Abstract
Preexposure prophylaxis (PrEP) against HIV using oral regimens based on the nucleoside reverse transcriptase inhibitor tenofovir disoproxil fumarate (TDF) has been effective to various degrees in multiple clinical trials, and the CCR5 receptor antagonist maraviroc (MVC) holds potential for complementary efficacy. The effectiveness of HIV PrEP is highly dependent on adherence. Incorporation of the TDF-MVC combination into intravaginal rings (IVRs) for sustained mucosal delivery could increase product adherence and efficacy compared with oral and vaginal gel formulations. A novel pod-IVR technology capable of delivering multiple drugs is described. The pharmacokinetics and preliminary local safety characteristics of a novel pod-IVR delivering a combination of TDF and MVC were evaluated in the ovine model. The device exhibited sustained release at controlled rates over the 28-day study and maintained steady-state drug levels in cervicovaginal fluids (CVFs). Dilution of CVFs during lavage sample collection was measured by ion chromatography using an inert tracer, allowing corrected drug concentrations to be measured for the first time. Median, steady-state drug levels in vaginal tissue homogenate were as follows: for tenofovir (TFV; in vivo hydrolysis product of TDF), 7.3 × 102 ng g−1 (interquartile range [IQR], 3.0 × 102, 4.0 × 103); for TFV diphosphate (TFV-DP; active metabolite of TFV), 1.8 × 104 fmol g−1 (IQR, 1.5 × 104, 4.8 × 104); and for MVC, 8.2 × 102 ng g−1 (IQR, 4.7 × 102, 2.0 × 103). No adverse events were observed. These findings, together with previous pod-IVR studies, have allowed several lead candidates to advance into clinical evaluation.
INTRODUCTION
Preexposure prophylaxis (PrEP) using FDA-approved antiretroviral (ARV) drugs holds significant promise as a strategy in the prevention of HIV infection. By analogy to highly active antiretroviral therapy (HAART), a combination of ARV agents likely is essential for optimally effective HIV PrEP (1, 2). Multiple HIV PrEP clinical trials have demonstrated that vaginal and oral ARV regimens based on the nucleoside reverse transcriptase inhibitor (NRTI) tenofovir (TFV) can be effective in susceptible men, women, and partners of HIV-infected individuals (3–9), but other studies based on analogous drug regimens were unsuccessful at reducing the rates of HIV acquisition (10–12). A critical factor driving success in these trials appears to involve sustaining high adherence to frequent dosing (13).
Adherence to therapy was found to be inversely related to dosing periods across different delivery methods (14–17). Topical delivery of ARV drugs using intravaginal rings (IVRs) is believed to improve adherence (18) while maintaining sustained mucosal microbicide levels independently of coitus and daily dosing (19). A recent phase 3, randomized, double-blind, placebo-controlled trial involving 2,629 African women evaluating a monthly IVR delivering the nonnucleoside HIV-1 reverse transcriptase inhibitor dapivirine (DPV) showed that this dosing modality can be effective at preventing HIV-1 infection (20). Overall, the incidence of HIV-1 infection in the DPV group was lower by 37% than that in the placebo group, following the exclusion of data from two sites that exhibited lower-than-expected protocol and product adherence. The efficacy of HIV-1 prevention was as high as 61% among women 25 years of age or older. However, the delivery of two or more ARV drugs by the use of conventional IVR designs, such as the DPV IVR, involves significant technological and manufacturing hurdles. To meet these challenges, we have developed a novel IVR technology, the pod-IVR (19, 21), that enables rapid development of devices capable of delivering multiple agents over a wide range of target delivery rates and levels of aqueous solubility (22–25).
Here, we report on the pharmacokinetics (PK) and preliminary local safety in an ovine model of a pod-IVR delivering the prodrug TFV disoproxil fumarate (TDF) in combination with maraviroc (MVC), an entry inhibitor/antagonist of chemokine receptor CCR5. Steady-state drug levels for both ARV agents in cervicovaginal fluids (CVFs) were sustained over the 28-day study with corresponding vaginal tissue (VT) concentrations above the levels required for putative efficacy in preventing HIV infection.
MATERIALS AND METHODS
Materials.
Tenofovir disoproxil fumarate (TDF) was kindly provided by Gilead Sciences, Inc. (Foster City, CA), under a Material Transfer Agreement (MTA) dated 8 August 2011. Maraviroc (MVC; ViiV Healthcare, Brentford, Middlesex, United Kingdom) was kindly provided by the International Partnership for Microbicides, Inc. (IPM; Silver Spring, MD), under an MTA dated 12 October 2010 and was used as an analytical reference standard. For formulation into IVRs, MVC was isolated from the commercial formulation (Pfizer, Inc., New York, NY), which consists of film-coated tablets for oral administration containing 300 mg of MVC and inactive ingredients, as described previously (25). Polyvinyl alcohol (PVA) with a mean weight-average molecular weight (Mw) of 85,000 to 124,000 (98% to 99% hydrolyzed) was obtained from Sigma-Aldrich (St. Louis, MO). Tenofovir (adenine-13C5) (TFV-13C5) was obtained from Moravek Biochemicals, Inc. (Brea, CA), and maraviroc-D6 (MVC-D6) was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX). All other reagents were obtained from Sigma-Aldrich, unless otherwise noted.
Manufacture of combination pod-IVRs.
Human-sized polydimethylsiloxane (PDMS; silicone) pod-IVRs were prepared in a multistep process that has been described in detail elsewhere (21, 23, 25). Ten pods per combination IVR were used: 6 pods of TDF and 4 pods of MVC (Table 1). Each pod contained a single drug. The drug powder, admixed with 0.5% (wt/wt) magnesium stearate in the case of TDF, was compacted into cores (3.2-mm outer diameter) in a manual tablet press (MTCM-I; Globe Pharma, New Brunswick, NJ). Drug cores were coated with polymer to afford so-called “pods” (Table 1), placed in the corresponding IVR cavities, and sealed in place by back-filling with room-temperature cure silicone. Each pod was matched with the appropriate configuration of a mechanically punched delivery channel(s) according to Table 1.
TABLE 1.
Physical characteristics of TDF-MVC pod-IVRs used in the animal study
| Physical characteristic | IVR configuration(s)a |
|---|---|
| TDF drug loading (mg)b | 230.8 ± 0.6 (6 pods) |
| MVC drug loading (mg)b | 153.6 ± 1.4 (4 pods) |
| Per-pod delivery channel cross-sectional area (mm2) | TDF, 0.79; MVC, 5.30c |
Each pod was coated with poly(vinyl alcohol); data represent total drug loading in IVR.
Data represent means ± SD.
For TDF, one 0.79-mm2 channel per pod; for MVC, three 1.77-mm2 channels per pod.
In vitro studies.
All in vitro release studies were designed to mimic sink conditions using methods reported previously (21). Briefly, the IVRs were placed in a simplified vaginal fluid simulant (VFS) (26) dissolution medium (100 ml) consisting of 25 mM acetate buffer (pH 4.2) with NaCl added to achieve 220 mOs. The vessels were agitated in an orbital shaker at 25 ± 2°C and 60 rpm. Aliquots (100 μl) were removed at predetermined time points and were replaced with an equal volume of dissolution medium. Samples were stored at −30°C prior to analysis. The concentrations of TDF and its hydrolysis products TFV isoproxil [mono(POC)TFV], TFV, and MVC were measured by high-performance liquid chromatography (HPLC) with UV detection as described previously (25, 27).
Ovine PK and local safety studies.
The PK and safety study was carried out at the Preclinical Surgical Research Laboratory, Colorado State University (Fort Collins, CO), under approval by the Institutional Animal Care and Use Committees at Colorado State University (Animal Welfare Assurance no. A3572-01). The study timeline and biological sample collection points are shown in Fig. 1 and were based on published protocols (22, 24, 28). Briefly, eight skeletally mature, multiparous, and nonpregnant female Rambouillet X Columbian ewes were used in the study; four received TDF-MVC pod-IVRs, and four received placebo IVRs. A piece of nonabsorbable nylon suture, several inches longer than the sheep's vaginal tract, was tied to the IVR (Fig. 2). IVRs were inserted on day 0 into the posterior vagina and were removed on day 28 (Fig. 1) with the sheep under general anesthesia in dorsal recumbency. The IVRs were inserted into the cranial vagina using a gloved finger lubricated with medical-grade lubricant gel. The end of the suture was allowed to hang outside the vulva to serve as an external visual check that the IVR had not been expelled; the suture also helped IVR removal during necropsy. Following insertion of the IVR, a speculum was used to view the vagina and determine that the device was placed correctly. Subsequently, vaginal colposcopy was used to confirm placement and retention of the IVRs and to examine the integrity of the cervicovaginal epithelium (Fig. 2), as described below.
FIG 1.
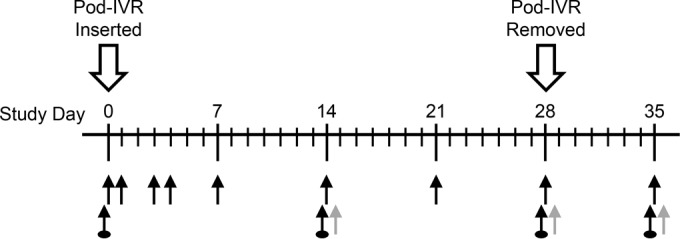
Sheep TDF-MVC pod-IVR study timelines and biological sample collection points (n = 4). Regular black arrows, in order of collection, blood, vaginal fluid (two Weck-Cel samples per time point—one dorsal, one ventral), and cervicovaginal lavage fluid; gray arrows, collection of vaginal tissue samples (four pinch biopsy samples per time point on day 14 and day 28; whole tissues on day 35); arrows with circles, colposcopic/laparoscopic examination.
FIG 2.
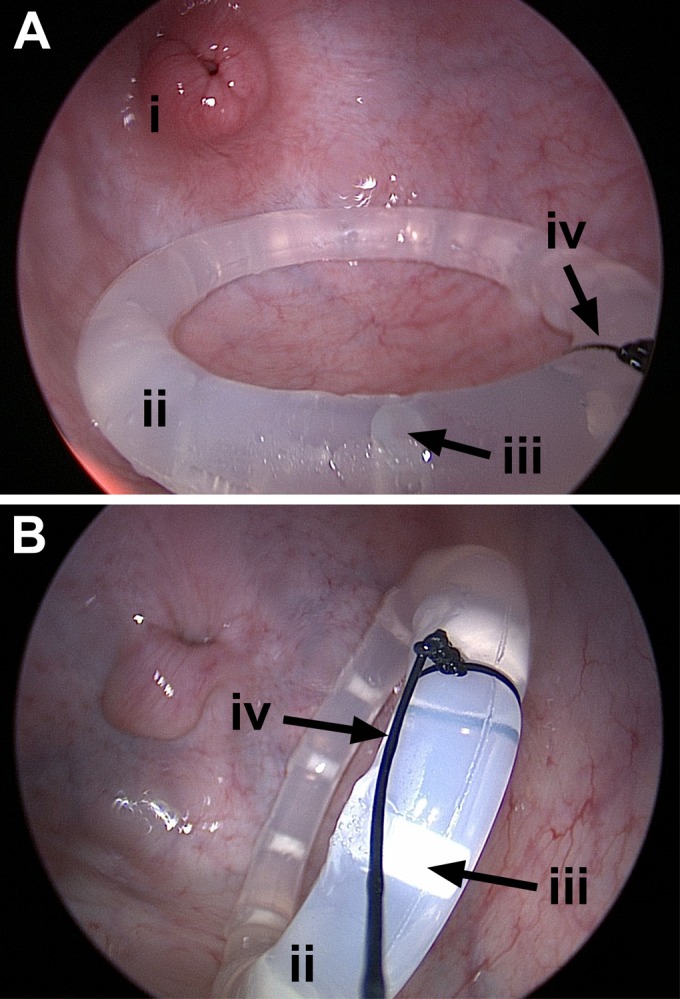
High-definition colposcopy images of the vaginal vault showing the cervix (i) and the mucous-covered pod-IVR (ii) in place. The location of one of the 10 pod cavities is identified (iii). The nylon suture (iv) attached to the IVR facilitated removal and was used to monitor expulsion(s).
Blood, local CVFs—collected by the use of a Weck-Cel sponge 0 to 2 cm from the IVR—and cervicovaginal lavage (CVL) fluid samples were collected at the time points shown in Fig. 1, stored, and transported at −80°C. For each CVL fluid sample, a polyvinyl chloride urethral catheter, or a sterile pediatric Foley catheter (size, 5 or 8 French) of adjusted length, was coated on one end with surgical lubricant and inserted 10 to 20 cm into the sheep's vagina. A 10-ml syringe was loaded with sterile phosphate-buffered CVL fluid saline solution containing 1 mM LiCl, and the saline solution was gently infused through the catheter into the vaginal vault. The saline solution was left in the vagina for 1 min and then drawn back through the catheter into the syringe.
At predetermined time points (Fig. 1), a single-incision laparoscopic surgery (SILS) port was placed through the vaginal opening to the level of the introitus, under conditions of general anesthesia, in dorsal recumbency. A 10-mm-long and two 5-mm-long cannulas were inserted into the SILS portals, and the vagina was insufflated to approximately 6 mm Hg. A 10-mm-long 30° rigid laparoscope was inserted through the 10-mm-long cannula into the vagina to evaluate the location of the IVR and to record images of the vaginal wall (Fig. 2). Following a colposcopy procedure, laparoscopic uterine biopsy forceps were used to obtain partial-thickness VT biopsy samples from the right and left anteriolateral walls and the right and left posterolateral walls (four samples in total), approximately 3 to 5 cm from the cervix. Following vaginal biopsy specimen collection, the rectum was voided of manure and prepped with povidone iodine. Uterine biopsy forceps were used to obtain partial-thickness rectal biopsy samples (four in total) of the left and right lateral and anterior and posterior rectal mucosa. Vaginal and rectal tissue samples were immediately flash-frozen in liquid nitrogen and stored and transported at −80°C. Animals in the medicated group were euthanized on day 35, while the animals in the unmedicated group were used in a follow-on study.
Used IVRs were analyzed for residual drug content using published methods (25). The HPLC methods were the same as those used to analyze aliquots from the in vitro studies.
Levels of TFV in CVF, CVL fluid, and plasma samples and TDF levels in CVF and CVL fluid samples were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using published and unpublished methods (29, 48). For analysis of MVC in CVF, CVL fluid, and plasma samples, the following method was used. Samples were thawed on ice, and 100-μl aliquots were dispensed into 96-well plates, along with a minimum of six standards and a minimum of three quality controls prepared in the appropriate matrix in accordance with FDA guidelines (30). Samples were spiked with 10 μl of internal standard (IS) solution (1 μg ml−1 MVC-D6). Sample purification was carried out in a 96-well format using a protein and phospholipid removal system (Phree; Phenomenex, Inc., Torrance, CA) according to the manufacturer's instructions. The purified samples were dried in vacuo using a SpeedVac concentrator system (Savant SC210A Plus; Thermo Fisher Scientific, Inc.) and were reconstituted in 0.1% (vol/vol) formic acid in water (100 μl) prior to analysis.
The concentration of MVC was measured by LC-MS/MS using a 5-μl injection volume and an HPLC system consisting of a model G1367A well plate autosampler and a model G1312A binary pump (1200 Series; Agilent Technologies, Santa Clara, CA) operating at 0.8 ml min−1 interfaced to an API 3000 triple-quadrupole tandem mass spectrometer (AB Sciex, Framingham, MA) with a Turbo Ion Spray electrospray ionization (ESI) source. An Agilent Zorbax Eclipse XDB-C18 Rapid Resolution column (2.1 by 50 mm; 3.5 μm pore size) controlled at 40°C was used for the stationary phase. The following gradient program was used (solution A, 0.1% [vol/vol] formic acid–water; solution B, 0.1% [vol/vol] formic acid–acetonitrile): 0.25 min 100% A; 1.25 min ramp from 100:0 A:B to 70:30 A:B; 1.0 min ramp from 70:30 A:B to 50:50 A:B; 0.5 min hold at 50:50 A:B; 2 min ramp from 50:50 A:B to 95:5 A:B; and 0.5 min ramp from 95:5 A:B to 100:0 A:B (total run time, 5.5 min; MVC retention time, 2.90 min). The measured transition ions, m/z, in positive ESI mode were as follows: for MVC, 514.7 atomic mass units (amu) (parent) and 280.6 amu (product); for MVC-D6 (IS), 520.7 amu (parent) and 280.6 amu (product).
Concentrations of TFV, TFV diphosphate (TFV-DP), and MVC in VT homogenate were measured by LC-MS/MS at Johns Hopkins University using established methods (31–33). The lower limits of quantitation (LLQs) for these analytes in the sample matrices described above are presented (see Table 3).
TABLE 3.
Summary of ARV drug concentrations in CVF, CVL, and VTa
| Analyte, matrix | n | LLQ | % >LLQb | Median (IQR)c |
|---|---|---|---|---|
| TDF, CVF | 56 | 1.5 ng ml−1d | 39 | 26 (17–43)e; 36 (21–53)f |
| TFV, CVF | 56 | 5 ng ml−1d | 98 | 5.6 × 103 (3.1 × 103–8.6 × 103)e; 4.4 × 103 (3.3 × 103–9.1 × 103)f |
| MVC, CVF | 56 | 8 ng ml−1d | 100 | 1.4 × 104 (5.6 × 103–3.3 × 104)e; 1.5 × 104 (5.5 × 103–3.9 × 104)f |
| TDF, CVL | 28 | 0.5 ng ml−1g | 96 | 42 (27–64)h |
| TFV, CVL | 28 | 5 ng ml−1g | 100 | 8.4 × 103 (3.6 × 103–2.2 × 104)h |
| MVC, CVL | 28 | 8 ng ml−1g | 100 | 2.4 × 104 (1.3 × 104–4.7 × 104)h |
| TFV, VT | 8 | 0.05 ng g−1 | 100 | 7.3 × 102 (3.0 × 102–4.0 × 103) |
| TFV-DP, VT | 8 | 50 fmol g−1i | 88 | 1.8 × 104 (1.5 × 104–4.8 × 104) |
| MVC, VT | 8 | 0.05 ng g−1 | 100 | 8.2 × 102 (4.7 × 102–2.0 × 103) |
| TFV, plasma | 36 | 2 ng ml−1 | 0 | BLQj |
| MVC, plasma | 36 | 3 ng ml−1 | 0 | BLQ |
All values correspond to time points with IVR in place (n = 4).
Data represent proportions of samples that contained quantifiable drug levels.
Interquartile range (25th to 75th percentile).
Data represent nanograms per sample.
Sample collected from the midvagina, ventral.
Sample collected from the midvagina, dorsal.
Data represent CVL fluid uncorrected for CVF dilution.
Data correspond to drug levels in CVF compensated for dilution during the CVL procedure.
Data represent femtomoles per sample.
BLQ, below LLQ.
The vaginal fluid volume collected during the CVL as an additive in the naive CVL fluid was measured by ion chromatography (IC) and calculated using LiCl (1 mM) according to methods discussed in detail elsewhere (34).
Statistical analysis.
Data were analyzed using GraphPad Prism (version 6.05; GraphPad Software, Inc., La Jolla, CA) with statistical significance defined as a P value of <0.05.
RESULTS
In vitro studies.
In vitro cumulative release profiles for the IVR formulation exhibited linear, sustained drug release (TDF, R2 = 0.93; MVC, R2 = 0.95), as is typical for pod-IVRs (21–25, 27). The daily release rates obtained from the slopes of the 28-day cumulative release profiles are presented in Table 2.
TABLE 2.
In vitro and in vivo daily release rates and in vitro-in vivo correlation
| Drug (IVR formulation)a | Release rate (mg day−1)b |
IVIVCc | |
|---|---|---|---|
| In vitro | In vivo | ||
| TDF | 0.88 ± 0.04 | 0.31 ± 0.13 | 0.35 |
| MVC | 2.72 ± 0.08 | 0.67 ± 0.19 | 0.25 |
n = 4.
Data represent means ± SD.
IVIVC, in vitro-in vivo correlation; defined as in vivo release rate divided by in vitro release rate.
In vivo release rates.
The mean daily in vivo TDF and MVC release rates are given in Table 2. The calculation is based on the residual drug mass remaining in the used IVRs and the assumption, supported by in vitro data, that drug release was linear over the 28-day period. Paired TDF and MVC daily in vivo release rates were weakly correlated (R2 = 0.42) for the four sheep, indicating that interanimal physiological differences (e.g., vaginal fluid pH, vaginal fluid volume, and mucous level differences) affect the in vivo release rates. Importantly, >98% of the residual TDF in the used IVR pods was present as the prodrug; i.e., no hydrolysis to mono(POC)TFV or TFV was observed following 4 weeks of use in vivo.
IVIVC.
The use of in vitro-in vivo correlation (IVIVC) to guide in vitro experiments during the development of sustained release formulations allows drug target levels to be achieved with a minimum number of in vivo studies. The calculated IVIVCs are given in Table 2 and are comparable to those measured previously in pigtailed macaques (TDF, 0.20 and 0.47; MVC, 0.66) (25). The IVIVC values suggest that the in vitro system provides an accelerated model of the in vivo release rates.
Local safety measures.
On the basis of intermittent physical examinations and twice-daily cage-side observations, all sheep remained healthy, maintained appropriate appetite and body condition, and had no test article-related adverse events throughout the study. No IVR expulsions or adverse events related to treatment with the test article were noted by colposcopy during the course of the study, and no significant, unusual abnormalities were observed. Figure 2 shows a high-resolution colposcopy image of the pod-IVR in place. The devices were located in a position proximal to the cervix in the upper vagina. Colposcopic examinations did not reveal subjective changes of the vaginal vault or mucosa as a result of implantation of medicated or unmedicated IVRs.
Measurement of CVF dilution during collection of lavage samples.
The CVF volume collected in CVL fluid samples was determined by measuring the dilution of Li+ ions by IC. The distribution of CVF volumes over the course of the study is shown in Fig. 3 for sheep receiving medicated and unmedicated IVRs. The median (interquartile range [IQR]) CVF volume collected in the 10-ml CVL fluid samples was 346 μl (185 to 541 μl). There was no statistically significant difference in collected CVF volumes across all eight sheep according to results of an ordinary one-way analysis of variance (ANOVA) (P = 0.3438). There also was no statistically significant difference in collected CVF volumes between the medicated and unmedicated groups according to results of an unpaired, two-tailed Student t test with Welch's correction (P = 0.9413).
FIG 3.
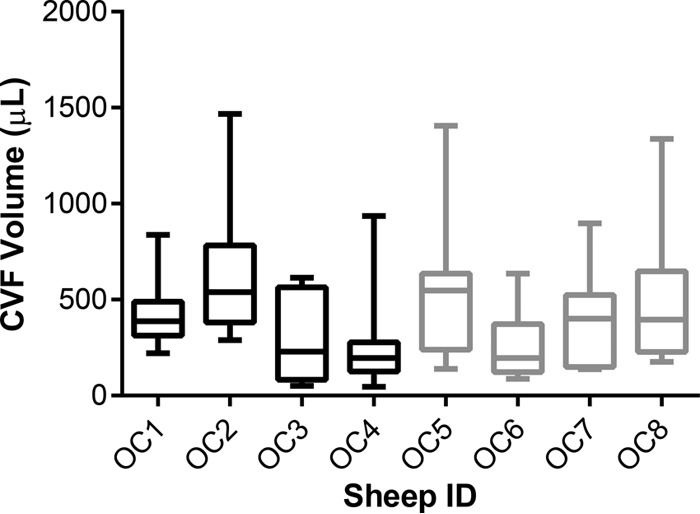
Box plots (8 or 9 CVL fluid samples collected over the 35-day study; see Fig. 1) of CVF volumes collected using the lavage procedure. The box extends from the 25th to 75th percentiles, with the horizontal line in the box representing the median; whiskers represent the lowest and highest datum. OC1 to OC4, medicated IVRs; OC5 to OC8, unmedicated IVRs.
Summary of PK measurements.
The PK parameters for ARV drugs and drug metabolites across key anatomic compartments are summarized in Table 3. All drug measurements in plasma were below the analytical LLQ (Table 3).
ARV drug CVF levels.
Vaginal fluid drug levels as a function of time are shown in Fig. 4 and 5. A one-way ANOVA with Geisser-Greenhouse's correction performed on paired data across all time points showed no statistically significant variation of [TFV plus TDF] (on a molar basis, P = 0.4848) and MVC (P = 0.4313) normalized CVL fluid concentrations over the 28 days of pod-IVR use, suggesting that the steady state was reached within 24 h and was maintained until IVR removal. This result is supported qualitatively by the data shown in Fig. 2 and 4. The day 35 (i.e., 7 days following IVR removal) vaginal fluid drug levels were at or below the LLQ of the analytical method.
FIG 4.
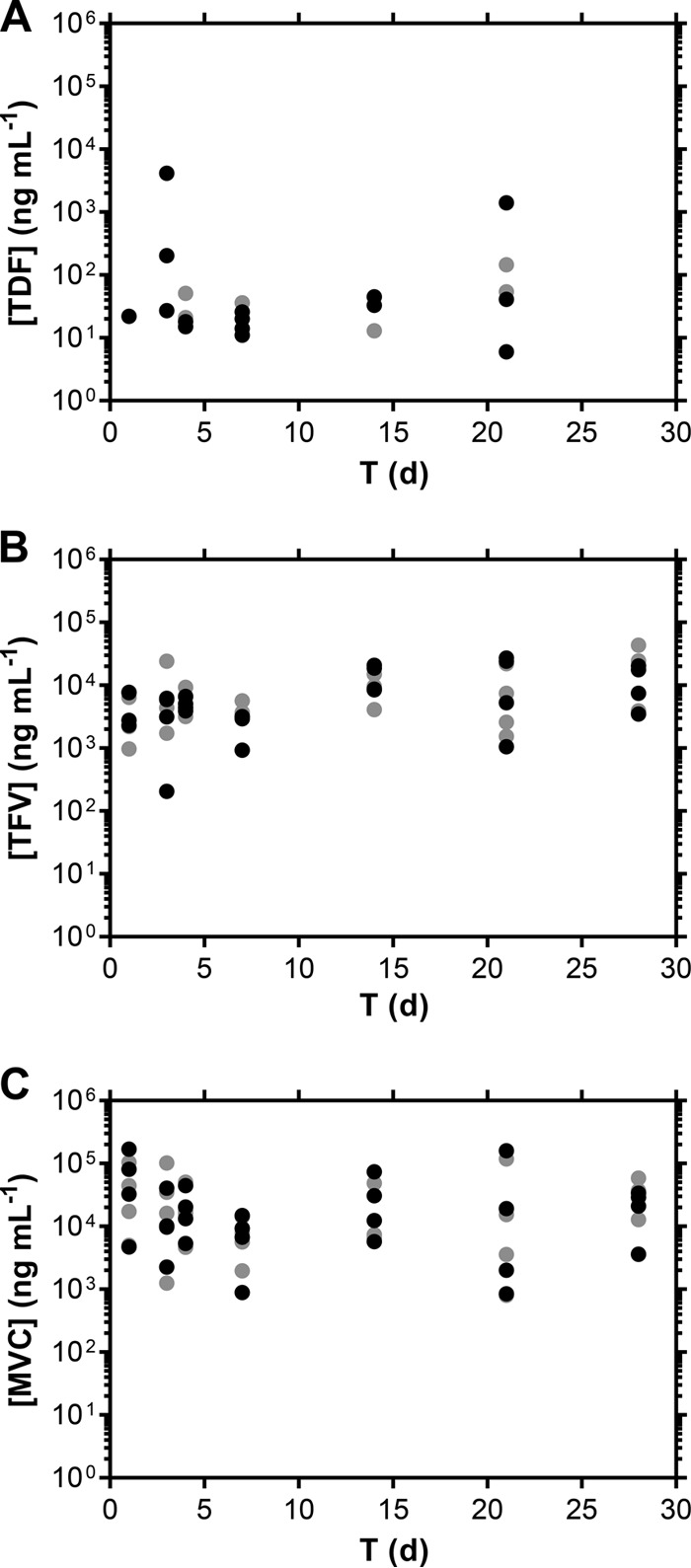
Distribution of ARV drug levels (A, TDF; B, TFV; C, MVC) in undiluted vaginal fluids collected ventrally (black symbols) and dorsally (gray symbols) in the midvagina using Weck-Cel sponges. d, day.
FIG 5.
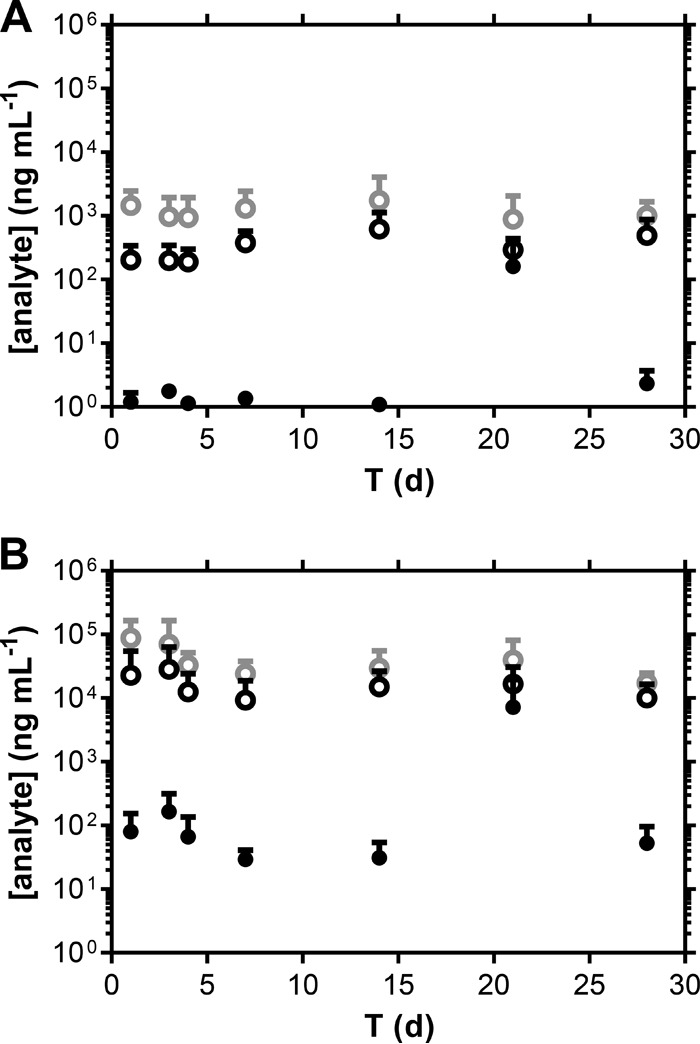
Distribution of ARV drug levels (means + standard deviations [SD], n = 4) in CVL fluid samples that were uncorrected (A) and normalized for dilution using a Li+ tracer added to the CVL fluid (B). Black closed circles, TDF; black open circles, TFV; gray open circles, MVC.
Levels of the prodrug TDF in Weck-Cel samples were low and variable (Fig. 4A) due to various degrees of hydrolysis to TFV (27). On average, TDF made up less than 2% of the molar sum of TDF and TFV in CVL fluid samples, where both analytes were quantifiable in >95% of the samples. It is unclear if TDF hydrolyzed to TFV in vivo or after collection or if the results reflect a combination of the two.
Median ARV drug levels in normalized (i.e., compensated for dilution) CVL fluid samples were higher than in the corresponding Weck-Cel samples. [TDF+TFV] levels, on a molar basis, were uncorrelated across paired Weck-Cel and CVL fluid samples. A moderate linear correlation was found in plotting MVC Weck-Cel concentrations (y axis) versus matched MVC CVL fluid concentrations (x axis): for ventral Weck-Cel, slope, 1.449 ± 0.2006, R2 = 0.6848; for dorsal Weck-Cel, slope, 1.085 ± 0.1639, R2 = 0.6460. Paired t tests were used to compare Weck-Cel and normalized CVL fluid drug levels. [TDF+TFV] levels, on a molar basis, were significantly different in Weck-Cel ventral (P = 0.0198) and dorsal (P = 0.0289) samples from the levels in the matched CVL fluid samples. MVC CVF levels were not significantly different from those in the matched CVL fluid samples in both ventral (P = 0.2378) and dorsal (P = 0.1011) Weck-Cel sampling locations.
Vaginal tissue ARV drug levels.
TFV, TFV-DP, and MVC concentrations at day 14 and day 28 in biopsy specimen VT homogenate are summarized in Table 3 and Fig. 5. The ratio of CVF (measured in nanograms per milliliter) to VT (measured in nanograms per gram) drug concentrations provides a simple measure of xenobiotic partitioning between the two anatomic compartments: the lower the ratio, the more the ARV agent distributes into the vaginal mucosa. The median (IQR) CVF/VT ratios, based on mean Weck-Cel concentrations, were as follows: TFV, 14.5 (6.6 to 26.5); MVC, 21.0 (12.2 to 34.4). The ratios for the two ARV drugs were not significantly different (P = 0.1258) according to a paired, two-tailed Student t test.
DISCUSSION
The primary objectives of the current study were to develop a novel, human-sized IVR for the delivery of ARV drug combinations and to study the PK and safety of a lead candidate delivering TDF with MVC in sheep.
Preclinical evaluation of biomedical vaginal products in sheep.
The ovine model embodies a number of important benefits in the preclinical PK and safety evaluation of vaginal products (19, 24). The vaginal epithelium of humans and sheep consists of stratified squamous tissue but is thinner in sheep, providing a more sensitive model of toxicity. The sheep vaginal cavity is slightly smaller than that of a human but can accommodate human-sized IVRs, an advantage over other models, such as the macaque and rabbit models. Differences between ovine and human vaginal microbiomes lead to a more alkaline CVF (pH 7.5 to 8.5) in sheep (35). Women with lactobacillus-dominated vaginal microbiota typically have a CVF pH of 3.5 (36). We were the first to study the PK and safety of an IVR delivering antiviral agents in sheep (24) and used this model to compare the vaginal bioavailability levels of TDF and TFV (22). Our finding that TDF was nearly 100 times more efficient than TFV at distributing into the vaginal mucosa from CVFs has led us and others to shift from TFV to TDF for vaginal delivery. We also used the ovine model to investigate the PK of a five-drug pod-IVR as a proof-of-concept, advanced multipurpose prevention technology (MPT), combining three ARV drugs from different mechanistic classes (TFV, nevirapine, and saquinavir) with a proven estrogen-progestogen contraceptive for prevention of HIV infection and unintended pregnancy (28). Here, the PK and safety of a novel combination pod-IVR delivering TDF and MVC—ARV drugs from different mechanistic classes under investigation in HIV PrEP—were evaluated in the ovine model. No local toxicity concerns were observed.
Advancing pharmacokinetic analyses in sheep.
The CVL fluid method has the advantage of collection of a sample integrated over the entire lower vaginal tract rather than collection of the local sample obtained with swabs, sponges, and tear test strips. In addition, the CVF sample does not need to be recovered from a sampling device at the time of analysis, decreasing errors due to weighing and other inaccuracies resulting from the collection of low CVF volumes. A fundamental drawback in prior studies involving CVL fluid sample analysis lies with the unknown amount of CVF collected, which can vary over more than 1 order of magnitude in women (37, 38). This uncertainty can lead to large errors in the calculation of CVF drug concentrations from CVL fluid measurements, confounding the interpretation of results. We have developed a novel method for quantifying the amount of CVF collected by CVL that uses IC to precisely and accurately measure the dilution of Li+ added to the CVL fluid (34) and used this method for the first time to determine the CVF volume collected in a preclinical in vivo PK study. The median CVF volume (0.35 ml [IQR, 0.19 to 0.54 ml]) collected in the lavage procedure in this sheep study was similar to the volumes collected from women at different phases of the menstrual cycle (37) (0.30 ± 0.22 ml [follicular phase] and 0.45 ± 0.21 ml [luteal phase]) and from HIV-1-infected women (38) (median, 0.51 ml; IQR, 0.33 to 0.69 ml). These results further validate the usefulness of the ovine model in the preclinical PK evaluation of vaginal drug delivery devices.
A sensitive LC-MS/MS assay for TFV-DP, the pharmacologically active metabolite of TFV, in vaginal tissue homogenate was extended to sheep and used to provide the first measure (Fig. 6) of this important surrogate of active drug, presumably TFV-DP, in CD4+ cells circulating deep in the vaginal mucosa (39).
FIG 6.
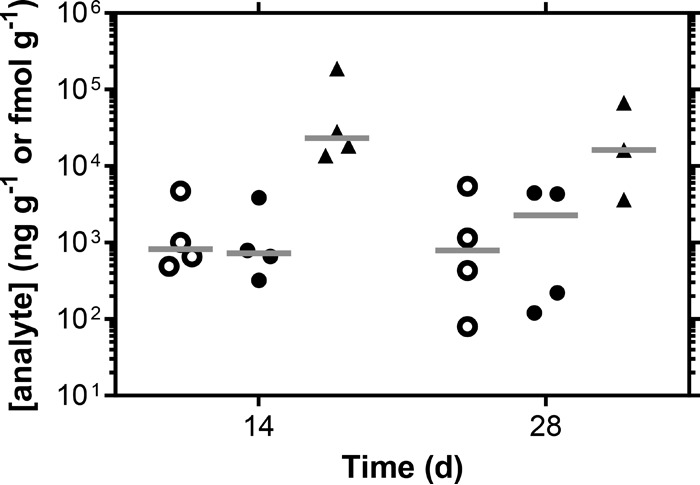
Distribution of ARV drug levels in vaginal tissue biopsy samples. Open circles, MVC (quantified in nanograms per gram); closed circles, TFV (nanograms per gram); triangles, TFV-DP (femtomoles per gram) (1 fmol g−1 TFV-DP is equivalent to 4.5 ×10−4 ng g−1). Horizontal bars represent means.
Combination pod-IVR pharmacokinetics.
The pod-IVRs provided linear, sustained delivery of both ARV drugs at independently controlled rates (Table 2). Cervicovaginal fluid drug levels reached the steady state in less than 24 h and were maintained for the length of the 28-day study (Fig. 4 to 6). Systemic exposure to TFV and MVC drugs was below the analytical LLQ, an advantage of topical dosing via IVR, as the risk of systemic toxicity and emergence of drug resistance is reduced. Median TFV CVF (ventral, 5.6 × 103 ng ml−1; dorsal, 4.4 × 103 ng ml−1) and undiluted CVL fluid (8.4 × 103 ng ml−1) levels (Table 3) were approximately 1 order of magnitude lower than in our pigtailed macaque studies using combination pod-IVRs delivering TDF with emtricitabine (FTC) and TDF-FTC-MVC (25). Median MVC CVF (ventral, 1.4 × 104 ng ml−1; dorsal, 1.5 × 104 ng ml−1) and undiluted CVL fluid (2.4 × 104 ng ml−1) levels (Table 3) were also approximately 10 times lower than in our macaque study. The in vivo ARV drug release rates can readily be modified if necessary by changing the IVR delivery channel configuration and pod polymer coating, a major advantage of the pod-IVR design, as discussed in detail elsewhere (19, 21, 27).
Implications to HIV prevention outcomes.
The two drugs evaluated here target different aspects of the HIV life cycle (40). TDF, a prodrug of TFV, is an NRTI, while MVC is an entry inhibitor/antagonist of chemokine receptor CCR5. The threshold drug levels required for complete protection from productive HIV infection in women remain largely unknown.
A pharmacokinetic-pharmacodynamic (PK-PD) analysis of data from the CAPRISA 004 clinical trial, where a 1% TFV vaginal gel was applied pericoitally, suggests that women with TFV vaginal fluid concentrations greater than 1 μg ml−1 were significantly protected from HIV infection (41), although the time between gel application and CVF measurement is not known. This protective concentration is 4 to 8 times lower than the median TFV CVF levels measured here. MTN-001 was a crossover clinical study designed to directly compare oral to vaginal steady-state TFV PKs in key anatomic compartments (31). Median TFV-DP tissue levels in samples collected at end-of-period visits for women receiving 1% TFV gel were 100 times higher than the corresponding concentrations measured here. However, TFV-DP concentrations in vaginal tissue samples from the oral TDF group were below 2.5 × 104 fmol g−1 and were, therefore, comparable to or lower than the levels in the current study. Because a number of HIV PrEP clinical trials based on oral TDF administration showed that the administration was efficacious (5, 6, 9), it is plausible that the in vivo TDF release rate obtained in the current study is sufficient to provide protection from HIV infection.
No clinical efficacy data for HIV PrEP using intravaginal MVC currently exist, and the levels in the pharmacologically relevant compartments required to afford protection are, therefore, unknown. Dorr and colleagues measured the antiviral potencies of MVC against HIV-1 primary and laboratory-adapted isolates in peripheral blood mononuclear cells (PBMCs) and reported a range of inhibitory concentrations: for 50% inhibitory concentrations (IC50s), 0.1 to 4.5 nM; for 90% inhibitory concentrations (IC90s), 0.5 to 13.4 nM (42). The observed median, steady-state MVC level of 8.2 × 102 ng g−1 (1.6 μM) in vaginal tissues was more than 100 times higher than the highest IC90, suggesting possible favorable pharmacodynamic outcomes.
A randomized clinical trial (MTN-013/IPM 026) in 48 HIV-negative women evaluating matrix-IVRs delivering MVC or MVC in combination with dapivirine (DPV) measured a level of MVC CVF of 2.5 × 103 or 1.1 × 103 ng ml−1, respectively, at day 28 when the IVRs were removed (33). These levels are 10 times lower than those obtained at the steady state in the current study (Table 3), although it should be noted that matrix-IVRs tend to have a drug release burst in the first week. Vaginal tissue MVC levels were undetectable for all subjects using the DPV-MVC IVR and were quantifiable in only 4 of the 12 MVC IVR users, with a 0.13 × 103 to 4.4 × 103 ng g−1 concentration range, comparable to the VT MVC levels measured here. In sheep, the levels of drug distribution from CVFs into VTs were similar for MVC and TDF. Assuming no gross differences between sheep and humans in this regard, MVC in vaginal fluids should partition favorably in the vaginal mucosa.
It should be noted that standard allometric scaling between sheep and human based on body weight does not apply in the context of topical HIV PrEP because efficacy is related to drug concentrations in the vaginal compartment (i.e., fluids and tissues) and not to systemic drug exposure. Human and sheep vaginal tracts have similar physical dimensions (35, 43–46), with comparable vaginal lengths (human, 8 to 12 cm; sheep, 9 to 13 cm) and vaginal epithelial cell thicknesses (human, 86 to 114 μm; sheep, 175 to 284 μm). Additionally, CVF volumes are similar in humans and sheep (see above). Direct comparison of CVF and VT drug levels in the context described above therefore is acceptable without scaling, albeit as part of a preliminary analysis.
An inefficient drug delivery profile of the matrix-IVR, combined with low release rates after the first week of use, could explain the low MVC tissue levels encountered clinically (33). This explanation seems more likely than that of significant active efflux from vaginal epithelial cells mediated by the membrane transporter p-glycoprotein (ABCB1), as it has been found to be underexpressed in VTs (40, 47).
Conclusion and future directions.
Topical administration of ARV combinations from pod-IVRs in sheep demonstrated preliminary local safety results and exhibited sustained, controlled drug release over 28 days. The successful completion of this and other pod-IVR studies (22, 25, 48) delivering TDF and/or MVC have enabled us to obtain an open Investigational New Drug (IND) application (no. 123099) submitted under section 505(i) of the Federal Food, Drug, and Cosmetic Act (FDCA) to advance several candidates into clinical evaluation, which is currently ongoing with a triple combination pod-IVR delivering TDF, FTC, and MVC, among others (ClinicalTrials registration no. NCT02431273).
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award no. R33AI079791 and U19AI113048.
The content of this article is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.D'Cruz OJ, Uckun FM. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des 10:315–336. doi: 10.2174/1381612043386374. [DOI] [PubMed] [Google Scholar]
- 2.Shattock RJ, Warren M, McCormack S, Hankins CA. 2011. Turning the tide against HIV. Science 333:42–43. doi: 10.1126/science.1206399. [DOI] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C; Partners PrEP Study Team. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, Grp TDFS . 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, Chiamwongpaet S, Kitisin P, Natrujirote P, Kittimunkong S, Chuachoowong R, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Hendrix CW, Vanichseni S. 2013. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 8.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, Sullivan AK, Clarke A, Reeves I, Schembri G, Mackie N, Bowman C, Lacey CJ, Apea V, Brady M, Fox J, Taylor S, Antonucci S, Khoo SH, Rooney J, Nardone A, Fisher M, McOwan A, Phillips AN, Johnson AM, Gazzard B, Gill ON. 9 September 2015. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, Hare CB. 2015. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 61:1601–1693. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Yuan A, Chuchuen O, Ham A, Yang KH, Katz DF. 2015. Vaginal deployment and tenofovir delivery by microbicide gels. Drug Deliv Transl Res 5:279–294. doi: 10.1007/s13346-015-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, Voice Study Team . 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. 2013. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav 17:2143–2155. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse W, Eggertkruse W, Rampmaier J, Runnebaum B, Weber E. 1991. Dosage frequency and drug compliance behavior—a comparative study on compliance with a medication to be taken twice or 4 times daily. Eur J Clin Pharmacol 41:589–592. doi: 10.1007/BF00314990. [DOI] [PubMed] [Google Scholar]
- 15.Sershen S, West J. 2002. Implantable, polymeric systems for modulated drug delivery. Adv Drug Deliv Rev 54:1225–1235. doi: 10.1016/S0169-409X(02)00090-X. [DOI] [PubMed] [Google Scholar]
- 16.Kutilek VD, Sheeter DA, Elder JH, Torbett BE. 2003. Is resistance futile? Curr Drug Targets Infect Disord 3:295–309. [DOI] [PubMed] [Google Scholar]
- 17.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. 2009. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, Bekker L, Ganesh S, Young K, Romano J, Nel A, Woodsong C. 2012. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav 16:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- 19.Moss JA, Baum MM. 2014. Microbicide vaginal rings, p 221–290. In das Neves J, Sarmento B (ed), Drug delivery and development of anti-HIV microbicides. Pan Stanford Publishing, Singapore. [Google Scholar]
- 20.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, MTN-020–ASPIRE Study Team . 22 February 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. 2012. An intravaginal ring for the simultaneous delivery of multiple drugs. J Pharm Sci 101:2833–2843. doi: 10.1002/jps.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis R, Vincent KL, Motamedi M, Smith TJ. 2012. Tenofovir and tenofovir disoproxil pharmacokinetics from intravaginal rings. AIDS 26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau C-P, Srinivasan P, Smith JM, Baum MM. 2012. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob Agents Chemother 56:5952–5960. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. 2012. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother 56:875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. 2014. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother 58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. doi: 10.1016/S0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 27.Baum MM, Butkyavichene I, Churchman SA, Lopez G, Miller CS, Smith TJ, Moss JA. 2015. An intravaginal ring for the sustained delivery of tenofovir disoproxil fumarate. Int J Pharm 495:579–587. doi: 10.1016/j.ijpharm.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss JA, Malone AM, Smith TJ, Kennedy S, Nguyen C, Vincent KL, Motamedi M, Baum MM. 2013. Pharmacokinetics of a multipurpose pod-intravaginal ring simultaneously delivering five drugs in the ovine model. Antimicrob Agents Chemother 57:3994–3997. doi: 10.1128/AAC.00547-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, Hendrix CW, Beliveau M, Moss JA, Smith TJ, Baum MM. 20 April 2015. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US FDA. 2001. Guidance for industry: bioanalytical method validation. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD. [Google Scholar]
- 31.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, Salata R, Soto-Torres L, Patterson K, Minnis AM, Gandham S, Gomez K, Richardson BA, Bumpus NN. 2013. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons TL, Emory JF, Seserko LA, Aung WS, Marzinke MA. 2014. Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J Pharm Biomed Anal 98:407–416. doi: 10.1016/j.jpba.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, Husnik MJ, Soto-Torres L, Nel A, Johnson S, Richardson-Harman N, Rabe LK, Dezzutti CS. 2015. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 70:242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Churchman SA, Moss JA, Baum MM. 2016. Accurate measurement of female genital tract fluid dilution in cervicovaginal lavage samples. J Chromatogr B Analyt Technol Biomed Life Sci 1017:75–81. doi: 10.1016/j.jchromb.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent KL, Bourne N, Bell BA, Vargas G, Tan A, Cowan D, Stanberry LR, Rosenthal SL, Motamedi M. 2009. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: a promising model for testing safety of candidate microbicides. Sex Transm Dis 36:312–318. doi: 10.1097/OLQ.0b013e31819496e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hanlon DE, Moench TR, Cone RA. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bélec L, Meillet D, Lévy M, Georges A, Tévi-Bénissan C, Pillot J. 1995. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin Diagn Lab Immunol 2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. 2011. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol 49:735–736. doi: 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix CW, Cao YJ, Fuchs EJ. 2009. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol 49:349–375. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- 40.Pyles RB, Moss JA, Baum MM. 2015. Vaginal mucosal HIV prep: fundamental insights and practical considerations, p 33–165. In Atta-ur-Rahman (ed), Frontiers in clinical drug research: HIV. Bentham Science Publishers, Oak Park, IL. [Google Scholar]
- 41.Karim SSA, Kashuba ADM, Werner L, Karim QA. 2011. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan ZY, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL. 1999. The effect of one injection of Depo-Provera(R) on the human vaginal epithelium and cervical ectopy. Contraception 60:15–24. doi: 10.1016/S0010-7824(99)00058-X. [DOI] [PubMed] [Google Scholar]
- 44.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. 2000. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol 183:967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 45.Pendergrass PB, Belovicz MW, Reeves CA. 2003. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol Obstet Invest 55:110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 46.Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. 2006. Baseline dimensions of the human vagina. Hum Reprod 21:1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 47.Gunawardana M, Mullen M, Moss JA, Pyles RB, Nusbaum RJ, Vincent KL, Wang C, Guo C, Yuan Y-C, Warden CD, Baum MM. 2013. Global Expression of molecular transporters in the human vaginal tract: implications for HIV chemoprophylaxis. PLoS One 8:e77340. doi: 10.1371/journal.pone.0077340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan P, Moss JA, Gunawardana M, Churchman SA, Yang F, Dinh CT, Mitchell JM, Zhang J, Fanter R, Miller CS, Butkyavichene I, McNicholl JM, Smith TJ, Baum MM, Smith JM. Topical delivery of tenofovir disoproxil fumarate and emtricitabine from pod-intravaginal rings protect macaques from multiple SHIV exposures. PLoS One, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


