Abstract
The majority of hospitalized patients receiving mold-active triazoles are at risk of drug-drug interactions (DDIs). Efforts are needed to increase awareness of DDIs that pose a serious risk of adverse events. Triazoles remain the most commonly utilized antifungals. Recent developments have included the mold-active triazoles (MATs) itraconazole, voriconazole, and posaconazole, which are first-line agents for the treatment of filamentous fungal infections but have the potential for DDIs. This objective of this study was to evaluate the prevalence of triazole DDIs. Hospitalized U.S. adults with MAT use were identified in the Cerner HealthFacts database, which contained data from over 150 hospitals (2005 to 2013). The severities of DDIs with MATs were categorized, using drug labels and the drug information from the Drugdex system (Thompson Micromedex), into four groups (contraindicated, major, moderate, and minor severity). DDIs of minor severity were not counted. A DDI event was considered to have occurred if the following two conditions were met: (i) the patient used at least one drug with a classification of at least a moderate interaction with the MAT during the hospitalization and (ii) there was a period of overlap between the administration of the MAT and that of the interacting drug of at least 1 day. A total of 6,962 hospitalizations with MAT use were identified. Among them, 88% of hospitalizations with voriconazole use, 86% of hospitalizations with itraconazole use, and 93% of hospitalizations with posaconazole use included the use of a concomitant interacting drug. A total of 68% of hospitalizations with posaconazole use, 34% of hospitalizations with itraconazole use, and 20% of hospitalizations with voriconazole use included the use of at least one drug with a DDI of contraindicated severity. A total of 83% of hospitalizations with posaconazole use, 61% of hospitalizations with itraconazole use, and 82% of hospitalizations with voriconazole use included the use of at least one drug that resulted in a severe DDI. The findings of this study demonstrate that a majority of hospitalized patients receiving MAT are at risk for severe drug-drug interactions and highlight the need for antifungal stewardship.
INTRODUCTION
Triazoles are widely used for the prophylaxis and treatment of invasive fungal infections (IFIs). Fluconazole, the first triazole agent, was introduced in 1990 and quickly became one of the most commonly prescribed antifungal agents. Itraconazole was the first agent with activity against mold pathogens. Triazoles that have been developed more recently have included drugs with predominant activity against molds that cause opportunistic infections (e.g., Aspergillus spp.). These mold-active triazoles, voriconazole, posaconazole, and, most recently, isavuconazole, have become first-line options for both the treatment and prophylaxis of filamentous fungal infections (1, 2).
Although these newer triazoles have a broader spectrum of activity than fluconazole, they exhibit complex pharmacokinetics, including a propensity for interactions with coadministered drugs (3). All mold-active triazoles are inhibitors of one or more phase 1 (cytochrome P-450) biotransformation enzymes and may also be inhibitors or substrates of phase 2 biotransformation enzymes or transporter proteins, such as P-glycoprotein (4). Mold-active triazoles may alter the absorption, distribution, excretion, and metabolism of coadministered agents, such as benzodiazepines, anxiolytics, dihydropyridine calcium channel blockers, sulfonylureas, calcineurin inhibitors, prednisone, or anticoagulants (3). Mold-active triazoles are also associated with QTc prolongation, so they should be used with caution with other agents having similar effects (5).
The definition of drug-drug interactions (DDIs) varies across the literature. Some studies define a DDI to be the concomitant use of interacting drugs, while others define DDIs on the basis of clinical evidence of interactions that are confirmed either by laboratory tests or by symptoms. In this paper, we use the term DDI to describe the concomitant use of interacting drugs. DDIs account for 3 to 5% of preventable in-hospital adverse drug reactions and are an important cause of emergency room and hospital visits (6). DDIs are associated with significant clinical consequences and higher resource use and costs (7, 8). DDIs are common among patients taking multiple medications, with the prevalence estimated to range from 60.0% to 70.3% among hospitalized patients, and are associated with increased lengths of stay, higher rates of morbidity and mortality, and higher health care costs (7–14). The risk of DDIs among mold-active triazoles has been an important factor contributing to the recommendation of consistent therapeutic drug monitoring of mold-active triazoles in the guidelines from the Infectious Diseases Society of America.
Although mold-active triazoles are often used in patients with multiple comorbid conditions experiencing polypharmacy, to date, limited information regarding the rates of DDIs among patients receiving mold-active triazoles in real-world settings is available (15). To fill this gap, this retrospective study aimed to evaluate the prevalence of DDIs among hospitalized patients with evidence of mold-active triazole utilization. Understanding the real-world occurrence of DDIs associated with mold-active triazoles may inform antimicrobial stewardship efforts to enhance patient safety.
MATERIALS AND METHODS
Data source.
The Cerner HealthFacts electronic medical records (EMR) data set (April 2005 to December 2013), an electronic data capture system containing information from clinical and laboratory systems at over 150 hospitals, was used for this study. The database contains data on 110 million encounters across the majority of states in the United States and includes patient demographic variables (age, gender, and race) and diagnoses and procedures (both of which are coded using the codes in the International Classification of Diseases, ninth revision, clinical modification [ICD-9]). Pharmacy data, including the national drug codes (NDCs), the dates and times that the drugs were dispensed, and the route of administration, are available. Only medications administered through the hospital pharmacies are captured. The data are deidentified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Interacting drugs.
A list of drugs that have been reported to interact with each mold-active triazole agent (itraconazole, posaconazole, and voriconazole) was collected from the Drugdex system (Thompson Micromedex) and supplemented with relevant prescribing information (PI). The Drugdex system contains evidence-based, expert-reviewed documents covering U.S. Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved, investigational, and nonprescription drugs. These databases include information on cautions, DDIs, clinical applications, and DDI-related adverse effects.
The Drugdex system classifies DDIs into four groups according to their severity: contraindicated (the DDI is life-threatening and the concomitant use is contraindicated), major (the DDI is potentially life-threatening or the DDI is capable of causing permanent damage), moderate (the DDI might cause deterioration in a patient's clinical status; additional treatment, hospitalization, or extension of the hospital stay might be necessary), and minor (the DDI causes mild effects that do not significantly affect the therapeutic outcomes) (16, 17). Drugs with interactions classified as being of contraindicated, major, and moderate severity were considered in this study; drugs with interactions classified as being of minor severity were excluded.
Sample selection.
Data for hospitalized patients who received a systemic mold-active triazole (itraconazole, voriconazole, or posaconazole) during the hospitalization were selected from the database. Patients were included in the study if they met the following inclusion/exclusion criteria: they received at least one administration/dose of itraconazole, voriconazole and/or posaconazole intravenously (i.v.) or orally during the hospitalization, they were at least 18 years of age at the time of hospital admission, they did not receive multiple mold-active triazoles (an i.v. or oral formulation of the same drug is counted as one drug), and they had nonmissing drug initiation and discontinuation dates for the mold-active triazole and its interacting drug(s), where present. The mold-active triazole used during the hospital stay was defined as the index triazole, and the corresponding hospital stay was defined as the index hospitalization. Among patients with multiple eligible hospitalizations, a random encounter was selected.
Analyses.
A DDI event was considered to have occurred if patients used at least one drug reported to interact with the index triazole during the index hospitalization and there was at least a 1-day overlap between the use of the index triazole and the use of the interacting drug. Only dispensed drugs were considered in the analysis (i.e., drugs that were prescribed but not dispensed were excluded). The half-lives of the mold-active triazoles ranged from 6 h (voriconazole) to 35 h (posaconazole) (3); thus, at least a 1-day overlap of drug use was required in the current study to allow a window of opportunity for interaction. At least a 1-day overlap of drug use has commonly been used to define DDIs in studies described in the literature (7, 15) and was used in the primary analysis described in the current study. As a sensitivity analysis, the use of overlaps of drug use of at least 2 and 3 days to define DDIs was explored.
The prevalence and frequency of DDI events during the index hospitalization are summarized and are reported for all patients and by index triazole. The prevalence of DDIs was defined as the proportion of hospitalizations with any DDI events that occurred during the index hospitalization. The frequency of DDI events was defined as the total number of DDI events of a unique pair of the triazole and its interacting drug that occurred during the index hospitalization. The prevalence and frequency of DDI events were further summarized by the severity of all DDI events (contraindicated, major, and moderate severity) observed during the index hospitalizations.
Patient characteristics, including demographic, treatment, and hospital characteristics, were described for all patients and stratified by DDI and non-DDI cohorts. Disease conditions based on ICD-9 codes for both the primary and secondary diagnoses were summarized. The top 5 most commonly observed IFIs based on ICD-9 diagnosis codes or microbiology lab test results were also reported. The differences in these characteristics between the DDI and non-DDI cohorts were compared using chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
In addition, the 25 drugs that most frequently interacted with each triazole were summarized. For each interacting drug, the frequency was measured as the total number of hospital admissions with the use of the interacting drug and the interacting triazole. The rate was defined as the frequency divided by the total number of hospitalizations with DDIs for each triazole.
Subgroup analysis.
The overall patient population that met the sample selection criteria described above was further selected into subgroups of patients who, we hypothesized, had a higher risk for DDIs and were more vulnerable to the detrimental effects of DDIs. These subgroups were critically ill patients or patients with multiple morbidities, who often require several concomitant medications, leading to an increased risk of DDIs. The following subgroups of patients were identified using ICD-9 diagnosis codes, procedure codes, generic product identifier codes, and care setting identifiers: patients with renal failure, hematologic malignancies, diabetes, neutropenia, hospitalization involving an intensive care unit (ICU), corticosteroid use, severe sepsis, transplantation (lung, liver, and kidney), chemotherapy, liver disease (moderate or severe), and allogeneic hematopoietic stem cell transplantation (HSCT) or bone marrow transplantation (BMT). The prevalence and frequency DDIs were reported for all patients in each subgroup overall and by stratification by DDI severity.
RESULTS
Sample selection.
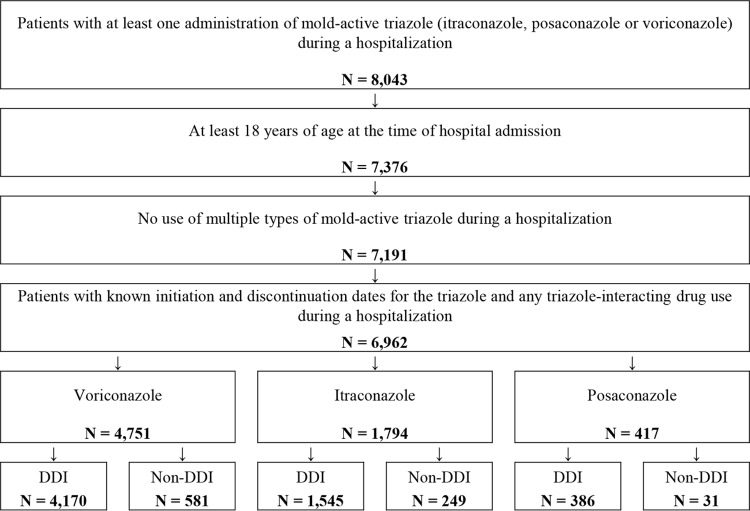
A total of 6,962 hospital admissions with mold-active triazole drug use were included in this study (Fig. 1). Voriconazole was the most frequently used triazole (n = 4,751 hospital admissions), followed by itraconazole (n = 1,794 hospital admissions) and then posaconazole (n = 417 hospital admissions).
FIG 1.
Sample selection for patients who received mold-active triazole therapies during hospitalization.
The study cohort had a mean age of 57.2 ± 16.9 years. About half of the patients (55.0%) were male, and the majority of the patients (73.3%) were Caucasian. Patients spent an average of 7.8 days in the hospital before receiving the triazole, and 74.9% of patients received the triazole within 10 days of admission. For the majority of patients (73.1%), treatment with the index triazole was initiated in the oral formulation. Among the patients for which information on the diagnosis was available (∼75%), disease conditions with a prevalence higher than 20% included respiratory complications, anemia, hematologic malignancies, hypertension, sepsis, and acute kidney failure. An invasive fungal infection (IFI) was identified in 36.6% of patients, and the most common IFIs were invasive candidiasis (20.4%) and aspergillosis (11.3%) (Table 1). The remaining patients were receiving either empirical or prophylactic antifungal therapy. Patient characteristics were mostly similar between patients with and patients without DDIs. Differences between the two cohorts included the following: patients with DDIs were older (57.4 years for patients with DDIs versus 55.5 years for patients without DDIs), perhaps reflecting polypharmacy as a risk; a higher proportion of patients with DDIs were Caucasian (74.0% for patients with DDIs versus 68.2% patients without DDIs); and initiation of fluconazole treatment began sooner after hospital admission in patients with DDIs than those without DDIs (7.5 versus 9.8 days).
TABLE 1.
Baseline characteristics stratified by patients with and without DDI evaluated during the index hospitalization
| Characteristic | Value(s) for: |
P valuea | ||
|---|---|---|---|---|
| All patients (n = 6,962) | Patients with DDIs (n = 6,101) | Non-DDI patients (n = 861) | ||
| Demographic characteristics | ||||
| Mean ± SD age (yr) | 57.2 ± 16.9 | 57.4 ± 17.0 | 55.5 ± 16.2 | 0.0003 |
| No. (%) of patients aged: | ||||
| 18–64 yr | 4,405 (63.3) | 3,802 (62.3) | 603 (70.0) | <.0001 |
| 65+ yr | 2,557 (36.7) | 2,299 (37.7) | 258 (30.0) | <.0001 |
| No. (%) of male patients | 3,829 (55.0) | 3,330 (54.6) | 499 (58.0) | 0.0624 |
| No. (%) of patients of the following race: | ||||
| Caucasian | 5,103 (73.3) | 4,516 (74.0) | 587 (68.2) | 0.0003 |
| African American | 1,385 (19.9) | 1,159 (19.0) | 226 (26.2) | <.0001 |
| Hispanic | 113 (1.6) | 100 (1.6) | 13 (1.5) | 0.7788 |
| Other | 272 (3.9) | 244 (4.0) | 28 (3.3) | 0.2894 |
| Unknown | 89 (1.3) | 82 (1.3) | 7 (0.8) | 0.1941 |
| Hospital characteristics | ||||
| No. (%) of patients in hospitals with the following bed sizes: | ||||
| <99 | 259 (3.7) | 242 (4.0) | 17 (2.0) | 0.0038 |
| 100–199 | 372 (5.3) | 332 (5.4) | 40 (4.6) | 0.3310 |
| 200–299 | 938 (13.5) | 822 (13.5) | 116 (13.5) | 0.9997 |
| 300–499 | 2,637 (37.9) | 2,422 (39.7) | 215 (25.0) | <.0001 |
| 500+ | 2,756 (39.6) | 2,283 (37.4) | 473 (54.9) | <.0001 |
| No. (%) of patients in hospitals within a teaching facility | 5,792 (83.2) | 5,039 (82.6) | 753 (87.5) | 0.0004 |
| No. (%) of patients in hospitals in urban areas | 6,961 (100.0) | 6,100 (100.0) | 861 (100.0) | 1.0000 |
| Treatment characteristics | ||||
| No. (%) of patients receiving the following type of index triazole: | ||||
| Itraconazole | 1,794 (25.8) | 1,545 (25.3) | 249 (28.9) | 0.0239 |
| Posaconazole | 417 (6.0) | 386 (6.3) | 31 (3.6) | 0.0016 |
| Voriconazole | 4,751 (68.2) | 4,170 (68.3) | 581 (67.5) | 0.6078 |
| No. (%) of patients receiving the following type of formulation | ||||
| Oral | 5,092 (73.1) | 4,494 (73.7) | 598 (69.5) | 0.0091 |
| Intravenous | 1,484 (21.3) | 1,289 (21.1) | 195 (22.6) | 0.3078 |
| Unknown and other | 386 (5.5) | 318 (5.2) | 68 (7.9) | 0.0013 |
| Mean ± SD no. of days from admission to triazole initiation | 7.8 ± 16.4 | 7.5 ± 14.8 | 9.8 ± 24.7 | <.0001 |
| No. (%) of patients with the following no. of days from admission to triazole initiation | ||||
| <1 | 2,294 (33.0) | 2,069 (33.9) | 225 (26.1) | <.0001 |
| 1–10 | 2,920 (41.9) | 2,540 (41.6) | 380 (44.1) | 0.1637 |
| ≥10 | 1,748 (25.1) | 1,492 (24.5) | 256 (29.7) | 0.0008 |
| No. (%) of patients with emergent/urgent hospital admission | 1,030 (14.8) | 932 (15.3) | 98 (11.4) | 0.0026 |
| Prevalent and relevant disease conditions, identified by ICD-9 codes | ||||
| No. (%) of patients with a diagnosis ofb: | 5,426 (77.9) | 4,750 (77.9) | 676 (78.5) | |
| Respiratory complications | 2,561 (47.2) | 2,256 (47.5) | 305 (45.1) | 0.2469 |
| Anemia | 1,715 (31.6) | 1,515 (31.9) | 200 (29.6) | 0.2270 |
| Hematologic malignancies | 1,606 (29.6) | 1,449 (30.5) | 157 (23.2) | 0.0001 |
| Hypertension | 1,295 (23.9) | 1,150 (24.2) | 145 (21.4) | 0.1151 |
| Sepsis | 1,321 (24.3) | 1,138 (24.0) | 183 (27.1) | 0.0776 |
| Acute kidney failure | 1,209 (22.3) | 1,053 (22.2) | 156 (23.1) | 0.5954 |
| IFIc | ||||
| Any IFI | 2,549 (36.6) | 2,237 (36.7) | 312 (36.2) | 0.8067 |
| Invasive candidiasis | 1,423 (20.4) | 1,245 (20.4) | 178 (20.7) | 0.8556 |
| Aspergillosis | 785 (11.3) | 710 (11.6) | 75 (8.7) | 0.0110 |
| Other and unspecified mycoses | 274 (3.9) | 242 (4.0) | 32 (3.7) | 0.7240 |
| Histoplasmosis | 248 (3.6) | 210 (3.4) | 38 (4.4) | 0.1500 |
| Blastomycosis | 55 (0.8) | 42 (0.7) | 13 (1.5) | 0.0108 |
P values comparing the DDI and non-DDI cohorts.
Proportion reported among patients for whom diagnosis information is available.
IFI, invasive fungal infection.
DDI prevalence and frequency.
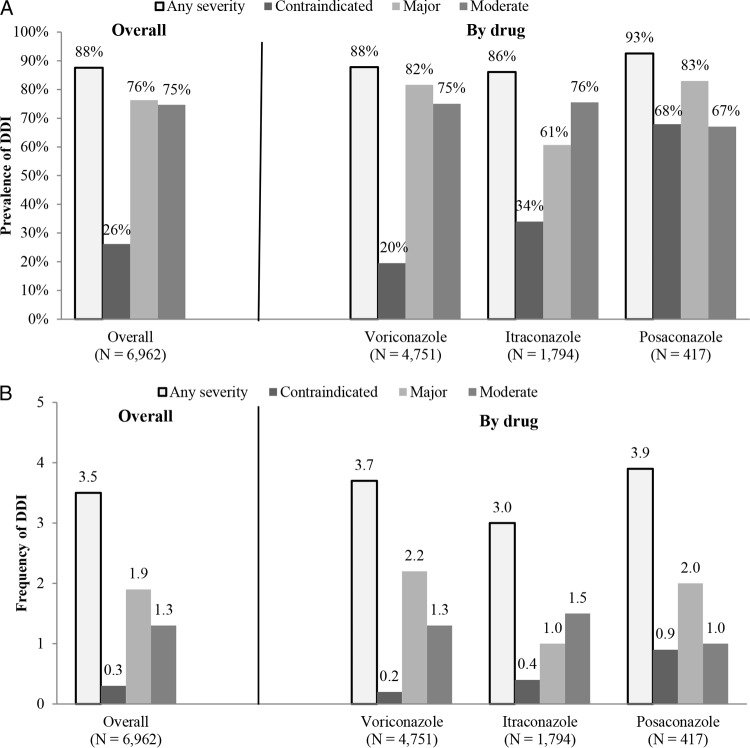
Among the hospitalizations included, 6,101 (87.6%) had DDIs. The prevalences were 87.8% for voriconazole, 86.1% for itraconazole, and 92.6% for posaconazole (Fig. 2a) when a DDI was defined by at least a 1-day overlap of drug use. The mean ± standard deviation (SD) number of DDI events per admission was 3.5 ± 2.4 among all admissions. The DDI frequency was relatively similar among the triazoles, with 3.9 ± 2.4 events per admission being observed in the posaconazole cohort, followed by 3.7 ± 2.5 events per admission in the voriconazole cohort and then 3.0 ± 2.2 events per admission in the itraconazole cohort (Fig. 2b). The frequency of contraindicated DDI events per admission was less than one among all cohorts receiving mold-active triazoles (0.3 ± 0.6 for all hospitalizations, 0.2 ± 0.5 for voriconazole, 0.4 ± 0.6 for itraconazole, and 0.9 ± 0.8 for posaconazole).
FIG 2.
(a) Overall prevalence of DDIs; (b) overall frequency of DDIs per admission.
When the prevalence of all DDI events within each hospitalization was summarized by severity, 26.2% of all hospitalizations had at least one DDI of contraindicated severity, 76.3% had at least one DDI of major severity, and 74.7% had at least one DDI of moderate severity. The prevalences of contradicted, major, and moderate severity DDIs were 19.5%, 81.6%, and 75.0%, respectively, for voriconazole; 34%, 60.7%, and 75.5%, respectively, for itraconazole; and 67.9%, 83.0%, and 67.1%, respectively, for posaconazole (Fig. 2a).
In the sensitivity analysis, when a DDI was defined by at least 2-day and 3-day overlaps of drug use, the prevalence of any DDI among all hospitalizations changed to 80.7% and 65.4%, respectively, and the prevalence of DDIs of contraindicated severity decreased to 18.4% and 13.4%, respectively.
Mold-active triazole-interacting drugs.
Among the hospital admissions, the most commonly used drug interacting with voriconazole was ondansetron, which was used in 47.6% of admissions with a voriconazole-related DDI event and classified as a DDI event of major severity on the basis of the risk of arrhythmia. The other commonly observed voriconazole-interacting drugs, which contributed to more than 20% of DDI events, were pantoprazole (used in 31.8% of admissions, moderate severity), esomeprazole (used in 29.2% of admissions, moderate severity), fentanyl (used in 28.2% of admissions, major severity), midazolam (used in 23.9% of admissions, moderate severity), and levofloxacin (used in 21.3% of admissions, major severity) (Table 2). The most commonly observed itraconazole-interacting drugs were ondansetron (used in 38.2% of admissions, major severity), pantoprazole (used in 27.0% of admissions, moderate severity), prednisone (used in 23.5% of admissions, moderate severity), and esomeprazole (used in 22.3% of admissions, moderate severity) (Table 3). The most commonly observed posaconazole-interacting drugs were ondansetron (used in 54.9% of admissions, contraindicated severity), lorazepam (used in 42.7% of admissions, moderate severity), promethazine (used in 31.1% of admissions, major severity), ciprofloxacin (used in 29.3% of admissions, major severity), prochlorperazine (used in 27.2% of admissions, major severity), esomeprazole (used in 21.5% of admissions, major severity), fentanyl (used in 20.7% of admissions, major severity), and pantoprazole (used in 20.7% of admissions, moderate severity) (Table 4). Among the drugs with interactions with mold-active triazoles of contraindicated and major severity, many are widely used for the treatment of common conditions and include warfarin (which prevents heart attacks and strokes), amiodarione (which is used to treat cardiac dysrhythmias), statins (i.e., simvastatin and atorvastatin, which are used to lower cholesterol), cyclosporine (which is used to treat rheumatoid arthritis and psoriasis and which prevents transplant rejection), and alprazolam (which is used to treat anxiety).
TABLE 2.
Top 25 voriconazole-interacting drugs by frequency of DDIsa
| Interacting medication | Severity of DDI | Frequency of DDIs | Rate of DDIs (%) |
|---|---|---|---|
| Ondansetron | Major | 1,986 | 47.6 |
| Pantoprazole | Moderate | 1,326 | 31.8 |
| Esomeprazole | Moderate | 1,218 | 29.2 |
| Fentanyl | Major | 1,175 | 28.2 |
| Midazolam | Moderate | 996 | 23.9 |
| Levofloxacin | Major | 888 | 21.3 |
| Oxycodone | Moderate | 700 | 16.8 |
| Promethazine | Major | 697 | 16.7 |
| Prochlorperazine | Major | 595 | 14.3 |
| Alprazolam | Major | 492 | 11.8 |
| Amlodipine | Moderate | 480 | 11.5 |
| Ciprofloxacin | Major | 434 | 10.4 |
| Tacrolimus | Major | 422 | 10.1 |
| Fluconazole | Contraindicated | 406 | 9.7 |
| Azithromycin | Major | 375 | 9.0 |
| Simvastatin | Contraindicated | 368 | 8.8 |
| Warfarin | Major | 311 | 7.5 |
| Amiodarone | Major | 280 | 6.7 |
| Omeprazole | Moderate | 275 | 6.6 |
| Lansoprazole | Moderate | 272 | 6.5 |
| Escitalopram | Major | 235 | 5.6 |
| Haloperidol | Major | 228 | 5.5 |
| Ibuprofen | Moderate | 198 | 4.7 |
| Clopidogrel | Major | 181 | 4.3 |
| Tamsulosin | Major | 179 | 4.3 |
Data are for 4,170 voriconazole-treated patients with DDIs.
TABLE 3.
Top 25 itraconazole-interacting drugs by frequency of DDIa
| Interacting medication | Severity of DDI | Frequency of DDIs | Rate of DDIs (%) |
|---|---|---|---|
| Ondansetron | Major | 590 | 38.2 |
| Pantoprazole | Moderate | 417 | 27.0 |
| Prednisone | Moderate | 363 | 23.5 |
| Esomeprazole | Moderate | 344 | 22.3 |
| Fentanyl | Major | 294 | 19.0 |
| Midazolam | Contraindicated | 279 | 18.1 |
| Amlodipine | Moderate | 184 | 11.9 |
| Famotidine | Moderate | 184 | 11.9 |
| Alprazolam | Contraindicated | 156 | 10.1 |
| Fluconazole | Contraindicated | 143 | 9.3 |
| Warfarin | Major | 134 | 8.7 |
| Oxycodone | Major | 122 | 7.9 |
| Simvastatin | Contraindicated | 121 | 7.8 |
| Hydrocortisone | Moderate | 105 | 6.8 |
| Omeprazole | Moderate | 97 | 6.3 |
| Digoxin | Major | 95 | 6.1 |
| Ciprofloxacin | Moderate | 94 | 6.1 |
| Magnesium oxide | Moderate | 94 | 6.1 |
| Escitalopram | Major | 84 | 5.4 |
| Tamsulosin | Major | 81 | 5.2 |
| Calcium carbonate | Moderate | 74 | 4.8 |
| Haloperidol | Moderate | 70 | 4.5 |
| Budesonide | Moderate | 66 | 4.3 |
| Lansoprazole | Moderate | 66 | 4.3 |
| Atorvastatin | Major | 63 | 4.1 |
Data are for 1,545 itraconazole-treated patients with DDIs.
TABLE 4.
Top 25 posaconazole-interacting drugs by frequency of DDIsa
| Interacting medication | Severity of DDI | Frequency of DDIs | Rate of DDIs (%) |
|---|---|---|---|
| Ondansetron | Contraindicated | 212 | 54.9 |
| Lorazepam | Moderate | 165 | 42.7 |
| Promethazine | Major | 120 | 31.1 |
| Ciprofloxacin | Major | 113 | 29.3 |
| Prochlorperazine | Major | 105 | 27.2 |
| Esomeprazole | Major | 83 | 21.5 |
| Fentanyl | Major | 80 | 20.7 |
| Pantoprazole | Moderate | 80 | 20.7 |
| Levofloxacin | Major | 67 | 17.4 |
| Midazolam | Major | 67 | 17.4 |
| Lansoprazole | Moderate | 56 | 14.5 |
| Tacrolimus | Contraindicated | 49 | 12.7 |
| Amlodipine | Moderate | 48 | 12.4 |
| Fluconazole | Major | 46 | 11.9 |
| Sirolimus | Contraindicated | 28 | 7.3 |
| Alprazolam | Major | 24 | 6.2 |
| Metoclopramide | Moderate | 24 | 6.2 |
| Simvastatin | Contraindicated | 20 | 5.2 |
| Azithromycin | Major | 18 | 4.7 |
| Tamsulosin | Major | 18 | 4.7 |
| Escitalopram | Contraindicated | 16 | 4.1 |
| Haloperidol | Contraindicated | 16 | 4.1 |
| Moxifloxacin | Major | 15 | 3.9 |
| Warfarin | Major | 13 | 3.4 |
| Cyclosporine | Major | 12 | 3.1 |
Data are for 386 posaconazole-treated patients with DDIs.
Subgroup analysis.
Across all subgroups of patients at higher risk for DDIs that were evaluated, the prevalence rate of any DDI ranged from 79.7% for hospitalizations with solid organ transplantation (lung, liver, or kidney) to 90.6% for hospitalizations involving an ICU (Table 5). The mean number of all DDI events per admission ranged from 3.5 for hospitalizations involving a diagnosis of neutropenia to 5.6 for hospitalizations involving allogeneic HSCT/BMT (Table 6).
TABLE 5.
DDI prevalence among subgroups
| Subgroup | Total no. of patients | No. (%) of patients with a DDI by severity |
|||
|---|---|---|---|---|---|
| Any | Contraindicated | Major | Moderate | ||
| Renal failure | 1,616 | 1,411 (87.3) | 472 (29.2) | 1,288 (79.7) | 1,262 (78.1) |
| Hematologic malignancies | 1,606 | 1,449 (90.2) | 406 (25.3) | 1,345 (83.7) | 1,209 (75.3) |
| Diabetes | 968 | 857 (88.5) | 323 (33.4) | 752 (77.7) | 739 (76.3) |
| Neutropenia | 674 | 608 (90.2) | 166 (24.6) | 554 (82.2) | 495 (73.4) |
| Hospitalization involving ICU | 616 | 558 (90.6) | 168 (27.3) | 499 (81.0) | 502 (81.5) |
| Corticosteroid use | 593 | 517 (87.2) | 164 (27.7) | 470 (79.3) | 455 (76.7) |
| Severe sepsis | 547 | 467 (85.4) | 136 (24.9) | 423 (77.3) | 426 (77.9) |
| Transplantation (lung, liver, or kidney) | 374 | 298 (79.7) | 93 (24.9) | 293 (78.3) | 267 (71.4) |
| Chemotherapy | 210 | 188 (89.5) | 58 (27.6) | 182 (86.7) | 150 (71.4) |
| Liver disease (moderate to severe) | 151 | 123 (81.5) | 35 (23.2) | 116 (76.8) | 118 (78.1) |
| Allogeneic HSCT/BMT | 88 | 71 (80.7) | 26 (29.5) | 70 (79.5) | 67 (76.1) |
TABLE 6.
DDI frequency among subgroups
| Subgroup | Mean ± SD no. of DDIs by severity |
|||
|---|---|---|---|---|
| Any | Contraindicated | Major | Moderate | |
| Renal failure | 4.0 ± 2.7 | 0.3 ± 0.6 | 2.1 ± 1.8 | 1.5 ± 1.2 |
| Hematologic malignancies | 3.9 ± 2.5 | 0.3 ± 0.6 | 2.3 ± 1.8 | 1.3 ± 1.0 |
| Diabetes | 3.8 ± 2.5 | 0.4 ± 0.6 | 1.9 ± 1.6 | 1.4 ± 1.2 |
| Neutropenia | 3.5 ± 2.3 | 0.3 ± 0.5 | 2.0 ± 1.6 | 1.2 ± 1.0 |
| Hospitalization involving ICU | 3.9 ± 2.5 | 0.3 ± 0.6 | 2.1 ± 1.7 | 1.5 ± 1.2 |
| Corticosteroid use | 4.0 ± 2.7 | 0.3 ± 0.6 | 2.2 ± 1.8 | 1.5 ± 1.3 |
| Severe sepsis | 3.9 ± 2.7 | 0.3 ± 0.6 | 2.2 ± 1.8 | 1.5 ± 1.2 |
| Transplantation (lung, liver, or kidney) | 4.3 ± 3.0 | 0.3 ± 0.6 | 2.5 ± 2.0 | 1.5 ± 1.3 |
| Chemotherapy | 4.1 ± 2.4 | 0.3 ± 0.5 | 2.6 ± 1.7 | 1.2 ± 1.0 |
| Liver disease (moderate to severe) | 3.7 ± 2.8 | 0.3 ± 0.5 | 2.0 ± 1.9 | 1.4 ± 1.1 |
| Allogeneic HSCT/BMT | 5.6 ± 3.5 | 0.4 ± 0.6 | 3.7 ± 2.3 | 1.6 ± 1.4 |
DISCUSSION
A DDI with triazoles poses a serious risk of adverse events, including QTc prolongation and subsequent arrhythmia, skeletal muscle toxicity, seizures, ergotism, respiratory depression, nephrotoxicity, hypoglycemia, and leukopenia (18–20). In addition, the risk of a DDI and the associated adverse events are particularly challenging among critically ill patients and patients with multiple morbidities, who typically require several concomitant medications and are vulnerable to the clinical signs and symptoms of DDIs (4, 21). Insightful case series studies and reviews have demonstrated the clinical importance of these interactions (3, 22, 23). The retrospective study described here used data from a large electronic medical records data set to investigate the prevalence and frequency of DDIs among hospital admissions involving the use of mold-active triazoles and found that more than 85% of hospitalizations with mold-active triazole use had at least one DDI event. Among all hospitalizations with mold-active triazole use, 26% had a DDI event of contraindicated severity (Fig. 2a) and 87% had at least one DDI of major or moderate severity (data not shown). Multiple DDI events per patient were common, with an average of 3.5 DDI events being observed. The prevalences of DDIs was greater than 90% in certain subgroups, such as patients with hospitalizations involving an ICU and admissions for hematologic malignancies and neutropenia, and the DDI frequency was greater than 5 events per admission among the subgroup of patients admitted for allogeneic HSCT/BMT.
The introduction of mold-active triazole agents during the past 2 decades (itraconazole in 1992 [24], voriconazole in 2002 [20], and posaconazole in 2006 [19]) and isavuconazole in 2015 has transformed the management of IFIs (25). Distinct from the yeast-only activity of fluconazole, these agents offer broad activity against both invasive candidiasis and invasive aspergillosis, which were the IFIs most commonly observed in this study (2, 26). Despite their efficacy against mold infections, these triazoles are often associated with frequent DDIs, due to both the long list of medications with which they interact and the frequency of comorbid conditions associated with their use, leading to polypharmacy. In this study, it was observed that patients had an average of 12 distinct disease conditions; the most common diagnoses included hematologic malignancies and critical illnesses, such as respiratory failure, acute kidney failure, and sepsis. In addition, on average, each patient was prescribed 36 distinct medications during each admission. The most frequently observed interacting drugs in the current study were ondansetron, pantoprazole, lorazepam, esomeprazole, prednisone, and promethazine. The majority of the frequently observed interacting drugs have major or moderate interactions with the triazole. The clinical relevance of some of these interactions has been debated. For example, the QTc prolongation linked to concomitant ondansetron use can be modest. However, the effect appears to be dose dependent and linked to intravenous use, which is more common among those receiving chemotherapy. Interestingly, patients receiving chemotherapy were the largest patient group in the current cohort. It is also noteworthy that much of the posaconazole use during the period of this study would have been with a formulation impacted by drugs which reduce gastric acidity and, thus, posaconazole absorption. If performed today, these interactions would not be listed. However, the higher absorption observed with the new oral tablet formulation may in turn increase the likelihood of more severe interactions for drugs with P-450 interactions.
Additionally, there were 2, 4, and 6 drugs with interactions of contraindicated severity with voriconazole, itraconazole, and posaconazole, respectively, among the 25 drugs most frequently observed to interact with each triazole. In addition, multiple interacting drugs observed in this study, such as warfarin, amiodarone, statins, and cyclosporine, are widely used to treat common conditions, including cardiovascular diseases and rheumatoid arthritis.
DDI events have been linked to severe clinical outcomes, including death; therefore, DDIs should be carefully considered in clinical practice when administering multiple drugs. A number of studies have shown that adverse events associated with DDIs from the concomitant use of triazoles and other drugs could result in increased toxicity or QTc prolongation (27–32). These interactions can be dangerous, especially among patients receiving chemotherapy or solid organ transplantation or critically ill patients treated in an ICU (33). This study provides real-world evidence indicating that the majority of patients treated with mold-active triazoles during hospitalizations have a risk of DDIs. Given that these patients often have multiple conditions requiring many treatments, full avoidance of DDIs might not be entirely feasible; however, greater awareness of DDIs could reduce the potential for clinically relevant interactions.
The high prevalence of DDIs observed in this study was generally consistent with the prevalence reported in the literature; however, the rates were even higher in this contemporary study. Egger et al. reported a rate of DDIs of 60% among consecutive hospital admissions with at least two prescriptions regardless of the underlying disease conditions and drugs used (13). Yu et al. found a DDI rate of 70.3% among hospitalizations in which fluconazole, itraconazole, or ketoconazole was used (14). Almost all patients in that study used fluconazole (94.5%), only a limited number of patients used itraconazole (n = 212) and ketoconazole (n = 68), and no patients used voriconazole or posaconazole.
The current study was subject to several limitations. First, this study was conducted with data from the Cerner HealthFacts electronic medical record database, which might limit the generalizability of the findings to all the hospitals in the United States. All the hospitalizations included in the DDI analysis were in urban hospitals, with the majority of the hospitals being affiliated with a teaching facility and having more than 300 beds. As a result, the findings of this study might not be generalizable to all the hospitals in the United States. Second, this study did not evaluate how many of the DDI events would eventually lead to an adverse event. Due to the limitations of electronic medical records data, it is not possible to evaluate the clinical outcomes and consequences of DDI events. Future research is warranted to address this question. Third, the study did not evaluate whether physicians adjusted the treatments, such as change the medications and reduce the dosage. This is particularly relevant for drugs that are commonly administered with therapeutic drug monitoring, such as warfarin and calcineurin inhibitors. Lastly, the current study used at least a 1-day overlap of drug use to define a DDI in the primary analysis. This length of overlap, which might cause an overestimation of the prevalence, given that the half-life of drugs might be less than 1 day, and dispending records instead of administration records were used for the evaluation.
Conclusion.
DDIs are highly prevalent among hospitalized patients receiving mold-active triazoles, and there are often multiple DDI events per admission. Efforts are needed to increase the awareness of DDIs and to decrease the risk of DDIs among mold-active triazole users via the use of electronic medical record tools and antifungal stewardship.
ACKNOWLEDGMENTS
This work was supported by Astellas Pharma Global Development, Inc.
David Andes has served as a consultant to Astellas. Nkechi Azie, Billy Franks, Rita Kristy, Edward Lee, and James Spalding are employed by Astellas Pharma Global Development, Inc. Nikhil Khandelwal was an employee of Astellas Pharma Global Development, Inc., at the time of the analysis. Hongbo Yang, Caroline Kelley, Ruo-Ding Tan, and Eric Q. Wu are employees of Analysis Group, Inc., which has received consultancy fees for research from Astellas.
Acknowledgments
Medical writing assistance was provided by Ana Bozas, an employee of Analysis Group, Inc.
REFERENCES
- 1.Lat A, Thompson GR III. 2011. Update on the optimal use of voriconazole for invasive fungal infections. Infect Drug Resist 4:43–53. doi: 10.2147/IDR.S12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clinic Proc 86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 4.Nivoix Y, Ubeaud-Sequier G, Engel P, Leveque D, Herbrecht R. 2009. Drug-drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr Drug Metab 10:395–409. doi: 10.2174/138920009788499012. [DOI] [PubMed] [Google Scholar]
- 5.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. 2012. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 73:293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. 2016. Preventable adverse drug reactions: a focus on drug interactions. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm110632.htm. [Google Scholar]
- 7.Pergolizzi JV Jr, Labhsetwar SA, Puenpatom RA, Ben-Joseph R, Ohsfeldt R, Summers KH. 2012. Economic impact of potential CYP450 pharmacokinetic drug-drug interactions among chronic low back pain patients taking opioids. Pain Pract 12:45–56. doi: 10.1111/j.1533-2500.2011.00503.x. [DOI] [PubMed] [Google Scholar]
- 8.Summers KH, Puenpatom RA, Rajan N, Ben-Joseph R, Ohsfeldt R. 2011. Economic impact of potential drug-drug interactions in opioid analgesics. J Med Econ 14:390–396. doi: 10.3111/13696998.2011.583302. [DOI] [PubMed] [Google Scholar]
- 9.Lubinga SJ, Uwiduhaye E. 2011. Potential drug-drug interactions on in-patient medication prescriptions at Mbarara Regional Referral Hospital (MRRH) in western Uganda: prevalence, clinical importance and associated factors. Afr Health Sci 11:499–507. [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PS, Rana DA, Suthar JV, Malhotra SD, Patel VJ. 2014. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J Basic Clin Pharm 5:44–48. doi: 10.4103/0976-0105.134983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li AP. 1998. The scientific basis of drug-drug interactions: mechanism and preclinical evaluation. Drug Information J 32:657–664. [Google Scholar]
- 12.Bertoli R, Bissig M, Caronzolo D, Odorico M, Pons M, Bernasconi E. 2010. Assessment of potential drug-drug interactions at hospital discharge. Swiss Med Wkly 140:w13043. doi: 10.4414/smw.2010.13043. [DOI] [PubMed] [Google Scholar]
- 13.Egger SS, Drewe J, Schlienger RG. 2003. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol 58:773–778. [DOI] [PubMed] [Google Scholar]
- 14.Yu DT, Peterson JF, Seger DL, Gerth WC, Bates DW. 2005. Frequency of potential azole drug-drug interactions and consequences of potential fluconazole drug interactions. Pharmacoepidemiol Drug Saf 14:755–767. doi: 10.1002/pds.1073. [DOI] [PubMed] [Google Scholar]
- 15.Andes D. 2013. Optimizing antifungal choice and administration. Curr Med Res Opin 29(Suppl 4):S13–S18. doi: 10.1185/03007995.2012.761135. [DOI] [PubMed] [Google Scholar]
- 16.Bergk V, Gasse C, Rothenbacher D, Loew M, Brenner H, Haefeli WE. 2004. Drug interactions in primary care: impact of a new algorithm on risk determination. Clin Pharmacol Ther 76:85–96. doi: 10.1016/j.clpt.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Micromedex. Micromedex healthcare series. Micromedex, Greenwood Village, CO. [Google Scholar]
- 18.U.S. Food and Drug Administration. 2001. Drug approval package. Sporanox (itraconazole) injection & oral solution. U.S. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-966S001_Sporanox.cfm Accessed 29 January 2016. [Google Scholar]
- 19.U.S. Food and Drug Administration. 2006. Drug approval package. Noxafil (posaconazole) oral suspension. U.S. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/022027_noxafil_toc.cfm Accessed 29 January 2016. [Google Scholar]
- 20.U.S. Food and Drug Administration. 2002. Drug approval package. Vfend (voriconazole) tablets & injection. U.S. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-266_21-267_Vfend.cfm Accessed 29 January 2016. [Google Scholar]
- 21.Hammann F, Drewe J. 2014. Data mining for potential adverse drug-drug interactions. Expert Opin Drug Metab Toxicol 10:665–671. doi: 10.1517/17425255.2014.894507. [DOI] [PubMed] [Google Scholar]
- 22.Gubbins PO. 2011. Triazole antifungal agents drug-drug interactions involving hepatic cytochrome P450. Expert Opin Drug Metab Toxicol 7:1411–1429. doi: 10.1517/17425255.2011.627854. [DOI] [PubMed] [Google Scholar]
- 23.Dodds-Ashley E. 2010. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy 30:842–854. doi: 10.1592/phco.30.8.842. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration. 2009. Pharmacology review(s). Application number 22-484. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022484Orig1s000PharmR.pdf Accessed 29 January 2016. [Google Scholar]
- 25.Gallagher JC, Dodds Ashley ES, Drew RH, Perfect JR. 2003. Antifungal pharmacotherapy for invasive mould infections. Expert Opin Pharmacother 4:147–164. doi: 10.1517/14656566.4.2.147. [DOI] [PubMed] [Google Scholar]
- 26.Carrillo-Munoz AJ, Quindos G, Ruesga M, Alonso R, del Valle O, Hernández-Molina JM, McNicholas P, Loebenberg D, Santos P. 2005. Antifungal activity of posaconazole compared with fluconazole and amphotericin B against yeasts from oropharyngeal candidiasis and other infections. J Antimicrob Chemother 55:317–319. doi: 10.1093/jac/dki022. [DOI] [PubMed] [Google Scholar]
- 27.Aypar E, Kendirli T, Tutar E, Ciftçi E, Ince E, Ileri T, Atalay S. 2011. Voriconazole-induced QT interval prolongation and torsades de pointes. Pediatr Int 53:761–763. doi: 10.1111/j.1442-200X.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- 28.Elbey MA, Cil H, Onturk E, Islamoglu Y. 2012. OTc prolongation and torsade de pointes ventricular tachycardia in a small dose voriconazole therapy. Eur Rev Med Pharmacol Sci 16:100–102. [PubMed] [Google Scholar]
- 29.Raad II, Graybill JR, Bustamante AB, Cornely OA, Gaona-Flores V, Afif C, Graham DR, Greenberg RN, Hadley S, Langston A, Negroni R, Perfect JR, Pitisuttithum P, Restrepo A, Schiller G, Pedicone L, Ullmann AJ. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis 42:1726–1734. doi: 10.1086/504328. [DOI] [PubMed] [Google Scholar]
- 30.Zeuli JD, Wilson JW, Estes LL. 2013. Effect of combined fluoroquinolone and azole use on QT prolongation in hematology patients. Antimicrob Agents Chemother 57:1121–1127. doi: 10.1128/AAC.00958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkan Y, Haefeli WE, Burhenne J, Stein J, Yaniv I, Shalit I. 2004. Voriconazole-induced QT interval prolongation and ventricular tachycardia: a non-concentration-dependent adverse effect. Clin Infect Dis 39:e49–e52. doi: 10.1086/423275. [DOI] [PubMed] [Google Scholar]
- 32.Hachem RY, Langston AA, Graybill JR, Perfect JR, Pedicone LD, Patino H, Raad II. 2008. Posaconazole as salvage treatment of invasive fungal infections in patients with underlying renal impairment. J Antimicrob Chemother 62:1386–1391. doi: 10.1093/jac/dkn401. [DOI] [PubMed] [Google Scholar]
- 33.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. 2006. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 50:1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]