Abstract
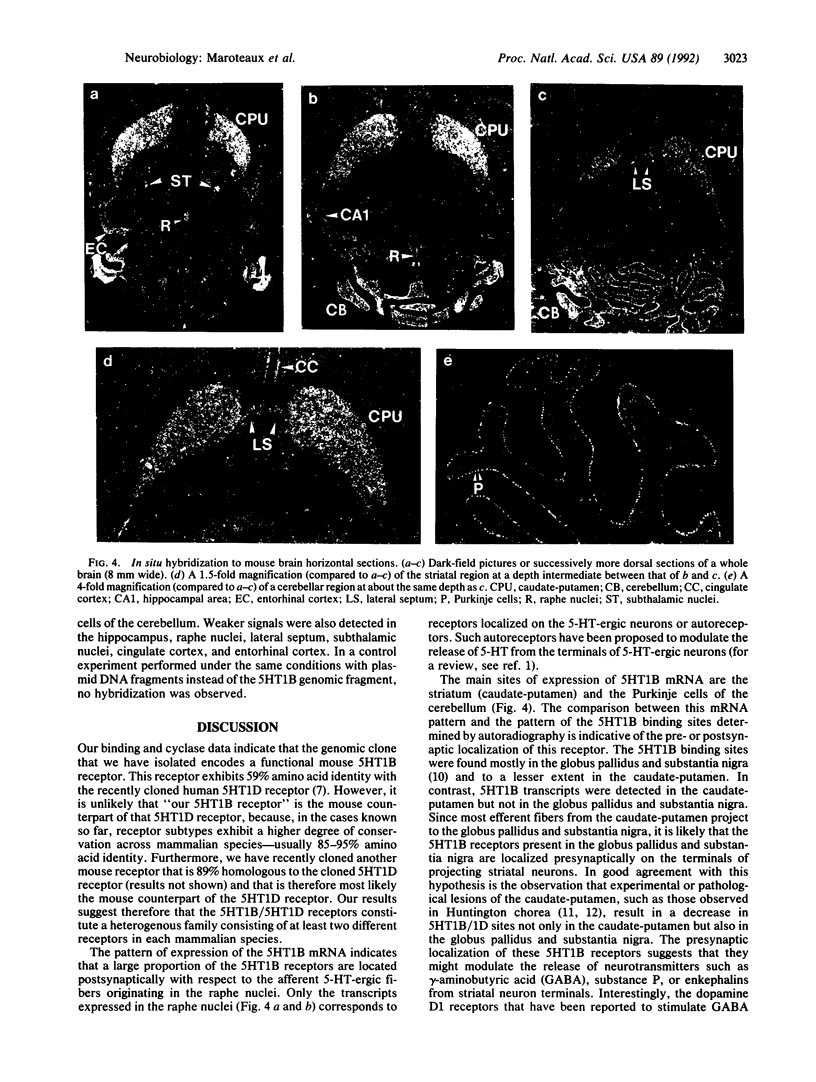
Serotonin is a neuromodulator that mediates a wide range of effects by interacting with multiple receptors. Using a strategy based on nucleotide sequence homology between genes encoding receptors that interact with guanine nucleotide-binding proteins, we have isolated a mouse gene encoding an additional serotonin receptor. When expressed in cultured cells, it displayed the pharmacological profile and coupling with adenylate cyclase characteristic of the 5HT1B receptor subtype. In NIH 3T3 cells expressing this receptor, serotonin induced a decrease in forskolin-stimulated cAMP levels. This effect was blocked by pertussis toxin, indicating that the 5HT1B receptor interacts with a pertussis toxin-sensitive guanine nucleotide-binding protein. To obtain clues as to the possible function of the 5HT1B receptor, we have analyzed its pattern of expression in the adult mouse brain by in situ hybridization. Our results, together with previous autoradiographic studies, suggest that the 5HT1B receptors are localized presynaptically on the terminals of striatal neurons and Purkinje cells and that they might modulate the release of neurotransmitters such as gamma-aminobutyric acid. The predominant expression of the 5HT1B receptor in the striatum and cerebellum points to an involvement of this receptor in motor control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amlaiky N., Caron M. G. Photoaffinity labeling of the D2-dopamine receptor using a novel high affinity radioiodinated probe. J Biol Chem. 1985 Feb 25;260(4):1983–1986. [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Frazer A., Maayani S., Wolfe B. B. Subtypes of receptors for serotonin. Annu Rev Pharmacol Toxicol. 1990;30:307–348. doi: 10.1146/annurev.pa.30.040190.001515. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Duncan G. E., Fornaretto M. G., Dearry A., Gingrich J. A., Breese G. R., Caron M. G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. W., Metcalf M. A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol. 1991 Aug;40(2):143–148. [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Oberlander C., Demassey Y., Verdu A., Van de Velde D., Bardelay C. Tolerance to the serotonin 5-HT1 agonist RU 24969 and effects on dopaminergic behaviour. Eur J Pharmacol. 1987 Jul 9;139(2):205–214. doi: 10.1016/0014-2999(87)90253-6. [DOI] [PubMed] [Google Scholar]
- Pazos A., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985 Nov 4;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Iversen L. L., Jessell T. M. Dopamine selectively increases 3H-GABA release from slices of rat substantia nigra in vitro. Nature. 1977 Aug 18;268(5621):652–654. doi: 10.1038/268652a0. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Hoyer D. 5-Hydroxytryptamine 5-HT1B and 5-HT1D receptors mediating inhibition of adenylate cyclase activity. Pharmacological comparison with special reference to the effects of yohimbine, rauwolscine and some beta-adrenoceptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1989 Sep;340(3):285–292. doi: 10.1007/BF00168512. [DOI] [PubMed] [Google Scholar]
- Voigt M. M., Laurie D. J., Seeburg P. H., Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991 Dec;10(13):4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C., Palacios J. M. Serotonin-1 receptor binding sites in the human basal ganglia are decreased in Huntington's chorea but not in Parkinson's disease: a quantitative in vitro autoradiography study. Neuroscience. 1989;32(2):337–347. doi: 10.1016/0306-4522(89)90082-1. [DOI] [PubMed] [Google Scholar]
- Waeber C., Schoeffter P., Palacios J. M., Hoyer D. 5-HT1D receptors in guinea-pig and pigeon brain. Radioligand binding and biochemical studies. Naunyn Schmiedebergs Arch Pharmacol. 1989 Nov;340(5):479–485. doi: 10.1007/BF00260601. [DOI] [PubMed] [Google Scholar]
- Waeber C., Zhang L. A., Palacios J. M. 5-HT1D receptors in the guinea pig brain: pre- and postsynaptic localizations in the striatonigral pathway. Brain Res. 1990 Oct 1;528(2):197–206. doi: 10.1016/0006-8993(90)91658-4. [DOI] [PubMed] [Google Scholar]






