status between Candida-instilled animals and controls (Fig. 1). These findings are consistent with colonization rather than clinical infection. However, instillation of C. albicans, but not of normal saline, elicited a strong host immune response (Fig. 5) similar to that observed previously (11). Therefore, even though there was no evidence of clinical disease, it seems that C. albicans in the respiratory tract is not just a commensal bystander.
Importantly, we found convincing evidence that C. albicans respiratory tract colonization in rats facilitated the subsequent development of severe A. baumannii infection, as evidenced by higher bacterial CFU counts, lung weights, histopathology (Fig. 2 to 4), and percentages of animals that developed pneumonia than for controls. To our knowledge, this is the first report of an association between Candida airway colonization and Acinetobacter pneumonia, which is consistent with animal studies (11) and clinical data (12, 13) for other pathogens. Such observations could be due to (i) modification of the host immune response by C. albicans and/or (ii) fungal-bacterial interactions resulting in enhanced bacterial virulence.
As noted above, C. albicans colonization increased IFN-γ secretion, in agreement with a previous report (11). In both studies, this effect was completely reversed by administration of antifungal agents (Fig. 7), which also led to significantly lower rates of bacterial pneumonia, lung weights, and A. baumannii CFU counts (Fig. 6). These effects were previously attributed to a strong TH1 response to the presence of C. albicans (11), leading to increased levels of IFN-γ. However, it should be noted that innate immune cell populations, such as NK cells, also produce IFN-γ in the context of pulmonary infection and likely contribute to cytokine production, especially at early time points before priming of TH cells.
IFN-γ has been shown to hamper host antibacterial defenses. Specifically, overnight incubation with IFN-γ decreased phagocytosis of Escherichia coli and Staphylococcus aureus by alveolar macrophages by >90% (11). Moreover, IFN-γ downregulated the expression of a class A scavenger receptor on the surfaces of alveolar macrophages, thus impairing phagocytosis of Pneumococcus, which was restored to normal by anti-IFN-γ specific antibodies or opsonization, which bypasses scavenger receptors (20). Therefore, upregulation of IFN-γ production is a potential determinant of the increased susceptibility to bacterial infections associated with C. albicans airway colonization.
IL-17 levels were significantly lower in Candida-colonized rats than in controls after A. baumannii instillation, in agreement with previous observations in a rat model of P. aeruginosa pneumonia (11). Similar to the study by Roux et al. (11), we found no difference in IL-17 lung concentrations between Candida-colonized rats treated with amphotericin B and untreated rats in the absence of A. baumannii instillation. However, in the prior study, the effect of antifungals on IL-17 production in Candida-colonized rats as a response to bacterial infection was not evaluated (11). Interestingly, in our study, treatment of C. albicans colonization with amphotericin B led to a significant increase in post-A. baumannii instillation IL-17 levels. These findings support the well-described role of IL-17 in host defenses against extracellular bacteria and fungi (21, 22). Notably, amphotericin B has antifungal as well as immunomodulatory effects, and further studies are needed to determine if our observations are the result of either or both (23).
In previous studies using the same rat model (11, 24), C. albicans airway colonization primed the host for development of S. aureus and P. aeruginosa pneumonia; however, the TH2 lineage did not seem to be activated, in agreement with our results (see Fig. S1 and S2 in the supplemental material). On the other hand, in BALB/c mice, C. albicans intranasal instillation had a beneficial effect on Pseudomonas pneumonia, with increased TH2 cytokine levels (25). Therefore, it seems that the interplay between Candida airway colonization, host immune response, and subsequent development of bacterial pneumonia is host dependent. In human cells, where a TH1-TH17 response is protective against Candida infections (26), IL-26 was recently identified as a potent mediator of the TH17 axis antibacterial properties (22). Since the gene for IL-26 is absent in rodents (21, 22), other mechanisms are likely implicated in our rat model observations, and it is not known whether they are conserved in humans, as well.
We demonstrated induction of the A. baumannii phospholipase (plc2 and pld2) and basC, basD (P = 0.066), and bauA genes in the presence of C. albicans (Table 1); basC, basD, and bauA are essential for the biosynthesis and utilization of acinetobactin, the main siderophore in A. baumannii. Furthermore, we observed induction of barB, also part of the siderophore-mediated iron acquisition system (27, 28). Siderophores facilitate iron acquisition from the host and consequently enhance bacterial fitness and virulence, given its limited availability as free iron in mammalian tissues (28). Therefore, the intensification of (i) iron uptake and (ii) use of carbon as a source of energy from host phosphatidylcholine breakdown by Acinetobacter phospholipases (29) are potential mechanisms through which C. albicans enhances the ability of A. baumannii to cause pneumonia. On the other hand, suppression of the ompA and abaI genes could be indicative of an antagonistic relationship between the two organisms. The ompA product has been found to adhere to hyphae, inhibiting C. albicans growth (17), whereas abaI encodes an autoinducer synthase that contributes to quorum sensing and biofilm formation (30, 31).
Our study has limitations that should be taken into consideration and addressed in future experiments. First, we used only one A. baumannii and one C. albicans strain. Also, we studied a model of acute infection in previously healthy animals, and it is difficult to draw firm conclusions that will be applicable to intensive care unit (ICU) patients, who are frequently immunosuppressed, are hospitalized for days or weeks, have structural lung abnormalities, and have received multiple antibiotics. Third, we studied cytokine levels in lung homogenate tissue, consisting of diverse immune and other cell populations. Fourth, we assessed only the short-term in vitro effect of A. baumannii coincubation with C. albicans on the expression of A. baumannii virulence genes, which might not adequately simulate the complex in vivo bacterial-yeast interactions in the setting of chronic airway colonization and biofilm physiology. Notably, though, in a recent clinical study of 618 intubated patients, we found that Candida colonization was significantly associated with the subsequent development of A. baumannii ventilator-associated pneumonia, after robust adjustment for all other confounders and propensity score matching (32).
In conclusion, we found that respiratory tract colonization with C. albicans facilitated the development of A. baumannii pneumonia in a rat model by modulating the expression of A. baumannii virulence genes and/or through modification of the host immune response. In the setting of established Candida colonization, antifungal treatment lowered the incidence of A. baumannii pneumonia, potentially by suppressing IFN-γ production and enhancing IL-17 production. Prospective clinical studies on the association between Candida sp. airway colonization, preemptive antifungal treatment, and subsequent A. baumannii ventilator-associated pneumonia should be considered.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Guangdong Natural Science Foundation (grant 2014A030313305), the Science and Technology Planning Project of Guangzhou, Guangdong Province (grant 201510010046), the Science and Technology Planning Project of Guangdong Province (grant 2015A050502026), the Science and Technology Innovation Fund of Guangdong Provincial Department of Education (grant 2014KTSCX039), and Projects of the National Natural Science Foundation of China (grant 81570012) to Xiaojiang Tan. We have no other financial disclosures.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02180-15.
REFERENCES
- 1.Harriott MM, Noverr MC. 2011. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol 19:557–563. doi: 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baena-Monroy T, Moreno-Maldonado V, Franco-Martinez F, Aldape-Barrios B, Quindos G, Sanchez-Vargas LO. 2005. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10(Suppl 1):E27–E39. [PubMed] [Google Scholar]
- 3.Hermann C, Hermann J, Munzel U, Ruchel R. 1999. Bacterial flora accompanying Candida yeasts in clinical specimens. Mycoses 42:619–627. doi: 10.1046/j.1439-0507.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Haque A, Mukhopadhyay G, Narayan RP, Prasad R. 2005. Interactions between bacteria and Candida in the burn wound. Burns 31:375–378. doi: 10.1016/j.burns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Tan X, Fuchs BB, Wang Y, Chen W, Yuen GJ, Chen RB, Jayamani E, Anastassopoulou C, Pukkila-Worley R, Coleman JJ, Mylonakis E. 2014. The role of Candida albicans SPT20 in filamentation, biofilm formation and pathogenesis. PLoS One 9:e94468. doi: 10.1371/journal.pone.0094468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon RD, Chaffin WL. 2001. Colonization is a crucial factor in oral candidiasis. J Dent Educ 65:785–787. [PubMed] [Google Scholar]
- 7.El-Azizi MA, Starks SE, Khardori N. 2004. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol 96:1067–1073. doi: 10.1111/j.1365-2672.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- 8.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother 50:1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cugini C, Calfee MW, Farrow JR, Morales DK, Pesci EC, Hogan DA. 2007. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol Microbiol 65:896–906. doi: 10.1111/j.1365-2958.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JR, Taylor GW, Rutman A, Hoiby N, Cole PJ, Wilson R. 1999. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol 52:385–387. doi: 10.1136/jcp.52.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux D, Gaudry S, Khoy-Ear L, Aloulou M, Phillips-Houlbracq M, Bex J, Skurnik D, Denamur E, Monteiro RC, Dreyfuss D, Ricard JD. 2013. Airway fungal colonization compromises the immune system allowing bacterial pneumonia to prevail. Crit Care Med 41:e191–e199. doi: 10.1097/CCM.0b013e31828a25d6. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, Adrie C, Garrouste-Orgeas M, Cohen Y, Mourvillier B, Schlemmer B. 2006. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129:110–117. doi: 10.1378/chest.129.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Nseir S, Jozefowicz E, Cavestri B, Sendid B, Di Pompeo C, Dewavrin F, Favory R, Roussel-Delvallez M, Durocher A. 2007. Impact of antifungal treatment on Candida-Pseudomonas interaction: a preliminary retrospective case-control study. Intensive Care Med 33:137–142. doi: 10.1007/s00134-006-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards AM, Abu KY, Lamont RJ. 2015. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol Oral Microbiol 30:2–15. doi: 10.1111/omi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medell M, Hart M, Duquesne A, Espinosa F, Valdes R. 2013. Nosocomial ventilator-associated pneumonia in Cuban intensive care units: bacterial species and antibiotic resistance. MEDICC Rev 15:26–29. [DOI] [PubMed] [Google Scholar]
- 17.Gaddy JA, Tomaras AP, Actis LA. 2009. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun 77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MG, Des Etages SG, Snyder M. 2004. Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol 24:3874–3884. doi: 10.1128/MCB.24.9.3874-3884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schortgen F, Bouadma L, Joly-Guillou ML, Ricard JD, Dreyfuss D, Saumon G. 2004. Infectious and inflammatory dissemination are affected by ventilation strategy in rats with unilateral pneumonia. Intensive Care Med 30:693–701. doi: 10.1007/s00134-003-2147-7. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Sun K, Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 22.Gaffen SL, Jain R, Garg AV, Cua DJ. 2014. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meller S, Di Domizio J, Voo KS, Friedrich HC, Chamilos G, Ganguly D, Conrad C, Gregorio J, Le Roy D, Roger T, Ladbury JE, Homey B, Watowich S, Modlin RL, Kontoyiannis DP, Liu Y, Arold ST, Gilliet M. 2015. TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol 16:970–979. doi: 10.1038/ni.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roux D, Gaudry S, Dreyfuss D, El-Benna J, de Prost N, Denamur E, Saumon G, Ricard J. 2009. Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit Care Med 37:1062–1067. doi: 10.1097/CCM.0b013e31819629d2. [DOI] [PubMed] [Google Scholar]
- 25.Ader F, Jawhara S, Nseir S, Kipnis E, Faure K, Vuotto F, Chemani C, Sendid B, Poulain D, Guery B. 2011. Short term Candida albicans colonization reduces Pseudomonas aeruginosa-related lung injury and bacterial burden in a murine model. Crit Care 15:R150. doi: 10.1186/cc10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drummond RA, Gaffen SL, Hise AG, Brown GD. 2015. Innate defense against fungal pathogens. Cold Spring Harb Perspect Med 5:a019620. doi: 10.1101/cshperspect.a019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiester SE, Actis LA. 2013. Stress responses in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol 8:353–365. doi: 10.2217/fmb.12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan T, Choi CH, Oh MH. 2015. Genes involved in the biosynthesis and transport of acinetobactin in Acinetobacter baumannii. Genomics Informatics 13:2. doi: 10.5808/GI.2015.13.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl J, Bergmann H, Göttig S, Ebersberger I, Averhoff B. 2015. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS One 10:e0138360. doi: 10.1371/journal.pone.0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu C, Clemmer KM, Bonomo RA, Rather FN. 2008. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anbazhagan D, Mansor M, Yan GO, Md Yusof MY, Hassan H, Sekaran SD. 2012. Detection of quorum sensing signal molecules and identification of an autoinducer synthase gene among biofilm forming clinical isolates of Acinetobacter spp. PLoS One 7:e36696. doi: 10.1371/journal.pone.0036696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan X, Zhu S, Yan D, Chen Y, Chen R, Zou J, Yan J, Zhang X, Farmakiotis D, Mylonakis E. 21 March 2016. Candida spp. airway colonization: a potential risk factor for Acinetobacter baumannii ventilator-associated pneumonia. Med Mycol. doi: 10.1093/mmy/myw009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.