Abstract
Introduction
A decreased cerebrospinal fluid (CSF) p-Tau181 to total tau ratio (p/t-tau) is a biomarker for frontotemporal lobar degeneration with TDP43 inclusions (FTLD-TDP) and for amyotrophic lateral sclerosis (ALS). CSF light chain neurofilaments (NfL) are increased in ALS. We examined whether CSF p/t-tau and NfL are related to ALS status in FTLD-TDP.
Methods
We compared CSF p/t-tau and NfL levels between patients with FTLD-TDP with ALS (n = 15), FTLD-TDP without ALS (n = 17), FTLD-Tau (n = 6), Alzheimer's disease (AD; n = 25), and subjective memory complaints (SMC, n = 24).
Results
Apart from FTLD-Tau, all groups differed significantly with increasing p/t-tau ratios from FTLD-TDP with ALS to FTLD-TDP without ALS to AD and SMC. CSF NfL was very high in FTLD-TDP with ALS followed by FTLD-TDP without ALS, AD, and SMC. Both biomarkers correlated with survival.
Discussion
CSF p/t-tau ratio and NfL levels are strongly driven by ALS status. These markers, therefore, appear to be more of prognostic than diagnostic significance.
Keywords: Frontotemporal dementia, Cerebrospinal fluid, Biomarker, Disease monitoring, Neurofilament, p/t tau ratio
1. Introduction
Frontotemporal dementia (FTD) is a clinical spectrum of neurodegenerative disorders mainly affecting the frontal and temporal lobes. When the disorder leads to severe personality and behavioral change, the resulting clinical syndrome is termed the behavioral variant of FTD (bvFTD), whereas both semantic dementia and progressive nonfluent aphasia are the FTD language variants [1], [2]. The clinical spectrum of FTD is associated with several distinct pathologies constituting the pathologic spectrum of frontotemporal lobar degeneration (FTLD). FTLD with TAR DNA-binding protein 43 inclusions (FTLD-TDP) and tau positive FTLD (FTLD-Tau) are the two most prevalent pathologies [3]. Clinico-pathologic correlations are not robust, except when the underlying pathology of FTD is predicted by the presence of an autosomal dominant mutation, where progranulin gene mutations and C9orf72 repeat expansions give rise to FTLD-TDP, and mutations in the tau gene are responsible for FTLD-Tau [4], [5]. In addition, FTD with amyotrophic lateral sclerosis (ALS) is virtually always associated with underlying FTLD-TDP [6].
In light of patient management and upcoming treatments, the identification of FTLD pathologic subtypes is important and cerebrospinal fluid (CSF) biomarker studies might aid in this. Ideally, CSF biomarkers would also be able to predict disease progression. So far, results of CSF biomarker studies in FTD have been conflicting, probably partly due to the fact that most studies included clinically diagnosed cases, thereby introducing etiopathologic inaccuracy [7]. A study in patients with known pathologies reported a reduced CSF tau to amyloid β-42 ratio in FTLD compared with Alzheimer's disease (AD) [8]. This finding was explained by the biomarker abnormalities in AD because FTLD cases did not differ from controls. More recently, a reduced CSF phospho-tau181 to total tau ratio (p/t-tau ratio) has been suggested to be useful to identify FTLD-TDP relative to FTLD-Tau [9]. In an independent patient group, it was confirmed that a reduced CSF p/t-tau ratio distinguished FTLD-TDP from FTLD-Tau, AD, and control subjects. This finding seems contra-intuitive from a pathologic point of view because tau pathology is not a hallmark of FTLD-TDP in contrast to FTLD-Tau and AD. The results might, however, be explained in the context of increased CSF tau as a nonspecific marker of neurodegeneration. In this light, another study addressing CSF markers of axonal degeneration in FTD pathologic subgroups found that CSF neurofilament light chain (NfL) levels were strikingly increased in FTLD-TDP and were correlated with disease severity and the degree of brain atrophy [10].
CSF NfL levels have been found to be strongly increased in ALS [11]. In line with this finding, the p/t-tau ratio has been found to be reduced in ALS, although in one study this was due to reduced p-tau levels, whereas another study reported increased tau levels relative to CSF pTau [12], [13]. Correlations with progression rate were found for CSF NfL, and correlations with disease severity were found for p/t-tau in ALS [13], [14]. Based on the mentioned information, we hypothesized that both CSF p/t-tau ratio and NfL levels are associated with the rate of neurodegeneration in FTLD. Because FTD with ALS has a more rapid disease course than FTD without ALS, we hypothesized that their levels are related to ALS status in FTLD-TDP. In this study, we set out to compare both CSF p/t-tau ratio and NfL levels between patients with FTLD-TDP with ALS, FTLD-TDP without ALS, FTLD-Tau, AD, and persons with subjective memory complaints (SMC). Moreover, we studied whether p/t-tau ratio and NfL were correlated with survival.
2. Methods
2.1. Patients
Patients were recruited from the Amsterdam Dementia Cohort [15]. The study has been approved by the Medical Ethical Review Board, and all patients or their representatives gave written informed consent. In 15 FTLD-TDP with ALS cases, the diagnosis had been confirmed by needle electromyography based on the El Escorial criteria [16]. Neuropathologic confirmation of FTLD-TDP pathology had taken place in one of these cases, whereas a C9orf72 repeat expansion was present in another of these cases. FTLD-TDP cases without ALS (n = 17) had been neuropathologically confirmed in 11 of 17 cases; moreover, underlying FTLD-TDP pathology was confirmed by the presence of a progranulin gene mutation in 3 of 17 cases and a C9orf72 repeat expansion in 5 of 17 cases. Two cases that had been confirmed by autopsy turned out to be carriers of a C9orf72 repeat expansion. There were three neuropathologically confirmed FTLD-Tau cases and three patients carrying a tau gene mutation. Eleven AD cases had been neuropathologically confirmed [17], [18], whereas the 19 cases with a clinical diagnosis of AD according to the criteria of McKhann et al. [19] had a positive 11C Pittsburgh Compound B (PiB) positron emission tomography (PET) [20]. Because clinical investigations in SMC are normal, we used this subgroup as controls [21]. All subjects in this group had CSF amyloid β-42 levels above the cut off of 550 pg/mL. Disease duration was defined as the time from reported symptom onset by a reliable informant till time of diagnosis. To calculate survival, the time of symptom onset was subtracted from the time of death. Survival data became available via The Netherlands Brain Bank or nursing homes.
2.2. CSF examination
CSF was obtained by lumbar puncture between the L3/L4 or L4/L5 intervertebral space, and 12 mL was collected in polypropylene tubes. Within 1 hour, CSF samples were centrifuged at 3000 rpm for 10 minutes at 4°C. CSF was aliquoted in polypropylene tubes of 0.5 or 1 mL, and stored at −80°C until analysis. For the determination of CSF phospho-tau181 and tau levels, we used commercial enzyme-linked immunosorbent assay (ELISAs; Innogenetics, Ghent, Belgium). The performance of these assays is monitored with two internal quality control pools of surplus CSF (high and low biomarker values). Interassay coefficients of variation (CV) (mean ± standard deviation [SD]) were 9.9 ± 2.1% for Tau, and 9.1 ± 1.8% for pTau, as analyzed in a high and low pool from 13 consecutive pool preparations of two surplus CSF specimens (one normal and one AD profile), used in total in 189–231 runs [15]. We determined CSF levels of light chain NfL using ELISAs (Uman) according to the manufacturer's instructions. The CV of duplicates (n = 149) was 1.5 ± 1.1%. In two internal quality control CSF samples, the between-assay variation was 17.4% at 999 pg/mL and 15.9% at 50,789 pg/mL (sample diluted ×50 in the assay) averaging at 16.7% analyzed over five independent runs [22]. Lumbar punctures were performed within 6 months of the time of diagnosis. CSF p/t-tau ratio was calculated in 15 cases with FTLD-TDP with ALS, 17 cases with FTLD-TDP without ALS, six cases with FTLD-Tau, 25 cases with AD, and 24 cases with SMC. CSF NfL levels could be determined in 12 cases with FTLD-TDP with ALS, 14 cases with FTLD-TDP without ALS, four cases with FTLD-Tau, 25 cases with AD, and 24 cases with SMC. Of three cases with FTLD-TDP with ALS, three cases with FTLD-TDP without ALS, and two cases with FTLD-Tau insufficient CSF were stored to perform these analyses.
2.3. Statistical analysis
For statistical analysis, the statistical package SPSS 22.0 for Mac was used. Group comparisons were made using analysis of variance followed by post-hoc Fisher Least Significant Difference tests, Kruskal-Wallis followed by post-hoc Mann-Whitney U tests, and chi-squared tests, where appropriate. Nonparametric correlations were calculated using Spearman's Rho. To determine diagnostic accuracy, receiver operating characteristic (ROC) curves were drawn and areas under the curve (AUCs) were calculated.
3. Results
Demographic data are presented in Table 1. Clinically, all subjects with FTLD had bvFTD, except for two semantic dementia cases that had pathologically confirmed FTLD-TDP. These patients did not have ALS. Groups did not differ regarding sex distribution and age. Reported disease duration at time of diagnosis was shorter in FTLD-TDP with ALS than in FTLD-TDP without ALS and AD (P < .001, P = .001, respectively).
Table 1.
Demographic data
| FTLD-TDP with ALS n = 15 | FTLD-TDP without ALS n = 17 | FTLD-tau n = 6 | AD n = 25 | SMC n = 24 | Significance | |
|---|---|---|---|---|---|---|
| Sex (M:F) | 7:8 | 11:6 | 2:4 | 15:10 | 15:9 | NS |
| Mean age (SD) (y) | 60.5 (6.0) | 60.6 (7.4) | 63.0 (7.8) | 62.2 (4.4) | NS | |
| Mean disease duration (SD) (y) | 1.6 (0.7) | 5.7 (5.0) | 3.6 (2.2) | — | FTLD-TDP with ALS versus FTLD-TDP without ALS P < .001 FTLD-TDP with ALS versus FTD-Tau P < .001 FTLD-TDP with ALS versus AD P = .01 |
|
| Follow-up duration (mo) | 3 (0–18) | 19 (0–80) | 10 (2–36) | 25 (0–85) | 12 (0–37) |
Abbreviations: FTLD-TDP, frontotemporal lobar degeneration with TDP43 inclusions; ALS, amyotrophic lateral sclerosis; AD, Alzheimer's disease; SMC, subjective memory complaints; M, male; F, female; NS, not significant; SD, standard deviation; ANOVA, analysis of variance.
NOTE. Values are displayed as mean (SD). Significant group differences were calculated using ANOVA followed by post hoc LSD t tests.
In 28 out of a total of 62 cases with dementia, the duration from symptom onset until death was known. Mean survival was 2.4 years (standard deviation 1.1) in seven patients with FTLD-TDP with ALS, 8.0 years (SD 3.8) in 11 patients with FTLD-TDP without ALS, and 8.0 years (SD 2.6) in nine patients with AD. One patient with pathologically proven FTLD-Tau had a survival of 12 years.
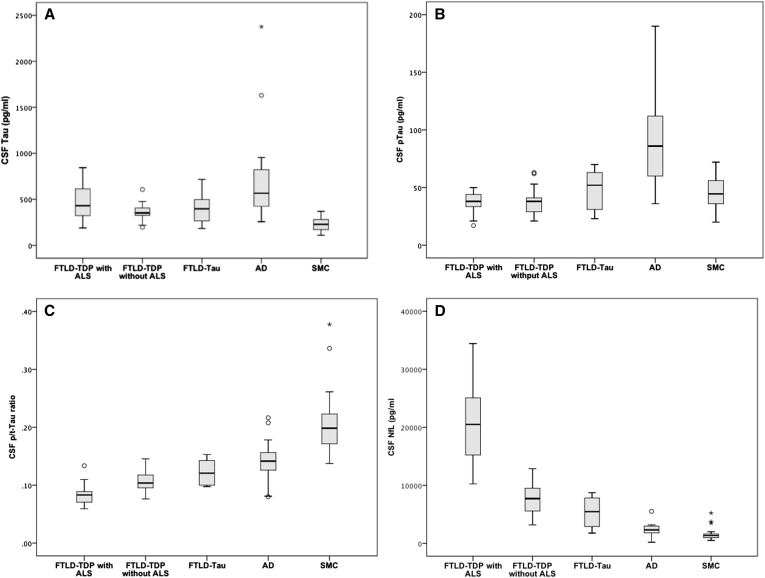
Results of CSF biomarker analyses are displayed in Fig. 1. Median CSF tau levels were 431 pg/mL (interquartile range [IQR] 330) in FTLD-TDP with ALS, 353 pg/mL (IQR 109) in FTLD-TDP without ALS, 398 pg/mL (IQR 308) in FTLD-Tau, 567 pg/mL (IQR 449) in AD, and 229 pg/mL (IQR 112) in SMC. As expected, CSF tau levels were highest in AD compared with both FTLD-TDP without ALS and SMC (P = .001, P < .001, respectively). CSF tau levels, however, were similar between AD, FTLD-TDP with ALS, and FTLD-Tau (AD vs. FTLD-TDP with ALS P = .14; AD vs. FTLD-Tau P = .10; and FTLD-TDP with ALS vs. FTLD-Tau P = .59). In FTLD-TDP with ALS, FTLD-TDP without ALS, and FTLD-Tau CSF Tau levels were higher than in SMC (P < .001, P < .001, and P = .01, respectively). CSF tau levels in FTLD-Tau without ALS did not differ from controls.
Fig. 1.
Biomarker levels per diagnostic group. (A) CSF tau. (B) CSF pTau181, (C) CSF p/t-tau ratio. (D) CSF NfL. Significant group differences were calculated using the Kruskal-Wallis test followed by the Mann-Whitney test. Abbreviations: CSF, cerebrospinal fluid; p/t-tau, p-Tau181 to total Tau ratio; NfL, neurofilaments. oOutliers of > 1.5 IQR. *Outliers of > 3 IQR.
Median CSF pTau181 levels were 38 pg/mL (IQR 13) in FTLD-TDP with ALS, 38 pg/mL (IQR 17) in FTLD-TDP without ALS, 52 pg/mL (IQR 36) in FTLD-Tau, 86 pg/mL (IQR 56) in AD, and 45 pg/mL (IQR 20) in SMC. CSF pTau181 levels were higher in AD compared with all FTLD subgroups and SMC (AD vs. FTLD-TDP with ALS P < .001, AD vs. FTLD-TDP without ALS P < .001, AD vs. FTLD-Tau P = .02, and AD vs. SMC P < .001).
The median p/t-tau ratio, however, was lowest in FTLD-TDP with ALS (0.08, IQR 0.02), followed by FTLD-TDP without ALS (0.10, IQR 0.03), FTLD-tau (0.12, IQR 0.05), AD (0.14, IQR 0.03), and SMC (0.20, IQR 0.06). The CSF p/t-tau ratio was significantly lower in FTLD-TDP with ALS compared with all other groups (P < .001 vs. FTLD-TDP without ALS, AD and SMC, P = .002 vs. FTLD-Tau). In FTLD-TDP without ALS, the CSF p/t-tau ratio was significantly lower than in AD (P = .001) and SMC (P < .001). In AD, the CSF p/t-tau ratio was significantly lower than in SMC (P < .001).
An opposite trend was seen in the levels of NfL where median NfL was very high in FTLD-TDP with ALS (20,509 pg/mL, IQR 10,622), compared with FTLD-TDP without ALS (7712 pg/mL, IQR 4150), followed by FTLD-tau (5462 pg/mL, IQR 5946), AD (2314 pg/mL, IQR 1314), and SMC (1342 pg/mL, IQR 653). CSF NfL levels were significantly higher in FTLD-TDP with ALS compared with all other groups (P < .001 vs. FTLD-TDP without ALS, AD, and SMC, P = .001 vs. FTLD-Tau). CSF NfL levels were significantly higher in FTLD-TDP without ALS compared with FTLD-Tau, AD, and SMC (for all comparisons, P < .001). In AD, CSF NfL levels were significantly higher than those in SMC (P < .001).
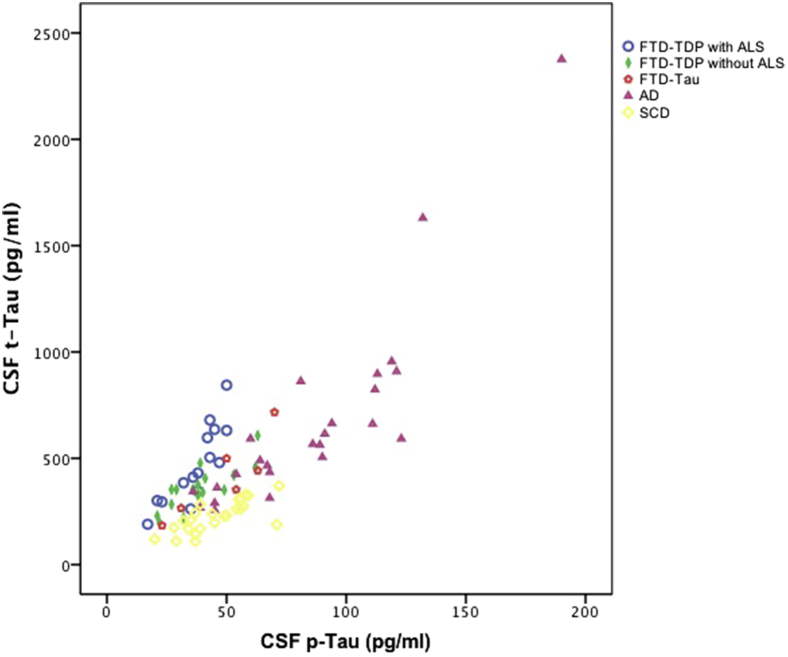
Fig. 2 presents CSF tau relative to CSF pTau levels per disease group, showing that the reduced ratio was grossly due to increased tau relative to normal or slightly increased pTau. In the total group, CSF tau was significantly correlated to CSF pTau (r = −0.67, P < .001). The p/t-tau ratio was negatively correlated with CSF NfL levels (r = −0.69, P < .001).
Fig. 2.
CSF tau relative to CSF pTau levels per diagnostic group. Abbreviations: CSF, cerebrospinal fluid; FTD-TDP, frontotemporal dementia with TDP43 inclusions; ALS, amyotrophic lateral sclerosis; AD, Alzheimer's disease.
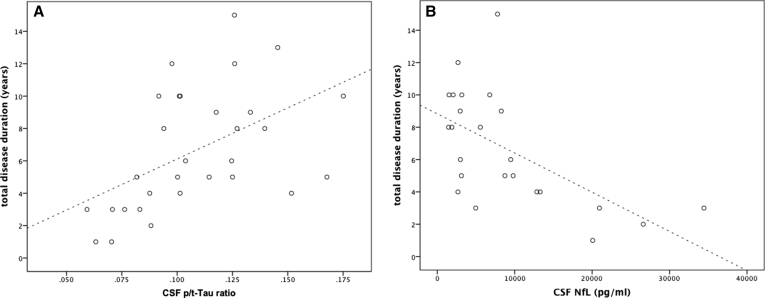
No significant correlations between any of the CSF biomarkers and disease duration at time of diagnosis were found. In the total patient group with known survival, CSF p/t-tau ratio positively correlated with survival (r = 0.60, P = .001), whereas CSF NfL levels were negatively correlated with survival (r = −0.62, P = .01; Fig. 3). This finding also holds true within the FTLD-TDP group (r = 0.81, P < .001 and r = −0.71, P = .005, respectively).
Fig. 3.
Correlation of CSF p/t-ratio (A) and CSF NfL (B) with survival. Abbreviations: CSF, cerebrospinal fluid; p/t-tau, p-Tau181 to total tau ratio; NfL, neurofilaments.
CSF tau and pTau were significantly correlated with each other (r = 0.67, P < .001), but these individual biomarkers were not correlated with survival (r = −0.12, P = .54 for CSF tau and r = 0.28, P = .13 for CSF pTau).
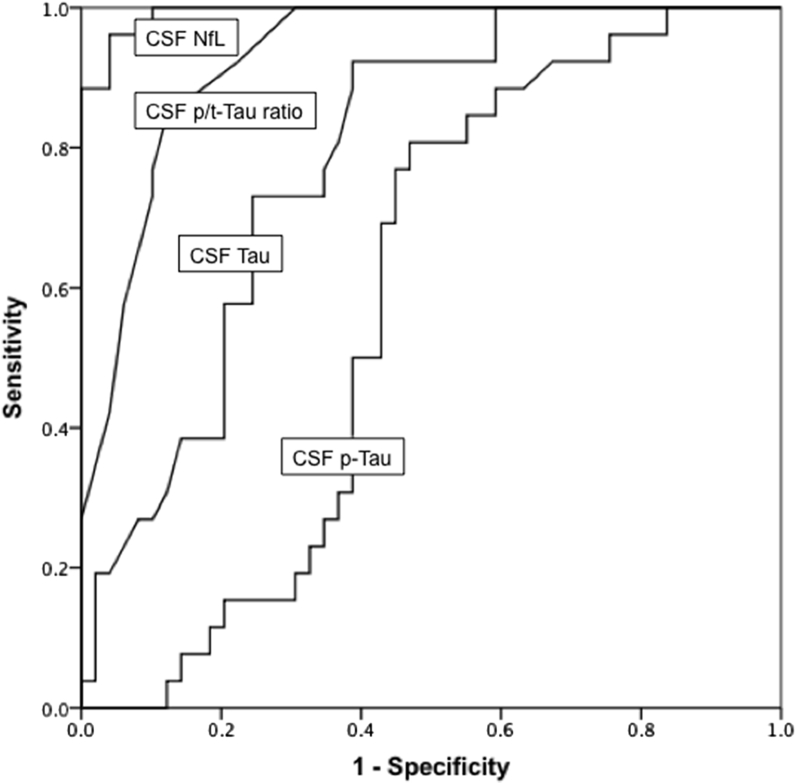
ROC curves are displayed in Fig. 4. The diagnostic accuracy of CSF NfL for FTLD-TDP (with and without ALS) versus AD and SMC was very high with an AUC of 0.99 (95% confidence interval [CI]: 0.98–1.0), which was even higher than the AUC of 0.93 (95% CI: 0.87–0.98) for the CSF p/t-tau ratio.
Fig. 4.
ROC curves of various biomarkers for FTLD-TDP versus AD and SMC. Abbreviations: FTLD-TDP, frontotemporal lobar degeneration with TDP43 inclusions; AD, Alzheimer's disease; SMC, subjective memory complaints.
4. Discussion
Our study confirms that a reduced CSF p/t-tau ratio is an accurate biomarker for the total group of FTLD-TDP in the differential diagnosis with AD and subjects without dementia. The value of the CSF p/t-tau ratio in distinguishing between FTLD-TDP and FTLD-Tau is less clear because we clearly demonstrate that the presence of ALS has a major effect on this ratio. We found a similar and strongly distinctive pattern for CSF NfL, with extremely high levels of CSF NfL in FTLD-TDP with ALS, followed by FTLD-TDP without ALS, and subsequently by FTLD-Tau, AD, and SMC, respectively. P/t-tau ratio and NfL levels were negatively correlated and both biomarkers were related to survival.
Reduced CSF p/t-tau levels were found in ALS by Grossman et al. [13] where reduced CSF pTau levels were causing this effect. The finding of decreased CSF p/t-tau ratio in ALS was reproduced by another study; however, in this study, CSF pTau was not reduced, but CSF Tau was increased [12]. It was previously observed that a reduced CSF p/t-tau ratio has a very high accuracy in detecting Creutzfeldt-Jakob disease [23]. Because phosphorylation of tau occurs mainly in AD and not so much in other neurodegenerative disorders, the reduced p/t-tau ratio most likely reflects extensive neuronal degeneration in actively degenerating diseases leading to increased total CSF tau levels rather than a reduction in pTau levels. A similar mechanism might account for the reduced p/t-tau ratio in FTLD-TDP with ALS, which has a significantly shorter survival than FTD without ALS [24], [25]. Indeed, we found that FTLD-TDP with ALS cases had the highest total tau levels relative to their p-tau levels. We found no evidence for reduced CSF pTau levels in our sample.
Our finding of increased CSF tau levels in FTLD subgroups is in agreement with some other studies that included pathologically or genetically confirmed FTLD [10], [26]. Other studies, however, found CSF tau levels in the normal or low range in pathologically confirmed FTLD [8], [27]. These conflicting results might be the consequence of heterogeneity of the neurodegenerative process in FTLD. Another possible explanation might be interassay variation between different laboratories.
CSF NfL levels are increased in ALS, but also in multiple sclerosis, and are considered as a dynamic marker for axonal degeneration with the potential to monitor treatment effects [11], [28]. We previously reported on increased CSF NfL levels in a pathologically undefined subgroup of FTD patients [29]. More recently, elevated CSF NfL levels were found in clinically diagnosed FTD cases, particularly in those with evidence of underlying TDP43 pathology, and correlations were found with the degree of frontotemporal atrophy and disease severity [10], [30]. A large study in 3356 subjects demonstrated that CSF NfL levels were highest in FTD (of unspecified pathology) and vascular dementia, compared with other types of dementia and healthy control subjects [31]. Our findings stress that the degree of axonal degeneration is apparently higher in FTLD-TDP than in AD, and most prominent in FTLD-TDP with ALS.
Both CSF p/t-tau ratio and CSF NfL levels correlated strongly to survival. Therefore, our study tentatively confirms the prognostic potential of these biomarkers. An earlier study in AD cases revealing that the CSF p/t-tau ratio was associated with the rate of cognitive decline hints into the same direction [32]. Moreover, both in a large study including various dementia types and in ALS, a negative correlation between survival and CSF NfL levels has been established [31], [33].
Disease duration in FTD varies between 2 and 20 years [34]. FTD with ALS is associated with the shortest disease duration. On the other hand, disease courses with very gradual decline have been described in a proportion of subjects carrying the C9orf72 hexanucleotide expansion [35]. This heterogeneity in the rate of neurodegeneration appears to be reflected in the variability of CSF p/t-tau ratio and NfL levels in FTD.
Patients with FTLD-TDP with ALS had much shorter follow-up durations than the other patient groups, given the severity of their clinical picture. Two of 15 patients with FTLD-TDP with ALS developed ALS after an initial diagnosis of bvFTD after 6 and 18 months, respectively. Because nine of 17 patients with FTLD-TDP without ALS had follow-up durations <18 months, theoretically these patients might still have developed ALS. This is very unlikely, however, because FTD-ALS usually has a rapid disease course.
Among the strengths of our study is the inclusion of cases with known pathology by genetic and pathologic confirmation and by the use of read-outs strongly suggestive of the underlying pathology (electromyography for FTD-ALS and PiB-PET for AD). By the inclusion of post-mortem verified and PiB-positive AD cases instead of CSF profile-based cases, we avoided circular reasoning. Unfortunately, the FTLD-Tau subgroup was relatively small, so no firm conclusions can be drawn concerning this subgroup. The differences between FTLD-TDP and FTLD-Tau were strongly driven by the presence of ALS because CSF p/t-tau ratio and CSF NfL levels did not differ significantly between FTLD-TDP without ALS and FTLD-Tau. Larger study groups would be needed to confirm this. Because in the study by Hu et al. [9] the FTLD-TDP group was taken together, it remains possible that their finding of a decreased CSF p/t-tau ratio in FTLD-TDP compared with FTLD-Tau is driven by the inclusion of FTLD-TDP with ALS.
Our findings may have important implications for in vivo pathologic subtyping in clinical FTD, which is highly relevant in treatment development, and mainly address the FTLD subgroup with TDP-43 positive pathology. Interestingly, other in-vivo biomarkers of pathology have recently been developed, such as tau-PET imaging and future studies might clarify the relationship between CSF biomarkers, selective tau imaging, and neuropathology [36]. Even though tau positive inclusions are not a pathologic hallmark of FTLD-TDP, the reduced p/t-tau ratio in this FTLD subtype probably reflects the rate of neuronal degeneration. Moreover, the CSF p/t-tau ratio and CSF NfL levels have a high potential for predicting survival in FTD, which is of utmost importance for patient management and treatment monitoring.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using PubMed. Although there are a limited number of publications about the p-Tau181 to total tau (p/t-tau) ratio in frontotemporal dementia, there have been several publications about cerebrospinal fluid (CSF) neurofilaments in various neurodegenerative and neurologic diseases and their correlation with survival has been the subject of the study, although not in the same context as our study. The relevant citations are appropriately cited.
-
2.
Interpretation: Our findings show that the decreased CSF p/t-tau ratio as a biomarker for frontotemporal dementia with TDP43 inclusions is driven by the presence of amyotrophic lateral sclerosis. Both CSF p/t-tau ratio and neurofilaments (NfL) levels correlate with survival.
-
3.
Future directions: Our article warrants careful use of biomarkers for frontotemporal dementia subtypes for diagnostic purposes. Future research should point out if CSF p/t-tau ratio and NfL are suitable biomarkers for prognosis and monitoring of therapeutic effects.
Acknowledgments
The authors thank Femke H. Bouwman for data collection of neuropathologically confirmed cases, Annemieke J. Rozemuller for neuropathologic examinations, Hanne Meijers-Heijboer for support in genetic testing, Bart N. van Berckel for performing the PIB PET scans, and Rob L.M. Strijers for the performance of electromyographies in FTLD with ALS.
Research of the VUmc Alzheimer center is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The VUmc Alzheimer Center is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte.
Authors' contributions: Y.A.L.P. and P.S. designed the study. Y.A.L.P. and N.A.V. performed the data analysis. Y.A.L.P. drafted the article. N.A.V. performed data collection. W.V.D.F. assisted in statistical analysis. Y.A.L.P., N.A.V., P.S., and C.E.T. reviewed the article. C.E.T. performed the laboratory investigations and assisted in drafting.
Footnotes
Y.A.L.P. reports no conflicts of interest. W.V.D.F reports grants from Boehringer Ingelheim and Janssen Stellar outside the submitted work. P.S. reports grants from GE Healthcare, Merck, and Piramal Imaging, outside the submitted work. C.E.T. serves on the advisory board of Fujirebio and Roche; received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Mesoscale Discovery; performed contract research for IBL, Shire, Boehringer Ingelheim, Roche, and Probiodrug; and received grants from the European Commission, the Dutch Research Council (ZonMW), the ISAO and the Alzheimer's Drug Discovery Foundation.
References
- 1.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuman J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: Consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairns N.J., Bigio E.H., Mackenzie I.R., Neumann M., Lee V.M., Hatanpaa K.J. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenzie I.R., Frick P., Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014;127:347–357. doi: 10.1007/s00401-013-1232-4. [DOI] [PubMed] [Google Scholar]
- 6.Ng A.S., Rademakers R., Miller B.L. Frontotemporal dementia: A bridge between dementia and neuromuscular disease. Ann N Y Acad Sci. 2014;1338:71–93. doi: 10.1111/nyas.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin D.J., Trojanowsky D.Q., Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration form Alzheimer's disease. Front Aging Neurosci. 2013;5:6. doi: 10.3389/fnagi.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian H., Van Swieten J.C., Leight S., Massimo L., Wood E., Forman M. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu W.T., Watts K., Grossman M., Lah J.J., Hales C., Schelnutt M. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81:1945–1952. doi: 10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherling C.S., Hall T., Berisha F., Klepac K., Karydas A., Coppola G. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75:116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brettschneider J., Petzold A., Sussmuth S.D., Ludolph A.C., Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006;66:852–856. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- 12.Wilke C., Deuschle C., Rattay T.W., Maetzler W., Synofzik M. Total tau is increased, but phosphorylated tau not decreased, in cerebrospinal fluid in amyotrophic lateral sclerosis. Neurobiol Aging. 2015;36:1072–1074. doi: 10.1016/j.neurobiolaging.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Grossman M., Elman L., McCluskey L., McMillan C.T., Boller A., Powers J. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:442–448. doi: 10.1001/jamaneurol.2013.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelhak A., Junker A., Brettschneider J., Kassubek J., Ludolp A.C., Otto M. Brain-specific cytoskeletal damage markers in cerebrospinal fluid: Is there a common pattern between amyotrophic lateral sclerosis and primary progressive multiple sclerosis? Int J Mol Sci. 2015;16:17565–17588. doi: 10.3390/ijms160817565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: The Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 16.Brooks B.R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 17.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del T.K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack CR, jr, Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 21.Schoonenboom N.S., Reesink F.E., Verwey N.A., Kester M.I., Teunissen C.E., Van de ven P.M. Cerebrospinal fluid markers for dementia differential diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 22.Koel-Simmelink M.J., Vennegoor A., Killestein J., Blankenstein M.A., Norgren N., Korth C. The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated SinglePlex Luminex assay and ELISA. J Immunol Methods. 2014;402:43–49. doi: 10.1016/j.jim.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Skillback T., Rosen C., Asztely F., Mattsson N., Blennow K., Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: Results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 24.Hodges J.R., Davies J., Xuereb J., Kril J., Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 25.Roberson E.D., Hesse J.H., Rose K.D., Slama H., Johnson J.K., Yaffe K. Rate of progression differs in frontotemporal dementia and Alzheimer's disease. Neurology. 2005;65:719–725. [Google Scholar]
- 26.Clark C.M., Xie S., Chittams J., Ewbank D., Peskind E., Galasko D. Cerebrospinal fluid tau and beta-amyloid: How well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 27.Grossman M., Farmer J., Leight S., Work M, Moore P., Van Deerlin V. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 28.Teunissen C.E., Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler. 2012;18:552–556. doi: 10.1177/1352458512443092. [DOI] [PubMed] [Google Scholar]
- 29.Pijnenburg Y.A., Janssen J.C., Schoonenboom N.S., Petzold A., Mulder C., Stigbrand T. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer's disease and controls. Dement Geriatr Cogn Disord. 2007;23:225–230. doi: 10.1159/000099473. [DOI] [PubMed] [Google Scholar]
- 30.Landqvist W.M., Frizell Santillo A., Passant U., Zetterberg H., Rosengren L, Nillson C. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol. 2013;13:54. doi: 10.1186/1471-2377-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skillbäck T., Farahmand B., Bartlett J.W., Rosén C., Mattsson N., Nägga K. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 32.Kester M.I., van der Vlies A.E., Blankenstein M.A., Pijnenburg Y.A., van Elk E.J., Scheltens P. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- 33.Ganesalingam J., An J., Shaw C.E., Shaw G., Lacomis D., Bowser R. Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem. 2011;117:528–537. doi: 10.1111/j.1471-4159.2011.07224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snowden J.S., Neary D., Mann D.M. Churchill Livingstone; Edinburgh: 1996. Frontotemporal lobar degeneration: Fronto-Temporal Dementia, Progressive Aphasia, Semantic Dementia. Edinburgh: Churchill Livingstone. [Google Scholar]
- 35.Khan B.K., Yokoyama J.S., Takada L.T., Sha S.J., Rutherford N.J., Fong J.C. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villemagne V.L., Fodero-Tavoletti M.T., Masters C.L., Rowe C.C. Tau-imaging: Early progress and future directions. Lancet Neurol. 2015;14:114–124. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]