Abstract
The protozoan parasite Leishmania donovani is the causative agent of visceral leishmaniasis, a disease potentially fatal if not treated. Current available treatments have major limitations, and new and safer drugs are urgently needed. In recent years, advances in high-throughput screening technologies have enabled the screening of millions of compounds to identify new antileishmanial agents. However, most of the compounds identified in vitro did not translate their activities when tested in in vivo models, highlighting the need to develop more predictive in vitro assays. In the present work, we describe the development of a robust replicative, high-content, in vitro intracellular L. donovani assay. Horse serum was included in the assay media to replace standard fetal bovine serum, to completely eliminate the extracellular parasites derived from the infection process. A novel phenotypic in vitro infection model has been developed, complemented with the identification of the proliferation of intracellular amastigotes measured by EdU incorporation. In vitro and in vivo results for miltefosine, amphotericin B, and the selected compound 1 have been included to validate the assay.
INTRODUCTION
The leishmaniases are a complex of diseases, with visceral and cutaneous manifestations caused by protozoan parasites of the genus Leishmania. Visceral leishmaniasis (VL) has been the main focus for drug research and development over the past 2 decades, due to the large disease burden in East Africa and South Asia (1) and potential patient death if not treated. For VL, there has been progress in treatment over the past decade, with clinical evidence for efficacy of and registration for use of oral miltefosine, paromomycin, and the liposomal formulation of amphotericin B (AmBisome, Gilead, USA) in South Asia (2), as well as combinations of these standard drugs (3). The need for new drugs to treat VL remains, as (i) miltefosine is the only approved oral treatment but requires 28 days of treatment and potential teratogenicity limits its use (4), (ii) paromomycin requires 21 days of treatment and intramuscular administration (http://www.dndi.org/diseases-projects/diseases/vl/current-treatment/current-treatment-vl.html), and (iii) liposomal amphotericin B formulations, which have successful cure rates with a single dose (5), require intravenous (i.v.) infusion, have a high cost if not donated, and have a requirement for cold storage, limiting use in countries where the disease is endemic (6). As part of the drive to find new treatments, there has been a refocus on the assays and models used to identify and develop new molecules as antileishmanial drugs. For in vitro screens and assays, this has ranged from the need to develop methods that (i) are adaptable to and enable high-throughput screens against the replicative intracellular-macrophage amastigote stage of Leishmania donovani, one of the causative species of VL (7); and (ii) include high-throughput technologies that enable the collection of more information compared to the traditionally used assays based on manual counting and reporter genes (8, 9). For example, high-content screening (HCS) systems that permit the screening of large sets of compounds using imaging techniques that also capture information about compound toxicity against host cells and mode of action (10, 11) have been applied to antileishmanial drug discovery (12–17).
In this paper, we describe methods to overcome some of critical issues related to reproducibility and biological relevance and to the replication of the intracellular parasite.
The role of the replication rate of intracellular amastigotes on interpretation of data from assays is often ignored. In vivo, we know that, in the L. donovani mouse model, the parasite load in the liver increased 20-fold over the initial 8 days (18) and, in the L. donovani hamster model, the parasite burden increased more than 6 log in the spleen and 4 log in the liver over the 56 days of the study (19). Recent experiments reported a doubling time of 2 days in an ex vivo splenic explant model system established 21 days postinfection, developed by the same group (20). We determined the replication rate of intracellular amastigotes in our assay using an adaptation of a classical nucleotide analogue incorporation assay (21) to enable visual identification of cells actively replicating within macrophage vacuoles.
MATERIALS AND METHODS
Cell lines.
THP-1 cells (human monocytic leukemia) were made available by the GlaxoSmithKline (GSK) Biological Reagents and Assay Development Department (BRAD; Stevenage, United Kingdom) and were maintained in RPMI media (Life Technologies) supplemented with 1.25 mM pyruvate (Life Technologies), 2.5 mM glutamine (Life Technologies), 25 mM HEPES (Life Technologies), and 10% heat-inactivated fetal bovine serum (FBS) (Gibco).
Leishmania donovani (MHOM/SD/62/1SCL2D, LdBOB) expressing green fluorescence protein (GFP) (14) was kindly provided by Manu de Rycker, University of Dundee, United Kingdom. Axenic amastigotes were maintained at 37°C, 5% CO2, in media containing 15 mM KCl solution (Invitrogen), 10 mM KH2PO4 (Merck), 136 mM KH2PO4 (Merck), 0.5 mM MgSO4 (Sigma-Aldrich), 24 mM NaHCO3 (Invitrogen), 25 mM glucose (Sigma-Aldrich), 1 mM l-glutamine (Invitrogen), 1× RPMI vitamin solution (Sigma-Aldrich), 10 μM folic acid (Sigma-Aldrich), 100 μM adenosine (Sigma-Aldrich), 5 mg/liter hemin (Sigma-Aldrich), 1× RPMI amino acid solution (Sigma-Aldrich), 25 mM 4-morpholineethanesulfonic acid, 0.0004% phenol red, and 20% heat-inactivated FBS (Gibco) in Milli-Q water. The selection antibody nourseothricin (Jena Bioscience) was regularly added to the cultures of amastigotes. Promastigotes were maintained at 30°C in M199 media (Sigma-Aldrich) supplemented with 25 mM HEPES (Invitrogen), 12 mM NaHCO3 (Invitrogen), 1 mM l-glutamine (Invitrogen), 1× RPMI vitamin solution (Sigma-Aldrich), 10 μM folic acid (Sigma-Aldrich), 100 μM adenosine (Sigma-Aldrich), 5 mg/liter hemin, and 10% heat-inactivated FBS (Gibco) (14).
In vitro intramacrophage L. donovani assay.
The intramacrophage assay was adapted from de Rycker et al. (14) and Peña et al. (16). THP-1 cells were grown in Cell Master roller bottles (Greiner catalog no. 680048) at an initial seeding concentration of 2 × 105 cells/ml for 72 h. Cells were visually inspected with an optical microscope and counted with a Casy counter (model TT, Roche). Cells were differentiated in a 225-cm3 T-flask (80 ml) in the presence of 30 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) at a final concentration of 6 × 105 cells/ml. Following 24 h of incubation at 37°C, 5% CO2, differentiation was visually confirmed, checking the confluence of the differentiated adherent monolayer, and PMA-containing medium was removed washing twice with complete growth media, taking care to not disrupt the cell layer.
Each T-flask containing differentiated THP-1 cells was infected with 80 ml of a suspension of 6 × 106 parasites/ml in THP-1 complete growth media without PMA and incubated for an additional 24 h. The medium was removed and the cell monolayer washed with phosphate-buffered saline (PBS). The infected cells were harvested by treatment with a solution of 0.25% (wt/vol) trypsin-EDTA in PBS and seeded in assay plates (1.6 × 105 cells/ml, 50 μl/well) in assay media containing RPMI media supplemented with 2% heat-inactivated horse serum (HS) (Gibco) or fetal bovine serum (Gibco), 25 mM NaHCO3 (Invitrogen), and 30 nM PMA using a Multidrop Combi dispenser (Thermo Scientific). A parallel culture of uninfected differentiated THP-1 cells was treated as described for infected cells and used as control for 100% compound response. Assay plates were incubated at 37°C, 5% CO2, for the time required for the assay and then fixed with 4% formaldehyde for 30 min at room temperature, adding 50 μl of 8% (vol/vol) formaldehyde solution (Sigma-Aldrich) in PBS to each well containing 50 μl of media. After fixation, cells were washed twice with 100 μl of PBS using an EL406 multiwell platewasher (BioTek), stained with 30 μl of a solution of DAPI (4′,6-diamidino-2-phenylindole; 10 μg/ml) and 0.1% (vol/vol) Triton X-100 in PBS for 30 min at room temperature, and washed an additional two times with 50 μl of PBS. Finally, 50 μl of PBS was added to each well, and plates were sealed and stored at 4°C until analysis.
Image analysis.
Automated image analysis was performed with an image analysis algorithm developed on Acapella high-content imaging and analysis software (PerkinElmer). The THP-1 cell count (MAC) and the average number of amastigotes per macrophage (AM/MAC) were calculated for each well, using the building blocks included in the analysis program. Briefly, the nuclei and cytoplasm for each macrophage were selected using DAPI stain. Amastigotes were detected as spots using the GFP signal and were filtered using area and roundness. In EdU incorporation experiments, the number of parasite nuclei that were labeled was used to determine the incorporation of the thymidine analogue in the nuclei. Images were taken with a high-content screening system (Opera QEHS, PerkinElmer) with a 20× air objective, acquiring a minimum of four fields per well. Two or three sequential images were taken for each well exciting at 405 nm (DAPI), 488 nm (GFP), and 635 nm (EdU).
Compounds and assay plates.
Amphotericin B and miltefosine were purchased from Sigma-Aldrich. Compound 1 was available from the GSK collection of compounds (Table 1).
TABLE 1.
Physicochemical properties of compound 1
| Propertya | Description |
|---|---|
| MW | 419.228 |
| MF | C17H15Cl2F3N4O |
| SMILES string | FC(F)(F)c1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(= O)NC1CCCC1 |
| aring | 2 |
| clogp | 4.602 |
| hba | 3 |
| hbd | 2 |
| heavy | 27 |
| tpsa | 66.91 |
MW, molecular weight; MF, molecular formula; SMILES, simplified molecular-input line-entry system; aring, number of aromatic rings; clogp, calculated partition-coefficient between n-octanol and water; hba, hydrogen-bond acceptor; hbd, hydrogen-bond donor; heavy, number of heavy atoms (no hydrogen atoms); tpsa, total polar surface area.
Predispensed assay plates (Greiner microclear, black, 384-well) were prepared by adding 250 nl of compounds dissolved in 100% dimethyl sulfoxide (DMSO) or 250 nl of DMSO to each well by using an Echo liquid handler (Labcyte, Inc.). Eleven-point one-in-three dilution curves were generated from a top concentration of 50 μM.
Plates were stored at −20°C until use and allowed to equilibrate at room temperature before addition of the cell suspension.
Data analysis.
Data were normalized to percentage biological response by using positive (i.e., highest response represented by noninfected cells, RCtrl2) or negative (i.e., lowest response achieved in the absence of any testing compound, RCtrl1) controls by using the following equation:
| (1) |
where Rx is the assay response measured for each compound X. RCtrl1 and RCtrl2 were included in each assay plate and calculated as the average of the replicates.
Assay performance statistics, such as signal-to-background ratio and Z′ (22), were calculated using templates in ActivityBase XE (IDBS, Guilford, Surrey, United Kingdom). Activities were expressed as pEC50 [pEC50 = −log EC50 (50% effective concentration in molar units)]. Values of pEC50 were obtained using the ActivityBase XE nonlinear regression function in the full curve analysis bundle to fit the 4-parameter logistic equation.
Biosafety and animal use.
Experimental procedures with L. donovani were carried out following standard operating procedures in compliance with biosafety level 3 (BSL3) regulations. THP-1 cells were treated according to GSK policies for the manipulation of human biological samples.
The protocols used for animal studies were approved by the GSK Diseases of the Developing World ethical committee. The animal research complied with Spanish and European Union legislation (European directive 86/609/EEC) on animal research and GSK 3R policy on the care and use of animals: replacement, reduction, and refinement.
Additional in vivo experiments were carried out at the London School of Hygiene & Tropical Medicine. These were performed under license, issued by the United Kingdom Home Office Animal (Scientific Procedures) Act 1986 and European Union directive 2010/63/EU.
EdU incorporation.
THP-1 cells were differentiated, infected, and seeded in 384-well plates as previously described and incubated in horse serum containing assay media. For the optimization of EdU (Click-iT plus Alexa fluor 647 picolyl azide toolkit; Lifetech) conditions (13, 23), concentrations ranging from 1 to 100 μM were added to different wells at time zero and every 12 h for 72 h, when cells were fixed with 4% formaldehyde for 30 min.
For intracellular amastigote replication experiments, EdU was added 24 h after plating to a final concentration of 50 μM in 1% DMSO. Plates were fixed every 12 h from 0 to 72 h post EdU addition with 4% formaldehyde for 30 min. EdU detection was performed following manufacturer's indications, and cells were stained with DAPI as previously described. Controls of GFP signal quenching and EdU positive spots detection in the absence of EdU in infected and uninfected cells were included in each experiment.
In vivo activity against L. donovani.
Sodium stibogluconate sensitive (SSG) L. donovani (MHOM/ET/67/HU3) amastigotes were isolated from donor RAG1.B6 mouse. Freshly isolated parasites were resuspended in RPMI1649 media at a concentration of 1 × 108/ml.
On day 0, female BALB/c mice (20 g; Charles River, Margate, United Kingdom) were infected intravenously by the lateral tail vein with 2 × 107 amastigotes (0.2-ml inoculum) and randomly assorted into four groups of five members.
Drug treatment started 7 days postinfection and continued until day 11. Groups were treated with either (i) vehicle only, orally (p.o.), twice daily for 5 days, (ii) miltefosine (Paladin, Inc., Canada), 12 mg/kg of body weight, orally, once daily for 5 days, (iii) liposomal amphotericin B (AmBisome, Gilead, USA) 1 mg/kg intravenously for 3 days (days 7, 9, and 11 postinfection), and (iv) compound 1 50 mg/kg, orally, twice for 5 days.
At day 14 postinfection, all animals were sacrificed and the parasite burden was determined microscopically on Giemsa-stained liver smears after methanol fixation. The number of amastigotes per 500 cells was counted microscopically (100×, oil immersion), and the parasite load was normalized to untreated controls.
Pharmacokinetic studies.
Experimental compounds were administered to BALB/c female mice (25 g weight) by oral gavage at a dose of 50 mg/kg of body weight at a volume of 20 ml/kg. All mice were treated during the fed state. Drugs were administered as 10% 70:30 Tween 80:ethyl alcohol (EtOH)/double-distilled water (ddH2O) suspensions and the blood sampling scheme was 15, 30, and 45 min, and 1, 1.5, 2, 3, 4, 8, and 24 h. At each time point, 10 μl of blood was taken from the lateral tail vein from three animals. Liquid chromatography-mass spectrometry was used for the establishment of compound concentration in blood with a sensitivity of LLQ (lower limit of quantitation) = 1 to 5 ng/ml in 25 ml of blood. The concentration of each drug was calculated in the peripheral total blood compartment. The noncompartmental data analysis was performed with WinNonlin 5.0 (Pharsight), and supplementary analysis was performed with GraphPad Prism (GraphPad Software).
RESULTS
Assay development.
In this intramacrophage system, the infection process was performed in bulk prior to the dispensation of the cell suspension in the assay plates, to eliminate any possible intrawell variation and to increase the robustness of the assay.
Copies of identical plates were prepared to allow fixing and staining at different time points and plotting of the growth curve. Cells were fixed with formaldehyde prior to DAPI staining. DAPI was used to detect the nucleus of THP-1 cells, and GFP was used to detect intracellular amastigotes using the image analysis algorithm described in the Materials and Methods. When performed for large-scale screening of compounds, the assay had an average throughput of 40 plates/run (two runs/week and a total of 240,000 wells/week), and the average Z′ calculated at 96 h using the AM/MAC output was 0.59 ± 0.12.
Effect of horse serum on extracellular amastigotes.
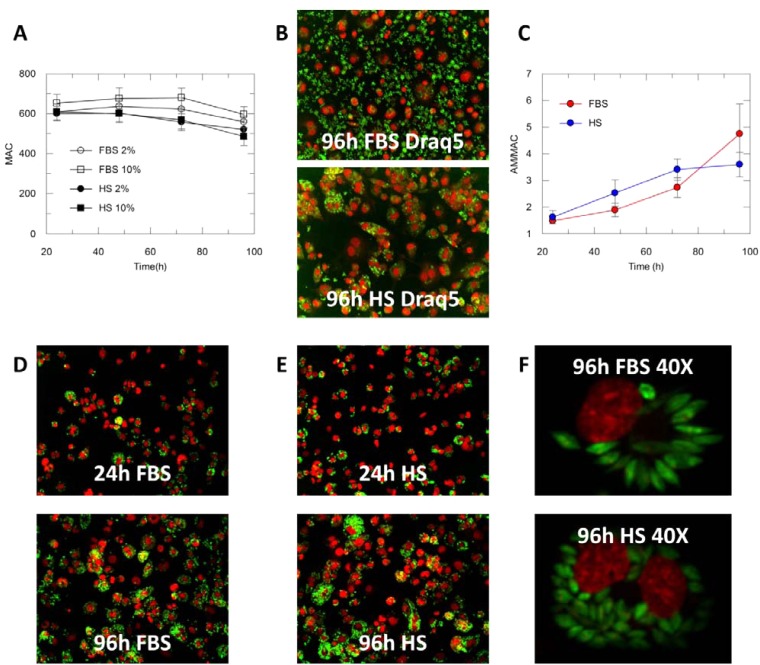
The presence of extracellular parasites was determined by visual inspection of the plates at each time point. The assay media used a reduced serum level, 2% serum instead of the 10% normally used in the complete growth media for culture of THP-1 cells, to minimize the growth of extracellular parasites. Neither the presence of HS nor the reduced quantity of FBS significantly affected the THP-1 counts (Fig. 1A). When cells were incubated with FBS, an increase in the extracellular parasites load could still be seen over the 4 days of incubation. In contrast, the few extracellular parasites present after seeding in the presence of HS-containing media were killed within a few hours of incubation. This difference could not be recorded when cells where stained with DAPI, since extracellular parasites were removed with the washing steps required to remove the dye after staining. This was overcome by the use of Draq5, a nuclear dye that can be added with formaldehyde in a single step and does not need to be washed out. Figure 1B illustrates the difference in the content of extracellular parasites when infected THP-1 cells were incubated for 4 days in the presence of FBS or HS (Draq5 staining).
FIG 1.
(A) Average number of THP-1 cells (6 fields) in assay media containing 2% FBS, 10% FBS, 2% HS, 10% FBS. (B) THP-1 cells (Draq5, red) infected with L. donovani (green) in the presence of FBS 2% or HS 2% at 96 h (20×, air objective. (C) Evolution of the number of amastigotes per macrophage (AM/MAC) in the presence of 2% HS (blue) or 2% FBS (red) and 0.5% DMSO. Number of amastigotes per total macrophages is represented; final percentage of infection: 86% in the presence of FBS and 78% in the presence of HS. (D) THP-1 cells (DAPI, red) infected with L. donovani (green) in the presence of 2% FBS at 24 and 96 h (20×, air objective). (E) THP-1 cells (DAPI, red) infected with L. donovani (green) in the presence of 2% HS at 24 and 96 h (20×, air objective). (F) THP-1 cells (DAPI, red) infected with L. donovani (green) in the presence of 2% FBS or 2% HS at 96 h (40×, water objective).
Effect of HS on L. donovani intracellular amastigotes.
The number of amastigotes per host cell (AM/MAC) at each time point (24 h, 48 h, 72 h, 96 h) was plotted to determine the growth of the intracellular amastigotes in the presence of FBS and of HS (Fig. 1C). Differentiated THP-1 cells do not replicate; therefore, the increase in the total number of amastigotes was not influenced by the increase of the number of host cells (24, 25).
In the presence of 2% FBS, the number of AM/MAC increased on average from 1.5 to 4.7 over 96 h. When HS was used in the assay media at the same concentration, the AM/MAC increased from 1.6 to 3.6 over the 96 h of incubation, with a linear increment in the initial 72 h post plating. When FBS was used, the presence of extracellular parasites and the potential of host cell reinfection prevented the replication rate to be accurately evaluated. At the same time, the use of HS ensured the elimination of any extracellular parasites after a few hours of incubation, removing the possible influence of reinfection in the observed increase and allowing any observed growth to be attributed to intracellular replication. Figure 1D and E show infected THP-1 cells fixed and stained with DAPI 24 h and 96 h post plating. These experiments were carried out in the presence of 0.5% DMSO, which is the concentration found in each well when compounds were screened. This concentration did not significantly affect either the number of host cells or the replication of intracellular amastigotes compared to a parallel experiment without DMSO (data not shown).
It was also observed that the shape of the intracellular parasite was influenced by the serum used. In the presence of FBS, the intracellular amastigotes were elongated (having similarity with extracellular amastigotes), while they were more round and amastigote-like when incubated in the presence of HS (Fig. 1F), an observation previously made (26).
EdU incorporation.
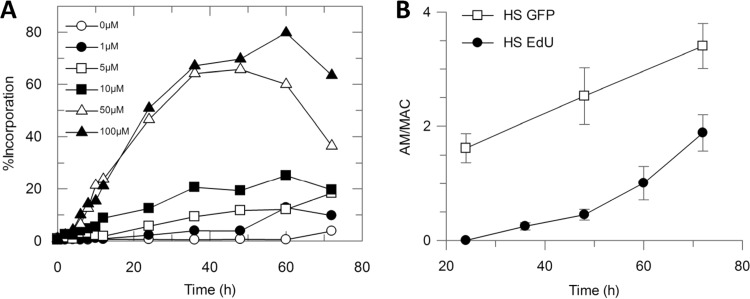
The optimal EdU concentration and exposure time were initially determined. THP-1 cultures infected with L. donovani amastigotes were incubated with increasing amounts of EdU for different periods of time in a single experiment that was processed at once to detect the EdU incorporated into amastigote DNA. Analyzed images showed that amastigotes were able to significantly incorporate EdU with an increasing and sustained rate when exposed to 50 μM EdU for at least 12 h (Fig. 2A); the incorporation rate achieved a plateau after 72 h of exposure without parasite number reduction and, thus, without apparent toxic effects. Uninfected cultures and cultures with no exposure to EdU were included as technical detection controls.
FIG 2.
(A) Percentage of intracellular L. donovani amastigotes incorporating EdU (100, 50, 10, 5, 1, 0 μM). (B) Number of amastigotes per macrophage in infected THP-1 cells processed for EdU detection. Amastigotes were detected as GFP-positive spots (white squares) or EdU (50 μM)-positive spots (black circles).
After the optimization of the experimental conditions, the incorporation of EdU over time by infected THP-1 cells maintained in the HS-containing medium was determined by adding 50 μM EdU 24 h after plating and measuring EdU incorporation in 12-h lapses from 24 to 72 h post plating. The number of amastigotes per macrophage was determined in both the GFP and the EdU channels. Not all amastigotes incorporated EdU during the course of infection, but the incorporation rate was consistent with the increase of intracellular parasite burden, reaching 40% parasites labeled as proliferating and demonstrating that the increase in the number of amastigotes per macrophage is to be attributed to replication (Fig. 2B).
In vitro activity.
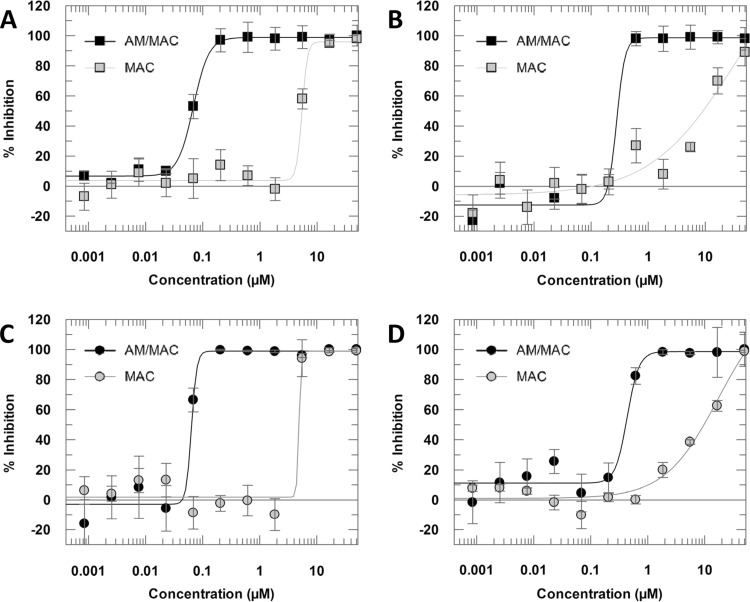
The activity of amphotericin B and miltefosine in FBS-containing medium was in accordance with previously reported data (12), showing a pEC50 [pEC50 = −log (EC50)] equal to 7.17 and 6.56, respectively, in the amastigotes/cell output. Both compounds maintained their activities when tested in the presence of HS (Fig. 3).
FIG 3.
Dose-response curves of amphotericin B-FBS (A), miltefosine-FBS (B), amphotericin B-HS (C), and miltefosine-HS (D). Curves were generated from 11-point one-in-three dilutions at a maximum concentration of 50 μM. Data are presented as means and standard deviations from 4 replicates.
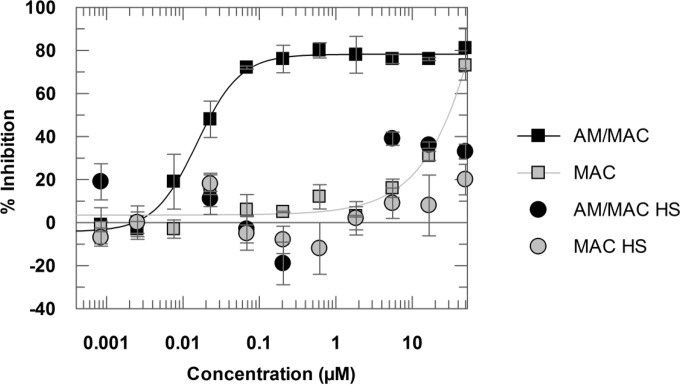
Compound 1 (Table 1) was assayed as part of the high-throughput screening campaign against the kinetoplastids L. donovani, Trypanosoma cruzi, and Trypanosoma brucei (16). This compound, when tested in the FBS-containing media, exhibited a pEC50 of 7.8 in the intramacrophage assay, as measured by the number of amastigotes/cell. Measuring the percentage of infected macrophages, the compound showed no significant activity, with a maximum asymptote of 40%. When the compound was assayed in the presence of HS, it was found to be inactive by both parameters (Fig. 4).
FIG 4.
Dose-response curves of compound 1 tested in the presence of FBS (squares) or HS (circles). Curves were generated from 11-point one-in-three dilutions at a maximum concentration of 50 μM. Data are presented as means and standard deviations from 4 replicates.
Pharmacokinetic studies.
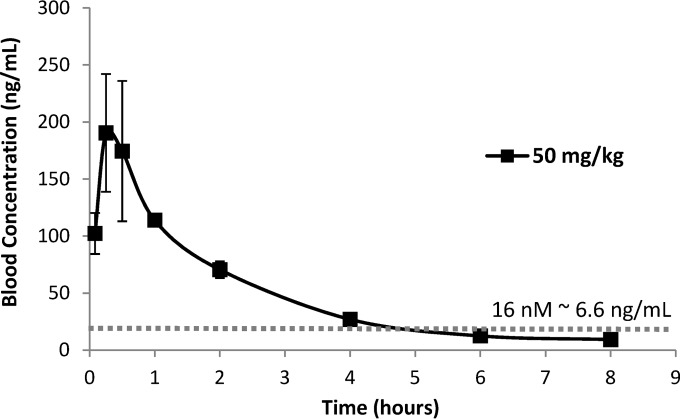
Compound 1 was administered to the mice by oral gavage in a single dose for 5 days, and no signs of pain, distress, or local or systemic toxicity were observed. Values for area under the curve (AUC) and plasma compound concentrations at peak and trough are given in Table 2. The values for AUC were high enough to ensure activity related to the calculated EC50 (Fig. 5). The exposure data were sufficiently favorable to warrant further in vivo testing.
TABLE 2.
Blood pharmacokinetic parameters in BALB/c mice after single oral gavage administration of compound 1
| Value | Actual dose (mg/kg of body weight) | Cmax (ng/ml) | Cmax/Da (kg · ng/ml/mg) | AUCall (h · ng/ml) | AUC∞ observed (h · ng/ml) | AUC∞/D observedb (h · kg · ng/ml/mg) |
|---|---|---|---|---|---|---|
| By animal ID | ||||||
| 1 | 50 | 227.0 | 4.5 | 401.0 | 438.1 | 8.8 |
| 2 | 50 | 154.0 | 3.1 | 339.7 | 358.4 | 7.2 |
| 3 | 50 | 95.9 | 1.9 | 227.6 | 238.8 | 4.8 |
| Overall | ||||||
| Average | 159.0 | 3.2 | 322.8 | 345.1 | 6.9 | |
| SDc | 65.7 | 1.3 | 87.9 | 100.3 | 2.0 |
Cmax/D, dose-normalized value of Cmax (Cmax/experimental dose).
AUC∞/D, dose-normalized value of AUC (AUC/experimental dose).
SD, standard deviation.
FIG 5.
Whole-blood levels in BALB/c mice after single oral gavage administration of compound 1 (actual dose, 50 mg/kg). The dotted line represents the 50% effective concentration (EC50) for compound 1.
In vivo antileishmanial activity.
Liposomal amphotericin B, miltefosine, and compound 1 were tested on L. donovani-infected BALB/c mice. Amphotericin B and miltefosine were active in vitro in the presence of FBS or of HS. In vivo, they decreased the parasite burden by 99.52% and 77.23% at 1 mg/kg i.v. and 12 mg/kg p.o., respectively, in accordance with previously reported data (27). In contrast, compound 1 at two daily doses of 50 mg/kg only reduced the parasite burden by 20% after 5 days of treatment (Table 3).
TABLE 3.
Activity of liposomal amphotericin B, miltefosine, and compound 1 against BALB/c mice infected with L. donovani HU3 (n= 5)
| Tested compound | Administration | % inhibition | 95% CIa |
|---|---|---|---|
| Liposomal amphotericin B | 1 mg/kg i.v. (days 7, 9, and 11) | 99.52 | 0.28 |
| Miltefosine | 12 mg/kg p.o. × 5 days | 77.23 | 12.41 |
| Compound 1 | 50 mg/kg BIDb × 5 days | 20.93 | 8.34 |
CI, confidence interval.
BID, twice a day.
DISCUSSION
Drug discovery for antileishmanial compounds has recently been focused on phenotypic rather than target-based screens, due to the limited number of fully validated targets and the issues of confirming on-target effects of active compounds (28, 29). However, the in vitro activity of test compounds frequently does not translate to in vivo activity, underlining the need for the development of new and more predictive in vitro assays adaptable to a high-throughput screening.
It has been demonstrated that the activity of antileishmanial drugs is host cell dependent (30). Primary host cells mimic the biological situation more accurately but are not compatible with the needs of a high-throughput screen. Instead, immortalized human monocytic THP-1 cells, that can be differentiated into macrophage-like cells, are able to develop and sustain L. donovani infections (24, 31). Different high-content screening assays using PMA-differentiated THP-1 cells infected with either promastigotes or amastigotes have been developed, confirming their suitability as L. donovani hosts (12-14, 16).
In comparison to traditional assays that provide information mainly on parasite viability, the use of HCS technologies permits the assessment of potential toxicity against the host cells and observation of morphological changes that can provide useful information to understand the mode of action of the compounds of interest (10). In our assay, THP-1 cells were differentiated and infected in bulk and dispensed into assay plates containing the compounds to be tested, as previously described (16). The use of cells that have been differentiated and infected in bulk ensured a homogeneous distribution of the infection throughout the plates, strongly reducing interwell variability, and eliminated the need of using intermediate plates loaded with test compounds.
One limitation of this protocol is that it does not allow any wash steps after the dispensation of cells in the plate, and that would remove extracellular parasites derived from the infection process or from the rupture of host cells during trypsinization or dispensing. This can be problematic, as axenic amastigotes are adapted to grow in culture with an average doubling time of 6 h; thus, after infection, any parasite that is not phagocytized by a host cell can grow over the incubation period and reinfect neighboring hosts. In addition, the pH of the assay medium is higher than the pH of the culturing media and could contribute to the differentiation of the amastigotes to an intermediate form of the parasite, similar to promastigotes. The primary objective in antileishmanial drug discovery is to identify compounds able to interfere with the growth and survival of the intracellular parasites rather than acting on the extracellular parasites. As the presence of HS in the medium was found to kill extracellular parasites within a few hours of incubation, HS was included in the assay media in order to prevent the growth and establishment of an extracellular culture, without affecting the viability of the hosts or of the intracellular amastigotes. The use of HS-containing media allowed a reduced number of washing steps following infection and ensured the elimination of any extracellular parasites deriving from a mechanical rupture of the host cell within a few hours following initial infection. We have also observed that, in the presence of HS, the intracellular parasites assumed a round shape, characteristic of the amastigote stage, whereas they were more elongated when incubated with FBS. The ability of HS to kill extracellular parasites and to push the differentiation of intracellular amastigotes toward a more amastigote-like form is in accordance with what was previously reported by Frothingham and Lehtimaki (26).
The antiparasitic effect of serum components has already been described. The trypanolytic factor present in human serum is responsible for the inability of Trypanosoma brucei brucei to infect humans (32). In the case of Leishmania and horse serum, there is no evidence of a similar mechanism. However, it is known that horse serum is less rich in nutrients and growth factors than fetal bovine serum, and this could contribute to the observed effect.
When FBS-containing medium was used in this assay, the presence of extracellular parasites, and hence the simultaneous contribution of replication and reinfection to the observed increase of the number of amastigotes/macrophages over time, did not permit us to conclusively establish the replication rate. A previous report on the doubling time of intracellular amastigotes in the presence of FBS and in the absence of extracellular parasites extrapolated a replication rate of approximately 12 days from the 7-day growth curve (14). In the assay developed in this work, when HS was included in the media, the number of amastigotes/macrophages doubled from 24 h to 72 h, and, since no extracellular parasites were visible, it was possible to attribute this proliferation solely to the replication of the intracellular parasite, as demonstrated with the EdU incorporation assay. The replication observed in this horse serum intramacrophage assay was lower than the one observed in the in vivo mouse model (18) or hamster model (19) but was similar to that observed in the ex vivo splenic explant culture from hamster infected with L. donovani described by Osorio et al. (20), where the number of amastigotes/macrophage doubled in the first 48 h post plating. The 2-day doubling time we observed in the in vitro system described in the present work is also in accordance with the doubling time observed by other groups when THP-1 cells were infected with L. donovani promastigotes (12, 31). Even if results obtained in different assays using different strains are difficult to compare, the fact that we observed and were able to quantify the replication of intracellular parasites in the horse serum in vitro system is of importance for the development of more predictive in vitro assays (7).
De Muylder et al. described the use of a media containing 5% HS and 5% FBS to wash differentiated THP-1 cells after infection with L. donovani promastigotes (12). The choice of use of HS in the washing medium was not discussed in this report, but, considering that differentiation and infection were performed in wells, it appears that HS was chosen to assist in the elimination of the extracellular parasites after infection, prior to addition of the compounds. In the same report, it appears that HS was not included in the assay media, and the effects of horse serum on the replication and appearance of intracellular amastigotes were not characterized.
DNA synthesis rate is highly upregulated during the replication process, representing a good biomarker for proliferation. The incorporation of thymidine analogues during the active S phase in dividing cells has been widely used as a molecular biomarker for proliferation (21). BrdU has been previously used to qualitatively identify the intracellular amastigotes as a replicating population, following THP-1 infection with L. donovani promastigotes (13). In the present work, to confirm that the increase in the number of amastigotes/macrophages observed when HS was included in the assay medium was attributable to replication, the EdU picolyl azide combined methodology was used, allowing the identification of those amastigotes that have entered into S phase while infecting macrophages, without compromising GFP fluorescence and amastigote identification. The increase of EdU incorporation over time specifically identified proliferation events that take place within the macrophages, since the addition of EdU after 24 h of incubation with horse serum ensured that only intracellular parasites had been exposed to the thymidine analogue. The EdU incorporation rate was similar to the estimated replication rate based on direct counting. The detection of nonlabeled parasites after long incubation periods suggests there might be a nondividing subpopulation of amastigotes, in accordance with observations by Kloehn et al. in murine L. mexicana lesions (33).
To validate this in vitro assay, two reference drugs, liposomal amphotericin B and miltefosine, and GSK compound 1 were tested in the intramacrophage assay in the presence of FBS or HS using an in vivo animal model, allowing for a comparison of the in vitro and in vivo activities. The in vitro activities of amphotericin B and miltefosine were in accordance with previous reports, and no significant difference between their activity in the presence of FBS or HS was observed. Compound 1 was selected as a proof-of-concept study, as it showed a pEC50 value higher than amphotericin B in the presence of FBS (pEC50, 7.8) but was inactive when tested in the presence of HS (pEC50 <4.3). When tested in vivo, liposomal amphotericin B and miltefosine confirmed their activities, reducing the parasite burden by 99.52% and 77.23%, respectively. In contrast, compound 1 was inactive when administered orally, reducing the parasite burden by 20.93% only. Since compound 1 possesses lead-like physicochemical properties (34) (Table 1) and reasonable bioavailability in mice in terms of Cmax and AUC (Table 2), we propose that factors other than pharmacokinetics might contribute to the lack of efficacy in the infection model, such as poor pharmacodynamics at the site of action. In particular, we suggest it could be linked to its lack of activity in the in vitro horse serum intramacrophage assay, in contrast with the high pEC50 value obtained when a media containing FBS was used (AM/MAC output). Several reasons could explain the lack of activity of compound 1 in HS: compound structure-related properties, the lack of activity against intracellular replicating amastigotes in horse serum, or activity of the compound only against the extracellular amastigotes forms found in the presence of FBS. Even though the exact mode of action of compound 1 has not been clarified, the correlation between the results obtained in the in vitro horse serum intramacrophage assay and the in vivo mouse model seem to suggest that the in vitro results obtained with horse serum translate to the in vivo animal model and that this assay mimics an in vivo L. donovani infection more accurately than the same assay with FBS. In fact, the standard drugs miltefosine and amphotericin B were active in in vitro and in vivo assays, and compound 1 was inactive both in vitro when horse serum was used, irrespective of the output used for the determination of its pEC50, and in vivo.
This is, to our knowledge, the first report on the inclusion of horse serum in the assay media for the whole assay, not only to completely remove the extracellular parasites and impede their growth over the incubation period but also to increase the replication rate of the intracellular amastigotes from the 12 days observed with FBS (14) to 2 days.
The activity of the test compounds in vivo correlated with what was observed in vitro in the intramacrophage horse serum assay. Although the causes of the different in vitro activities of compound 1 in FBS and HS are still not clear, these results suggest that the assay described here is a right step toward the development of a translational in vitro assay and represents an incentive for the deeper investigation of its application in antileishmanial drug discovery.
ACKNOWLEDGMENTS
Diana Tegazzini was funded by the European Commission FP7 Marie Curie Initial Training Network ParaMet (grant 290080). Rosario Diáz and Fernando Aguilar were funded by Tres Cantos Open Lab Foundation (project TC046). This research was conducted in collaboration with the Drugs for Neglected Diseases initiative (DNDi), and we acknowledge both financial support and permission to include the in vivo results for compound 1.
From GSK, we thank Dolores Jimenez-Alfaro and SMTech Department for supplying compounds predispensed in microtiter plates and managing compound logistics. We thank Maria Marco Martin, Ignacio Cotillo, and Emilio Alvarez for the technical assistance in the experiments and discussion of the results. We thank the DMPK department for its work in PK/PD studies and the LAS department for its support in the animal facilities. We also thank Manu de Rycker and scientists from Dundee University Drug Discovery Unit for providing the eGFP strain.
Funding Statement
This research was conducted in collaboration with the Drugs for Neglected Diseases initiative (DNDi), and we acknowledge both financial support and permission to include the in vivo results for compound 1.
REFERENCES
- 1.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, the Leishmaniasis Control Team WHO . 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft SL, Olliaro P. 2011. Leishmaniasis chemotherapy: challenges and opportunities. Clin Microbiol Infect 17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal CS, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, noninferiority, randomized controlled trial. Lancet 377:477–486. [DOI] [PubMed] [Google Scholar]
- 4.Sundar S, Olliaro PL. 2007. Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther Clin Risk Manag 3:733–740. [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, Chakravarty J. 2010. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis 2:159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. 2012. Visceral leishmaniasis treatment: what do we have, what do we need, and how to deliver it? Int J Parasitol Drugs Drug Resist 2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft SL. 1986. In vitro screens in the experimental chemotherapy of leishmaniasis and trypanosomiasis. Parasitol Today 2:64–69. doi: 10.1016/0169-4758(86)90157-2. [DOI] [PubMed] [Google Scholar]
- 8.Dube A, Gupta R, Singh N. 2009. Reporter genes facilitating discovery of drugs targeting protozoan parasites. Trends Parasitol 25:432–439. doi: 10.1016/j.pt.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Nishi. 2011. Visceral leishmaniasis: experimental models for drug discovery. Indian J Med Res 133:27–39. [PMC free article] [PubMed] [Google Scholar]
- 10.Zanella F, Lorens JB, Link W. 2010. High-content screening: seeing is believing. Trends Biotechnol 28:237–245. doi: 10.1016/j.tibtech.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Brodin P, Christophe T. 2011. High-content screening in infectious diseases. Curr Opin Chem Biol 15:534–539. doi: 10.1016/j.cbpa.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 12.De Muylder G, Ang KKH, Chen S, Arkin MR, Engel JC, McKerrow JH. 2011. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis 5:e1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siqueira-Neto JL, Moon S, Jang J, Yang G, Lee C, Moon HK, Chatelain E, Genovesio A, Cechetto J, Freitas-Junior LH. 2012. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl Trop Dis 6:e1671. doi: 10.1371/journal.pntd.0001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rycker M, Hallyburton I, Thomas J, Campbell L, Wyllie S, Joshi D, Cameron S, Gilbert IH, Wyatt PG, Frearson JA, Fairlamb AH, Gray DW. 2013. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrob Agents Chemother 57:2913–2922. doi: 10.1128/AAC.02398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aulner N, Danckaert A, Rouault-Hardoin E, Desrivot J, Helynck O, Commere PH, Munier-Lehmann H, Späth GF, Shorte SL, Milon G, Prina E. 2013. High-content analysis of primary macrophages hosting proliferating Leishmania amastigotes: application to anti-leishmanial drug discovery. PLoS Negl Trop Dis 7:e2154. doi: 10.1371/journal.pntd.0002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña I, Manzano MP, Cantizani J, Kessler A, Alonso-Padilla J, Bardera AI, Alvarez E, Colmenarejo G, Cotillo I, Roquero I, de Dios-Anton F, Barroso V, Rodriguez A, Gray DW, Navarro M, Kumar V, Sherstnev A, Drewry DH, Brown JR, Fiandor JM, Martin JJ. 2015. New compound sets identified from high-throughput phenotypic screening against three kinetoplastid parasites: an open resource. Sci Rep 5:8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagley MJ, Saunders EC, Simpson KJ, McConville MJ. 2015. High-content assay for measuring intracellular growth of Leishmania in human macrophages. Assay Drug Dev Technol 13:389–401. doi: 10.1089/adt.2015.652. [DOI] [PubMed] [Google Scholar]
- 18.Bradley DJ, Kirkley J. 1977. Regulation of Leishmania populations within the host: I—the variable course of Leishmania donovani infections in mice. Clin Exp Immunol 30:119–129. [PMC free article] [PubMed] [Google Scholar]
- 19.Melby PC, Chandrasekar B, Zhao W, Coe JE. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol 166:1912. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 20.Osorio Y, Travi BL, Renslo AR, Peniche AG, Melby PC. 2011. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. PLoS Negl Trop Dis 5:e962. doi: 10.1371/journal.pntd.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh BL, Walker T, Norazit A, Meedeniya AC. 2011. Thymidine analogues for tracking DNA synthesis. Molecules 16:7980–7993. doi: 10.3390/molecules16097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JH, Chung TDY, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 23.Cappella P, Gasparri F, Pulici M, Moll J. 2008. Cell proliferation method: click chemistry based on BrdU coupling for multiplex antibody staining. Curr Protoc Cytom Chapter 7:Unit7.34. doi: 10.1002/0471142956.cy0734s45. [DOI] [PubMed] [Google Scholar]
- 24.Ogunkolade BW, Colomb-Valet I, Monjour L, Rhodes-Feuillette A, Abita JP, Frommel D. 1990. Interactions between the human monocytic leukemia THP-1 cell line and Old and New World species of Leishmania. Acta Trop 47:171–176. doi: 10.1016/0001-706X(90)90023-S. [DOI] [PubMed] [Google Scholar]
- 25.Croft SL, Seifert K, Yardley V. 2006. Current scenario of drug development for leishmaniasis. Indian J Med Res 123:399–410. [PubMed] [Google Scholar]
- 26.Frothingham TE, Lehtimaki E. 1967. Leishmania in primary cultures of human amniotic cells. Am J Trop Med Hyg 16:658–664. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson L, Yardley V, Croft SL. 2014. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. J Antimicrob Chemother 69:1888–1891. doi: 10.1093/jac/dku069. [DOI] [PubMed] [Google Scholar]
- 28.Don R, Ioset JR. 2014. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 141:140–146. doi: 10.1017/S003118201300142X. [DOI] [PubMed] [Google Scholar]
- 29.Reguera RM, Calvo-Alvarez E, Alvarez-Velilla R, Balana-Fouce R. 2014. Target-based versus phenotypic screenings in Leishmania drug discovery: a marriage of convenience or a dialogue of the deaf? Int J Parasitol Drugs Drug Resist 4:355–357. doi: 10.1016/j.ijpddr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert K, Escobar P, Croft SL. 2010. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother 65:508–511. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- 31.Gebre-Hiwot A, Tadesse G, Croft SL, Frommel D. 1992. An in vitro model for screening antileishmanial drugs: the human leukemia monocyte cell line, THP-1. Acta Trop 51:237–245. doi: 10.1016/0001-706X(92)90042-V. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler RJ. 2010. The trypanolytic factor-mechanism, impacts and applications. Trends Parasitol 26:457–464. doi: 10.1016/j.pt.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Kloehn J, Saunders EC, O'Callaghan S, Dagley MJ, McConville MJ. 2015. Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog 11:e1004683. doi: 10.1371/journal.ppat.1004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hann MM, Oprea TI. 2004. Pursuing the leadlikeness concept in pharmaceutical research. Curr Opin Chem Biol 8:255–263. doi: 10.1016/j.cbpa.2004.04.003. [DOI] [PubMed] [Google Scholar]