Abstract
Introduction
Guidelines for the use of cerebrospinal fluid (CSF) biomarkers in the diagnosis of Alzheimer's disease (AD) establish that each laboratory must use internally qualified cutoff values. We determined the concentrations of biomarkers that discriminate cases from controls and combinations that predict the progression to dementia in a Brazilian cohort.
Methods
Concentrations of amyloid-beta peptide (Aβ1–42), total tau (T-tau), and 181Thr-phosphorylated-tau (P-tau) were determined in CSF samples from 184 older adults (68 mild cognitive impairment, 41 AD, 34 non-AD cognitive impairment, and 41 controls) by the INNO-BIA AlzBio3 assay.
Results
Cutoff values discriminating AD from controls are as follows: Aβ1–42: 416.0 pg/mL (sensitivity [SE]: 83%, specificity (SP): 70%); T-tau: 76.7 pg/mL (SE: 82%, SP: 67%); P-tau: 36.1 pg/mL (SE: 83%, SP: 49%); Aβ1–42/P-tau <9.53 (SE: 88%, SP: 78%); and Aβ1–42/T-tau <4.13 (SE: 80%; SP: 80%). Combining values Aβ1–42 <416.5 pg/mL and Aβ1–42/P-tau <9.5 best predicted the conversion in 2 years (Cox regression: hazard ratio 7.24 [2.09–25.06], P = .002, SE: 74%, Sp: 73%).
Discussion
Our findings are in line with most of the available evidence in this field; yet, our cutoff values are different from those derived from other laboratories.
Keywords: Alzheimer's disease, Mild cognitive impairment, Amyloid-β, Tau protein, Biomarkers, Cerebrospinal fluid
1. Introduction
Alzheimer's disease (AD) is the leading cause of dementia, affecting >35 million people worldwide [1]. The neuropathologic hallmarks of AD are the neuritic plaques and neurofibrillary tangles, which, respectively, arise from the extracellular deposition of the amyloid-beta (Aβ) peptide and from the intracellular accumulation of hyperphosphorylated tau protein in neurons. The pathophysiological changes that cause cognitive, functional, and behavioral impairment in AD allegedly start several years or perhaps decades before the onset of clinical symptoms [2]. Molecular and neuroimaging markers portray the presence of AD pathology [3], [4], [5]; therefore, AD biomarkers may play an important role in the diagnostic workup of patients with cognitive impairment, particularly among those with clinical symptoms compatible with prodromal AD [6].
Over the past years, multicenter task forces invested substantial resources in the integrated study of clinical, genetic, biochemical, and neuroimaging markers of AD and their relationship with clinical symptoms and rate of disease progression [7]. Many efforts have been expended to determine the specific pattern of changes that is found in AD and the predictive value of such findings in the diagnosis of predementia AD. The so-called “AD signature in the cerebrospinal fluid (CSF)” subsumes decreased concentrations of the Aβ1–42 peptide [8] and increased concentrations of total tau (T-tau) [9] and hyperphosphorylated tau (P-tau) [10]. Studies have shown an average decrease of 50% in CSF Aβ1–42 levels in AD compared with cognitively normal elders, along with a 300% increase in T-tau and a 200% increase in P-tau [5], [11], [12]. This set of biomarkers were accepted as proxy, in vivo evidence of the AD pathology, and incorporated into the revised diagnostic criteria of the National Institute of Neurological Disorders and Stroke, Alzheimer's and Related Disorders disease as supporting features to the diagnostic of AD [13], including its predementia and preclinical stages [14]. In combination, these biomarkers have a sensitivity (SE) and specificity (SP) profile in the 85%–95% range for the diagnosis AD at prodromal and dementia stages [15].
The availability of this technology reinforces the use of AD biomarkers in the selection of more homogeneous samples of patients for research purposes, particularly intervention trials with antidementia drugs. The translation of this method into a diagnostic tool for clinical purposes is expected in the near future, particularly to support the prediction of dementia among patients with subtle memory symptoms [15]. Therefore, it is relevant to determine the biomarker profile that distinguishes individuals at risk of AD among those diagnosed with mild cognitive impairment (MCI). The aim of the present study was to determine CSF concentrations of Aβ1–42, T-tau, and P-tau and the respective cutoff scores that best discriminate normal elders from patients with AD, as well as the combination of values that predicts the conversion from amnestic MCI to dementia in a cohort of older adults.
2. Methods
2.1. Subjects and assessment
The present study was conducted at the psychogeriatric clinic of a tertiary, university-based hospital in Brazil (Institute of Psychiatry, Faculty of Medicine, University of São Paulo). A total of 184 older adults were enrolled after signing informed consent. The study was approved by the local Ethics Committee and conducted under the tenets of the Helsinki Declaration. The study was designed to include a cross-sectional assessment of the whole sample at baseline, followed by the longitudinal reassessment of nondemented participants at 12-month intervals. Initial assessment was performed by psychiatrists and a neurologist through the Brazilian version of the structured interview for Cambridge mental disorders of the elderly examination [16], which provides scores for cognitive test Cambridge (CAMCOG), and mini-mental state examination (MMSE) [17]. Neuropsychological assessments were performed by trained neuropsychologists and included the Fuld object memory evaluation (FOME) [18], the trail making test (TMT) A and B [19], and the short cognitive test (SKT) [20], [21]. In view of the variability in educational level of the subjects in the sample, the cutoff scores of neuropsychological tests are also adjusted for age and educational level. To rule out cases with comorbid major depression, participants were assessed with the 21-item Hamilton depressive scale [22] and euthymia was defined as a score <8. All participants underwent blood tests (complete blood count, blood chemistry, thyroid function, blood lipid profile, folic acid and vitamin B12 dosage, and syphilis test), and neuroimaging (magnetic resonance imaging) studies, to exclude metabolic and vascular etiologies for MCI and dementia. Additional information on the assessment protocol can be found in previous publications from our group [23], [24]. Clinical diagnoses were established at consensus meetings, taking into account all clinical and laboratorial information gathered by a multidisciplinary team including physicians (psychiatrists, a geriatrician, and a neurologist), neuropsychologists, physical therapists, speech therapists, occupational therapists, and gerontologists.
The patient sample comprised 41 demented patients with mild AD [25], 68 subjects with MCI [26], and 34 patients with cognitive impairments due to other neuropsychiatric conditions, namely 17 with major depression [27], 6 with bipolar disorder [27], and 11 patients with non-AD neurodegenerative disorders (frontotemporal dementia, Huntington disease, multiple system atrophy, Lewy body dementia, and corticobasal degeneration). The comparison group comprised 41 healthy older adults with no evidence of cognitive impairment or psychiatric disorder at the time of clinical and neuropsychological assessment. Healthy controls were recruited with the aid of internal and media advertisements. We also included older adults who volunteered to join our cohort after becoming aware of this initiative from information provided by other participants in the study and their relatives. In any case, volunteers were only included in the cohort as healthy controls in the absence of any relevant memory complaints, medical comorbidities, and psychiatric history and if they had a normal performance in neuropsychological tests.
Healthy controls and subjects with MCI were annually reassessed (mean duration of follow-up: 24 ± 11 months) and had their diagnostic status adjusted depending, respectively, on the onset of cognitive deficits (incident MCI) or the progression from MCI to dementia. MCI subjects with conversion to AD during follow-up were reclassified as having MCI-AD. MCI subjects remaining cognitively stable over time were designated as having stable MCI (MCI-S). Conversion from MCI to incipient dementia was characterized by objective measures of functionality with the Brazilian version of the direct assessment of functional status [28], [29].
2.2. CSF biomarkers analysis
CSF samples were obtained by lumbar puncture in the L3/L4 or L4/L5 intervertebral space, with a 23-gauge needle and using polypropylene tubes, in the morning period without fasting. CSF samples (12–15 mL) were centrifuged at 3200 × g for 10 minutes at 4°C, split into 0.5-mL aliquots in cryotubes (Sarstedt), and immediately frozen and stored at −80°C until analysis, without being thawed and refrozen.
CSF concentrations of the 42 amino acid-long Aβ1–42, T-tau, and 181Thr-phospho-tau (P-tau) were determined in duplicates with the INNO-BIA AlzBio3 assay (Innogenetics, Ghent, Belgium), a multiplex microsphere-based xMAP platform that allows the simultaneous analysis of the three biomarkers. After prewetting the filter plate with a wash buffer, a suspension of microsphere carrying the corresponding capturing antibodies (AT120, AT270, and 4D7A3 for T-tau, P-tau, and Aβ1–42, respectively) was added to the plate. A mixture of biotinylated detection monoclonal antibodies, designed to detect specifically one of the capturing antibodies (HT7 for T-tau and P-tau and 3D6 for Aβ1–42), and 75 μL of CSF or standards were added to the plate and incubated overnight in the dark. Next, the plate was washed and a detection conjugate (phycoerythrin-labeled streptavidin) was added and incubated for 1 hour at room temperature. The plate was washed and after the addition of a reading solution (phosphate buffer saline) the assay was analyzed on a Luminex 100IS platform (Luminex, Austin, TX, USA). Standard curves were constructed for each biomarker using a sigmoidal curve fitting method, and the mean fluorescence values for the duplicate CSF samples were used to determine the concentration of Aβ1–42, T-tau, and P-tau.
2.3. Statistical analysis
Differences in sociodemographic characteristics, cognitive performance, and CSF biomarker concentrations at baseline were analyzed by analysis of variance tests with least square difference tests for pairwise post-hoc test between groups. Differences in gender distribution were analyzed by χ2 tests. Differences in baseline and follow-up neuropsychological scores for MCI-AD and MCI-S groups were analyzed by paired sample t test.
Receiver operating characteristic (ROC) curves were carried out to determine the SE, SP, and area under the curve of CSF biomarkers for the discrimination between AD and controls. Then, we evaluated the cutoff values generated in these analyses for the differential diagnosis between AD and other diagnostic groups (MCI and non-AD cognitive impairment/dementia). We also evaluated the power of these biomarkers and respective cutoff values to predict the risk of progression from MCI to AD in the longitudinal arm of the study. Additional analysis with Kaplan-Meier survival curves and time-dependent Cox regressions was carried out to assess the best biomarker combinations and predictors of progression to AD in MCI subjects.
3. Results
3.1. Sociodemographic characteristics, cognitive performance, and concentrations of CSF biomarkers at baseline
Patients with AD as expected were older and had lower scores on cognitive tests (MMSE and CAMCOG) compared with the other diagnostic groups. There were no significant differences in gender distribution and years of education between the diagnostic groups (Table 1). Table 2 displays the results of two consecutive neuropsychological evaluations performed at an interval of at least 1 year; in the MCI-AD group, the scores in all neuropsychological tests remained stable over time, whereas in the MCI-S group, there was a decline in TMT-A, FOME, and SKT scores in the same period, and the patients were now classified as mild AD.
Table 1.
Demographic characteristics, cognitive performance, and concentrations of cerebrospinal fluid biomarkers (Aβ1–42, T-tau, and P-tau) according to baseline diagnosis
| Diagnosis |
P | ||||
|---|---|---|---|---|---|
| Healthy controls | MCI | AD | Non-AD | ||
| Gender (%W) | 70% | 77% | 58% | 57% | .14 |
| Age | 69.68 (8.66) | 70.22 (10.23) | 74.23 (6.61) | 68.38 (9.38) | .04 |
| Years of education | 12.85 (5.81) | 10.59 (6.55) | 7.02 (4.99) | 10.25 (7.43) | .07 |
| MMSE | 29.54 (5.22) | 29.35 (9.96) | 23.81 (11.27) | 27.09 (9.40) | .02 |
| CAMCOG | 92.54 (13.63) | 85.40 (21.30) | 67.43 (21.92) | 78.08 (25.27) | <.001 |
| Aβ1-42 | 503.99 (156.67) | 410.91 (149.98) | 328.76 (110.54) | 474.11 (185.88) | <.001 |
| Total tau | 86.03 (47.47) | 88.38 (55.75) | 145.69 (80.22) | 104.59 (85.04) | <.001 |
| Phosphorylated tau | 41.59 (21.81) | 45.92 (27.11) | 66.72 (35.82) | 38.97 (26.41) | <.001 |
| Aβ1-42/P-tau | 14.38 (6.46) | 12.09 (7.34) | 7.39 (7.39) | 15.82 (8.43) | <.001 |
| Aβ1-42/T-tau | 7.12 (3.48) | 6.40 (3.82) | 3.45 (4.01) | 6.76 (4.73) | <.001 |
Abbreviations: Aβ1–42, amyloid-beta peptide; T-tau, total tau; P-tau, 181Thr-phosphorylated-tau; MCI, mild cognitive impairment; AD, Alzheimer's disease; Non-AD, cognitive impairment or dementia due to other etiologies; MMSE, mini-mental state examination; CAMCOG, cognitive test Cambridge.
NOTE. Biomarker concentrations are given in pg/mL. Values are presented as means and standard-deviations.
Table 2.
Neuropsychological performances of MCI-AD and MCI-S patients at baseline and follow-up
| Test | MCI-S |
MCI-AD |
||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P | Baseline | Follow-up | P | |
| MMSE | 27.30 (2.31) | 27.15 (2.86) | .76 | 25.18 (2.99) | 25.09 (3.47) | .90 |
| CAMCOG | 91.07 (7.61) | 91.69 (11.09) | .81 | 81.27 (7.15) | 82.18 (7.99) | .65 |
| VF | 12.57 (3.37) | 13.50 (3.23) | .14 | 10.41 (2.99) | 10.50 (2.84) | .92 |
| TMT-A | 73.46 (39.95) | 65.28 (28.97) | .18 | 72.16 (41.77) | 97.00 (13.69) | .04 |
| TMT-B | 152.44 (55.48) | 157.85 (73.82) | .60 | 167.55 (60.45) | 228.44 (94.33) | .05 |
| FOME | 40.42 (4.10) | 39.67 (7.60) | .60 | 38.00 (9.21) | 31.66 (9.41) | <.01 |
| SKT | 3.35 (2.83) | 4.17 (6.09) | .49 | 6.92 (4.09) | 9.00 (4.39) | .02 |
Abbreviations: MCI-AD, mild cognitive impairment subjects who progressed to Alzheimer's disease; MCI-S, stable cases of MCI; MMSE, mini-mental state examination; CAMCOG, cognitive test Cambridge; VF, verbal fluency test; TMT-A and -B, trail making test A and B; FOME, Fuld object memory evaluation; SKT, short cognitive test.
NOTE. Values are presented as means and standard deviation.
Subjects with AD also had significantly lower CSF concentrations of Aβ1–42 and higher concentrations of P-tau and T-tau compared with those of healthy controls and the other diagnostic groups; likewise, the Aβ1–42/P-tau and Aβ1–42/T-tau ratios were significantly lower in AD compared with the other groups. As expected, subjects with MCI had intermediate levels (i.e., between AD and controls) of all biomarkers (Table 3).
Table 3.
Cutoff scores and sensitivity/specificity values of cerebrospinal fluid biomarkers (Aβ1–42, T-tau, and P-tau) and combinations (Aβ1–42/tau ratios; pathologic signatures) discriminating patients with AD from controls, AD from cognitive impairment/dementia due to other etiologies, and MCI patients who progressed to dementia from stable cases of MCI
| CSF biomarker | Cutoff | AD (n = 41) versus controls (n = 41) |
AD (n = 41) versus non-AD (n = 35) |
MCI-AD (n = 19) versus MCI-S (n = 49) |
|||
|---|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | ||
| Aβ1–42 | <416.0 pg/mL | 83 | 70 | 83 | 54 | 79 | 42 |
| Phosphorylated tau | >36.1 pg/mL | 83 | 49 | 80 | 68 | 84 | 46 |
| Total tau | >76.7 pg/mL | 82 | 67 | 78 | 57 | 79 | 50 |
| Aβ1–42/P-tau | <9.53 | 88 | 78 | 85 | 71 | 90 | 65 |
| Aβ1–42/T-tau | <4.13 | 80 | 80 | 76 | 66 | 74 | 69 |
| Pathologic signature 1 | — | 78 | 83 | 78 | 74 | 79 | 69 |
| Pathologic signature 2 | — | 73 | 85 | 74 | 74 | 74 | 73 |
Abbreviations: Aβ1–42, amyloid-beta peptide; T-tau, total tau; P-tau, 181Thr-phosphorylated-tau; AD, Alzheimer's disease; Non-AD, cognitive impairment or dementia due to other etiologies; MCI, mild cognitive impairment; CSF, cerebrospinal fluid; MCI-AD, MCI subjects who progressed to AD; MCI-S, stable cases of MCI (i.e., subjects who retained the MCI diagnosis on follow-up); pathologic signature 1, Aβ1–42 <416.0 and Aβ1–42/P-tau <9.53; pathologic signature 2, Aβ1–42 <416.0 and Aβ1–42/T-tau <4.13.
3.2. CSF biomarkers: cut-off scores and diagnostic accuracy
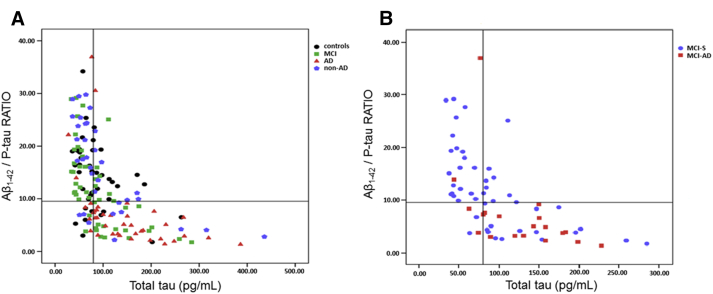
ROC curves were built to determine, for each CSF biomarker and combinations (ratios), the cutoff scores that best discriminated AD from controls based on SE and SP values (Table 3). CSF concentrations of Aβ1–42 <416.0 pg/mL (SE: 83%; SP: 70%) and Aβ1–42/P-tau ratio <9.53 (SE: 87.5%; SP: 78%) best differentiated AD from controls. We further determined whether the combination of biomarkers and their ratios might increase the diagnostic accuracy for the diagnosis of AD subjects compared with controls. Fig. 1 presents the distribution of subjects according to the values of T-tau (x-axis) and Aβ1–42/P-tau ratio (y-axis) in the whole sample (Fig. 1A), and in the subsample of patients with MCI (Fig. 1B), taking into account the longitudinal change in diagnostic status, i.e., conversion to dementia (MCI-AD) or stability of MCI diagnosis (MCI-S). It is noteworthy that MCI patients who converted to dementia on follow-up had a distribution similar to that observed among AD patients who were already demented at baseline, i.e., T-tau >76 pg/mL and Aβ1–42/P-tau ratio <9.5.
Fig. 1.
Distribution of subjects according to the values of T-tau (x-axis) and Aβ1–42/P-tau ratio (y-axis) in the whole sample (A) and in the subsample of patients with MCI (B) taking into account the longitudinal change in diagnostic status, i.e., conversion to dementia (MCI-AD) or stability of MCI diagnosis (MCI-S). Abbreviations: T-tau, total tau; Aβ1–42, amyloid-beta peptide; P-tau, 181Thr-phosphorylated-tau; MCI, mild cognitive impairment; MCI-AD, MCI subjects who progressed to Alzheimer's disease.
Cross validation using the “leave-one-out method” was used to determine the reliability of our CSF measures; in this method, each subject is removed, a new cutoff point is determined, and the subject is reclassified based on the new cutoff point. The same process is repeated for all subjects in the sample and the new generated classification is compared with the actual diagnosis. Aβ1–42/P-tau yielded SEs of 87% and 78%, and predictive values of 79.5% and 86% in classifying AD and controls, respectively; Aβ1–42/T-tau provided SEs of 72% and 76.5%, and predictive values of 87.5% and 76.5% in classifying AD and controls, respectively. These results have ensured an accuracy of 82.7% in discriminating AD × controls for the Aβ1–42/P-tau ratio, and of 81% for the Aβ1–42/T-tau ratio (Table 4).
Table 4.
Cross-validation values for sensitivity, predictive values, and accuracy in the classification of AD and controls
| CSF biomarker | Cutoff | AD (n = 41) |
Controls (n = 41) |
(AD × controls) |
||
|---|---|---|---|---|---|---|
| Sensitivity, % | Predictive value, % | Sensitivity, % | Predictive value, % | Accuracy, % | ||
| Aβ1–42 | <416.0 pg/mL | 80 | 68 | 63 | 76 | 72 |
| Phosphorylated tau | >36.1 pg/mL | 60 | 72 | 78 | 67 | 69 |
| Total tau | >76.7 pg/mL | 79 | 66 | 60 | 75 | 69.6 |
| Aβ1–42/P-tau | <9.53 | 87 | 79.5 | 78 | 86 | 82.7 |
| Aβ1–42/T-tau | <4.13 | 72 | 87.5 | 90 | 76.5 | 81 |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; Aβ1–42, amyloid-beta peptide; P-tau, 181Thr-phosphorylated-tau; T-tau, total tau.
NOTE. Biomarker concentrations are given in pg/mL.
The best diagnostic accuracy was obtained with the following combination of biomarkers: “pathologic signature 1” took into account the cutoff values of Aβ1–42 <416.0 pg/mL and Aβ1–42/P-tau <9.53 yielding an SE of 78% and an SP of 83%. The second combination (“pathologic signature 2”) took into account the cutoff values of Aβ1–42 <416.0 and Aβ1–42/T-tau <4.13 yielding an SE of 73% and an SP of 85%. Levels of Aβ1–42 <416.0 pg/mL were found in 12 controls (29.2%).
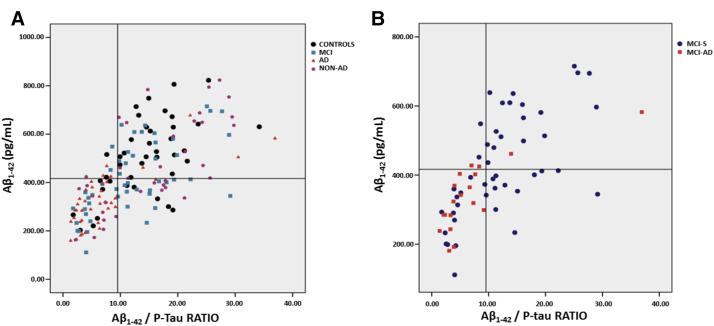
Next, we determined whether these cutoff scores and pathologic signatures could also distinguish AD from other groups of patients with cognitive impairment (MCI and non-AD) and also whether this set of information could help predict the progression from MCI and AD. Table 3 presents the SE and SP values for each biomarker and pathologic signatures. Fig. 2 depicts a scatter plot of “pathologic signature 1” in the whole sample (Fig. 2A) and in the subsample of subjects with MCI (Fig. 2B) according to the follow-up outcome (conversion to dementia), indicating that AD patients and MCI-AD concentrate in the left-lower quadrant considering their values of Aβ1–42/P-tau in the x-axis (<9.53) plotted against the values of Aβ1–42 in the y-axis (<416.0 pg/mL).
Fig. 2.
Scatter plot of the “pathologic signature 1” for the whole sample (A) and for the subsample of patients with MCI according to conversion status (B). Abbreviations: MCI, mild cognitive impairment; MCI-AD, MCI subjects who progressed to Alzheimer's disease; MCI-S, stable cases of MCI.
3.3. Survival analysis
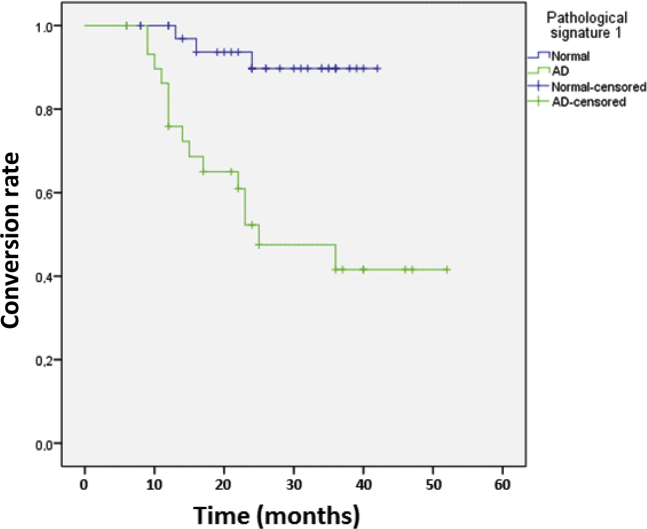
Kaplan-Meier curves and Cox regression analyses showed that patients with MCI who displayed at baseline the “pathologic signature 1” (Aβ1–42<416.0 pg/mL and Aβ1–42/P-tau <9.53) had a higher risk of progressing to AD as opposed to those without it (hazard ratio [HR], 7.24; 95% confidence interval [CI] [2.1–25.1]; P = .002). MCI subjects who had at baseline the “pathologic signature 2” (Aβ1–42 <416.0 and Aβ1–42/T-tau <4.13) had a higher risk of progressing to AD compared with those without it (HR, 6.5; 95% CI [2.1–19.8]; P = .009). The HR was reduced, but still significant after controlling for cognitive and demographic data (“pathologic signature 1”: HR = 5.6, 95% CI [1.5–20.9], P < .001; “pathologic signature 2”: HR = 4.80, 95% CI [1.5–15.6], P = .009). Fig. 3 shows the Kaplan-Meier curve for MCI subjects with “pathologic signature 1” compared with those without it and the progression to AD.
Fig. 3.
Kaplan-Meier curve for MCI subjects according to the “pathologic signature 1” (pathologic signature 1: Aβ1–42 <416.0 and Aβ1–42/P-tau <9.53). Abbreviations: MCI, mild cognitive impairment; Aβ1–42, amyloid-beta peptide; P-tau, 181Thr-phophorylated-tau; AD, Alzheimer's disease.
4. Discussion
In the present study, CSF concentrations of Aβ1–42 had SE and SP values similar to those reported in previous studies discriminating AD from controls [30], [31], whereas T-tau and P-tau values alone had a discriminatory power lower than that reported by other groups [32], [33], [34], [35], [36]. Yet, the combined analysis of biomarkers (“AD signatures”) yielded estimates of accuracy very close to those recommended in the literature as good for a diagnostic biomarker [37]: “pathologic signature 1” (plotting values of Aβ1–42 and Aβ1–42/P-tau ratio) and “pathologic signature 2” (plotting Aβ1–42 and Aβ1–42/T-tau ratio) provided estimates of SE >70% and SP >80% differentiating healthy elders from AD patients. Compared with literature data, we also found a similar performance of these combinations of biomarkers in the prediction of conversion from MCI to dementia (AD) [36], [37], [38], [39].
Both signatures also discriminated cases of AD from other (non-AD) forms of cognitive impairment or dementia, with sensitivities and specificities >70% in the present sample. The differential diagnosis between AD and other forms of neurodegenerative disorders is another important application of CSF biomarkers, particularly addressing cases with mild symptoms or at early stages of the disease process. Studies have already demonstrated that CSF biomarkers discriminate well cases of frontotemporal dementia from AD [40], with estimates of diagnostic accuracy ranging from 93% to 99% [41]. On the other hand, studies addressing vascular dementia, dementia with Lewy bodies, and Parkinson's disease–related dementia failed to provide a clear diagnostic discrimination from AD [42], [43]. This limitation can be attributed in part to the difficulties in establishing accurate diagnoses based solely on clinical criteria, and to the fact that many cases may display mixed etiologies, particularly in the presence of vascular burden [44]. In our study, the best diagnostic accuracy was obtained using the Aβ1–42/P-tau ratio, which discriminated AD from other dementias with an SE of 85% and an SP of 71%. In a recently published clinicopathologic study using this combination of biomarkers (i.e., P-tau/Aβ1–42), Seeburger et al. [45] reported a similar SE value discriminating AD from other dementias. It is important to mention that the group of non-AD cognitively impaired patients in the present study comprised cases of non-AD neurodegenerative dementia and also cases of cognitive impairment or dementia secondary to major psychiatric disorders, largely represented by cases of geriatric major depression and late-life bipolar disorder.
A limitation of our study is the relatively small sample size as compared with those used by large-scale multicentric studies, and the small number of cases of dementia due to other (non-AD) etiologies. Nonetheless, we included an important subsample of patients with cognitive impairment associated with primary psychiatric disorders, namely geriatric depression and late-life bipolar disorder. We understand that these are highly prevalent conditions at psychogeriatric services, which invariably present with cognitive symptoms that require diagnostic and prognostic elucidation. We also acknowledge that lumbar puncture is an invasive/aversive method that may render a proportion of older patients or their relatives prone to reject the procedure; nonetheless, available evidence supports its safe use in the diagnostic workup of cognitive impairment [46]. In the present study, we observed a very low incidence of complications related to lumbar puncture (2% of adverse events of any kind), including back pain (1%), headache (0.8%), and dizziness (0.7%). In all cases, complaints were mild, transient, and benign, except for one case requiring blood patch with full recovery. Participants in the AD group were less educated, although this difference did not reach statistical significance. This may be in part explained by the fact that poor education is a risk factor for dementia. This source of bias is common in studies conducted in developing countries such as ours, where social inequalities strongly impact on the access to education, which requires that this source of bias be treated statistically, in addition to using cognitive tests with cutoff scores adjusted for educational level.
A recent consensus from the Alzheimer's Biomarker Standardization Initiative stated that lumbar puncture for AD biomarker analysis in the CSF should be included in the routine assessment of patients with suspected early onset AD, atypical presentations of late-onset AD, and also prodromal AD [46]. Nonetheless, the routine clinical use of these biomarkers is still not recommended in spite of the encouraging results presented by many research groups internationally. Although good within-laboratory interday precision may be obtained with the establishment of robust protocols [47], there is still a large interlaboratory variability that prevents the establishment of universal cutoff scores, and no reliable values have been provided so far to guide the interpretation of single results in the clinical setting [48], [49]. Apart from random variation, different laboratories may present systematically higher or lower mean biomarker results, also when the same assay is used, depending on details in regard to exactly how the assay is performed. This type of bias can be detected in external control programs and could be dealt with by establishing more robust assays with fewer manual steps. Fagan et al. [50] compared two widely used methods for the determination of AD-related CSF biomarkers, namely the INNOTEST and the INNO-BIA AlzBio3 assay, which are based on enzyme-linked immunosorbent assay (ELISA) and Luminex platforms. The assay platforms yielded different (approximately twofold to sixfold) absolute measurements for CSF Aβ1–42, T-tau, and P-tau, but values were highly correlated. In addition, both assays yielded similar patterns of correlations between CSF concentrations of Aβ1–42 and intracerebral amyloid load, as determined by Pittsburgh Compound B (PIB) imaging with Positron Emission Tomography (PET). The authors concluded that both ELISA and Luminex-based platforms perform well, albeit with differences in absolute values, which reinforce the need for assay-specific diagnostic cutoff values. In our study, the AlzBio3-derived Aβ1–42 results were much higher than those reported in the Alzheimer's Disease Neuroimaging Initiative study in which the same assay was used [5], whereas T-tau and P-tau levels were similar. This result underscores that, for the time being, each laboratory must use internally validated cutoff values and make sure they maintain longitudinal stability in their measurements to be able to use them [51], [52].
Nonetheless, the analysis of CSF biomarkers may yield important diagnostic and treatment-decision insights at specialized memory clinics: clearly abnormal values of Aβ1–42, T-tau, and P-tau in patients with episodic memory impairment support a diagnosis of prodromal AD; conversely, the diagnosis of AD may be ruled out should all three biomarkers read within the normal range. Yet, most cases will probably display intermediate results and require longitudinal monitoring and reassessment [46].
5. Conclusion
CSF biomarkers represent a valuable tool in the differential diagnosis of cognitive impairment in the elderly and prediction of dementia due to AD. New guidelines have incorporated molecular, genetic, and neuroimaging biomarkers to clinical criteria to the benefit of diagnostic accuracy, i.e., increasing the certainty that AD pathology is present and is the most likely cause of dementia or, at earlier stages of the disease process, indicating the probability of progression from mild impairment to dementia. The analysis of CSF concentrations of Aβ1–42, T-tau, and P-tau provides good diagnostic accuracy, particularly when used in combination (“signatures”). Our results follow the trends of international reports.
Research in context.
-
1.
Systematic review: Cerebrospinal fluid (CSF) biomarkers illustrate in vivo the presence of Alzheimer's disease (AD) pathology and add accuracy to the diagnostic workup of patients with cognitive impairment. We reviewed the published literature addressing the use of CSF biomarker values for the early diagnosis of AD, in addition to position articles from key authors in this field. We conclude that methodological limitations still preclude a widespread clinical use of this technology. Several different sources of bias (preanalytical, analytical, and postanalytical) may affect the final reading of the concentrations of AD biomarkers, causing a significant intralaboratory and interlaboratory variability of results. Recent guidelines establish that each laboratory must warrant longitudinal stability in its measurements and use internally qualified cutoff values.
-
2.
Interpretation: We present CSF biomarker data generated in a memory clinic located in a tertiary hospital in Brazil. Our results follow the trends of international reports. The analysis of CSF concentrations of amyloid-beta peptide, total tau, and 181Thr-phosphorylated-tau provides good diagnostic accuracy, particularly when used in combination (“signatures”). Nonetheless, the cutoff values presented here are different from those derived from other laboratories, which reinforces the notion that the method, in spite of having a good overall consistency, is not ready for translation into clinical practice.
-
3.
Future directions: The present set of data adds to the limited body of information on AD-related CSF biomarkers derived from research groups in non-US/European countries. To the best of our knowledge, this is the first study to define cutoff values for AD-related CSF biomarkers in Brazil. Until the worldwide efforts toward the standardization of CSF biomarker values are complete, we understand that similar studies of diagnostic accuracy of AD-related biomarkers in distinct settings are welcome.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa de São Paulo (grant no 09/52825-8, Brazil), National Counsel of Technological and Scientific Development (CNPq, grant no. 466625/2014-6), Associação Beneficente Alzira Denise Hertzog da Silva, and JNK Empreendimentos e Incorporações.
Footnotes
There are no conflicts of interest to disclose.
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zetterberg H., Blennow K. Cerebrospinal fluid biomarkers for Alzheimer's disease: More to come? J Alzheimers Dis. 2013;33:S361–S369. doi: 10.3233/JAD-2012-129035. [DOI] [PubMed] [Google Scholar]
- 4.Koopman K., LeBastard N., Martin J.J., Nagels G., DeDeyn P.P., Engelborghs S. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau (181P) Neurochem Int. 2009;55:214–218. doi: 10.1016/j.neuint.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C., Alzheimer's Disease Neuroimaging Initiative Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forlenza O.V., Diniz B.S., Gattaz W.F. Diagnosis and biomarkers of predementia in Alzheimer's disease. BMC Med. 2010;8:89. doi: 10.1186/1741-7015-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller S.G., Weiner M.W., Thal L.J., Petersen R.C., Jack C., Jagust W. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–877. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motter R., Vigo-Pelfrey C., Kholodenko D., Barbour R., Johson-Wood K., Galasko D. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 9.Vandermeeren M., Mercken M., Vanmechelen E., Six J., van de Voorde A., Martin J.J. Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- 10.Blennow K., Wallin A., Agren H., Spenger C., Siegfried J., Vanmechelen E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K., Hampel H. Cerebrospinal fluid markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 13.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 14.Jack C.R., Jr., Alber M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höglund K., Fourier A., Perret-Liaudet A., Zetterberg H., Blennow K., Portelius E. Alzheimer's disease: Recent biomarker developments in relation to updated diagnostic criteria. Clin Chim Acta. 2015;449:3–8. doi: 10.1016/j.cca.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Roth M., Tym E., Mountjoy C.Q., Huppert F.A., Hendrie H., Verma S. CAMDEX: A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M., Folstein S., McHugh P.R. Mini-mental state: A practical method for grading the cognitive state of patients. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Fuld P. Guaranteed stimulus processing in the evaluation of memory and learning. Cortex. 1980;16:255–271. doi: 10.1016/s0010-9452(80)80061-x. [DOI] [PubMed] [Google Scholar]
- 19.Army individual test battery. Manual of directions and scoring. War Department, Adjutant General's Office; Washington, DC: 1944. [Google Scholar]
- 20.Erzigkeit H. 23rd ed. Geromed GmbH; Erlangen, Germany: 2001. SKT: A short cognitive performance test for assessing deficits of memory and attention. User's Manual. [DOI] [PubMed] [Google Scholar]
- 21.Flaks M.K., Yassuda M.S., Regina A.C., Cid C.G., Camargo C.H., Gattaz W.F. The short cognitive performance test (SKT): A preliminary study of its psychometric properties in Brazil. Int Psychogeriatr. 2006;18:121–133. doi: 10.1017/S1041610205002577. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diniz B.S., Nunes P.V., Yassuda M.S., Pereira F.S., Flaks M.K., Viola L.F. Mild cognitive impairment: Cognitive screening or neuropsychological assessment? Rev Bras Psiquiatr. 2008;30:316–321. doi: 10.1590/s1516-44462008000400003. [DOI] [PubMed] [Google Scholar]
- 24.Forlenza O.V., Diniz B.S., Talib L.L., Radanovic M., Yassuda M.S., Ojopi E.B. Clinical and biological predictors of Alzheimer's disease in patients with amnestic mild cognitive impairment. Rev Bras Psiquiatr. 2010;32:216–222. doi: 10.1590/s1516-44462010005000002. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . 4th ed. American Psychiatric Association; Washington DC: 2000. Diagnostic and statistical manual of mental disorders (DSM-IV) [Google Scholar]
- 28.Loewenstein D.A., Amigo E., Duara R. A new scale for the assessment of functional status in Alzheimer's disease and related disorders. J Gerontol. 1989;4:114–121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- 29.Pereira F.S., Oliveira A.M., Diniz B.S., Forlenza O.V., Yassuda M.S. Cross-cultural adaptation, reliability and validity of the DAFS-R in a sample of Brazilian older adults. Arch Clin Neuropsychol. 2010;25:335–343. doi: 10.1093/arclin/acq029. [DOI] [PubMed] [Google Scholar]
- 30.Buchhave P., Minthon L., Zetterberg H., Wallin A.K., Blennow K., Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 31.van Harten A.C., Visser P.J., Pijnenburg Y.A., Teunissen C.E., Blankenstein M.A., Scheltens P. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen N., Vanmechelen E., van de Voorde A., Davidsson P., Hesse C., Tarvonen S. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer's disease: A community based follow-up study. J Neurol Neurosurg Psychiatr. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galasko D., Clark C., Chang L., Miller B., Green R.C., Motter R. Assessment of CSF levels of tau protein in mildly demented patients with Alzheimer's disease. Neurology. 1997;48:632–635. doi: 10.1212/wnl.48.3.632. [DOI] [PubMed] [Google Scholar]
- 34.Galasko D., Chang L., Motter R., Clark C.M., Kaye J., Knopman D. High cerebrospinal fluid tau and low amyloid b42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 35.Hulstaert F., Blennow K., Ivanoiu A., Schoonderwaldt H.C., Riemenschneider M., De Deyn P.P. Improved discrimination of AD patients using b-amyloid (1-42) and tau levels in CSF. Neurology. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 36.Andreasen N., Minthon L., Davidsson P., Vanmechelen E., Vanderstichele H., Winbald B. Evaluation of CSF-tau and CSF-Aß42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 37.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C., Alzheimer's Disease Neuroimaging Initiative The Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2013;9:e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 39.Hertze J., Minthon L., Zetterberg H., Vanmechelen E., Blennow K., Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer's disease: A clinical follow-up study of 4.7 years. J Alzheimers Dis. 2010;21:1119–1128. doi: 10.3233/jad-2010-100207. [DOI] [PubMed] [Google Scholar]
- 40.Struyfs H., van Broeck B., Timmers M., Fransen E., Sleegers K., van Broeckhoven C. Diagnostic accuracy of cerebrospinal fluid amyloid-β isoforms for early and differential dementia diagnosis. J Alzheimers Dis. 2015;45:813–822. doi: 10.3233/JAD-141986. [DOI] [PubMed] [Google Scholar]
- 41.Irwin D.J., Trojanowski J.Q., Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci. 2013;5:6. doi: 10.3389/fnagi.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaerst L., Kuhlmann A., Wedekind D., Stoeck K., Lange P., Zerr I. Cerebrospinal fluid biomarkers in Alzheimer's disease, vascular dementia and ischemic stroke patients: A critical analysis. J Neurol. 2013;260:2722–2727. doi: 10.1007/s00415-013-7047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefani A., Brusa L., Olivola E., Pierantozzi M., Martorana M. CSF and clinical hallmarks of subcortical dementias: Focus on DLB and PDD. J Neural Transm. 2012;119:861–875. doi: 10.1007/s00702-012-0820-0. [DOI] [PubMed] [Google Scholar]
- 44.Rosén C., Hansson O., Blennow K., Zetterberg H. Fluid biomarkers in Alzheimer's disease—Current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeburger J.L., Holder D.J., Combrinck M., Joachim C., Laterza O., Tanen M. Cerebrospinal fluid biomarkers distinguish postmortem confirmed Alzheimer's disease from other dementias and healthy controls in the OPTIMA cohort. J Alzheimers Dis. 2015;44:525–539. doi: 10.3233/JAD-141725. [DOI] [PubMed] [Google Scholar]
- 46.Molinuevo J.L., Blennow K., Dubois B., Engelborghs S., Lewczuk P., Perret-Liaudet A. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: A consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Figurski M., Coart E., Blennow K., Alzheimer's Disease Neuroimaging Initiative Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattsson N., Andreasson U., Persson S., Arai H., Batish S.D., Bernardini S. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattsson N., Andreasson U., Persson S., Carrillo M.C., Collins S., Chalbot S., Alzheimer's Association QC Program Work Group CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagan A.M., Shaw L.M., Xiong C., Vanderstichele H., Mintun M.A., Trojanowski J.Q. Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmqvist S., Zetterberg H., Blennow K., Vestberg S., Andreasson U., Brooks D.J. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: A cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 52.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]