Abstract
Recent methodological and conceptual advances have led to a fundamental reappraisal of the nature of visual working memory (WM). A large corpus of evidence now suggests that there might not be a hard limit on the number of items that can be stored. Instead, WM may be better captured by a highly limited––but flexible––resource model. More resource can be allocated to prioritized items but, crucially, at a cost of reduced recall precision for other stored items. Expectations may modulate resource distribution, for example, through neural oscillations in the alpha band increasing inhibition of irrelevant cortical regions. Our understanding of the neural architecture of WM is also undergoing radical revision. Whereas the prefrontal cortex has previously dominated research endeavors, other cortical regions, such as early visual areas, are now considered to make an essential contribution, for example holding one or more items in a privileged state or “focus of attention” within WM. By contrast, the striatum is increasingly viewed as crucial in determining why and how items are gated into memory, while the hippocampus, it has controversially been argued, might be critical in the formation of temporally resilient conjunctions across features of stored items in WM.
Keywords: working memory, binding, precision, hippocampus, basal ganglia
Introduction
Working memory (WM)––the mechanisms through which we store and manipulate information over a short time period––is one of the most intensely studied areas in cognitive neuroscience. From the psychology of development and aging through to neuroimaging and neurophysiology, from neuropsychology and psychopharmacology through to animal behavior and computational modeling, it has become a central and fundamental part of very diverse research enterprises. However, being situated at the nexus of such different interests, it is also at risk of being pulled in disparate directions, its shape and character distorted by many diverse agendas. Even within the fields of perception, attention, and memory, the direction of travel is contested, with some researchers claiming that the neural basis of WM may closely resemble perception1 while others argue that WM and long‐term memory (LTM) can operate with the same constraints.2 There is a danger then that WM becomes different things to different people, and in this way its utility becomes diluted.
If there is one unifying theme across the collective endeavors of researchers in different fields, it might perhaps be the attempt to characterize and delineate capacity limits in WM, both in healthy individuals and in people with brain disorders. Ultimately, this is the currency with which findings are reported. In part, the focus on limits stems largely from the well‐established strong relationship between WM capacity and performance. For example, reading comprehension,3 scores on exams,4 and real world performance5 are all intimately related to WM capacity. This suggests that limits in WM might act as crucial bottlenecks in determining the ability of a person to coordinate behavior effectively over time. Thus, understanding the neural and psychological bases of such capacity limits might be crucial to unlocking constraints on human cognitive performance limitations––and also to improving them. Moreover, limits in WM have important consequences in terms of the mechanisms required to protect memory contents from distraction or to update them on the basis of new information.
Here, we review some recent work on WM and identify key trends that have advanced our state of knowledge (for other recent viewpoints, the reader is referred to reviews in Refs. 6–9). At the outset, we point out that, although similar mechanisms are likely to operate across other modalities, the focus of this review is visual WM, an area that has generated intense debate in recent years. For a more verbal WM focus, the reader is referred to Ref. 10; for other recent developments in the cognitive psychology of WM, there are several important contributions that have reviewed different aspects of the field.11, 12, 13
Here, we first discuss recent research in the field of visual WM that suggests that, although it is indeed highly limited, the limitation is not the number of items that can be retained but rather the resources that can be devoted to storing items. Second, we examine data that reveal how maintained items compete with each other for mnemonic resources and how recall can be corrupted (e.g., by misbinding features that belong to different stored objects). Next, we review recent findings that highlight the intimate relationship between attention and WM. These studies show that limited resources in memory can be deployed flexibly between objects, with some items––often only one––being held in a privileged state: the so‐called focus of attention. Fourth, we review recent reports on how expectations or predictions about the environment affect the temporal coordination of resources––or deployment of attention––in WM. Findings from this line of research suggest that distinct neurophysiological signals are responsible for preparing either to update the contents of WM or to ignore new information. Next, we discuss results that suggest that the basal ganglia are important in why and how we shift resources within WM. Finally, we integrate the above findings with a growing body of work that challenges a sharp demarcation between WM and LTM and their neural substrates, with a specific focus on the hippocampus.
WM is a highly limited resource
Perhaps the most fundamental and controversial aspect of WM research in the last few years has been a reappraisal of its underlying architecture. Recent theoretical and empirical approaches7, 14, 15, 16 have challenged the orthodox view that WM is effectively quantal and limited to a small, finite number of items (sometimes referred to as K) that are held in a fixed number of memory “slots,” each with a fixed resolution.17, 18, 19, 20 Evidence in favor of an alternative resource model of WM comes primarily from delayed reproduction or adjustment tasks that require participants to reproduce the exact feature of a remembered object using a continuous, analog response space (Fig. 1).
Figure 1.
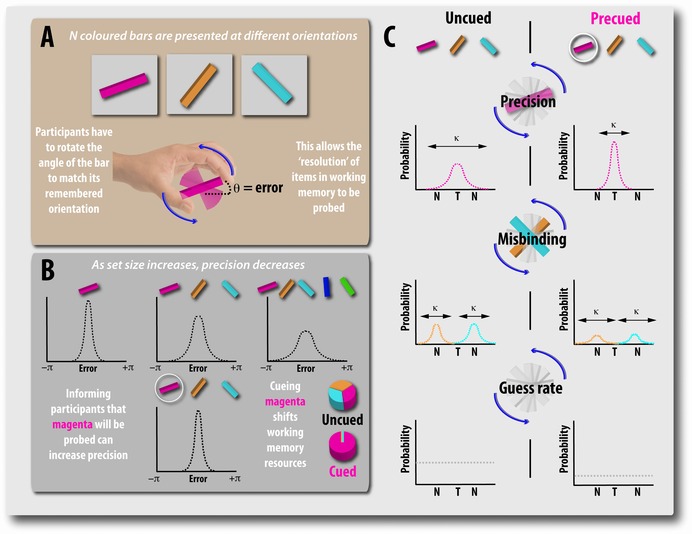
Recall precision: a new way to measure the fidelity of working memory. Sequential working memory task in which recall precision is measured on a continuous analog scale (e.g., Ref. 21). (A) Participants are presented with a series of bars, each of a different color. After a variable delay, they have to reproduce the orientation of the probed item (in this case, the magenta bar) by rotating it to match their memory. (B) Using such methods, it has become apparent that, as the number of items increases, the precision of recall decreases. Moreover, precueing the item by telling the participant which of the bars is going to be probed restores precision levels to that observed for one item. (C) These methods also allow participants’ recall error to be decomposed into three components. First, error can be due to imprecise memory of the target item, T (top). Second, error may result from being corrupted by other nontarget items (labeled N) in the sequences (middle). These so‐called misbinding errors occur when the orientation of one of the nonprobed items is erroneously reported (e.g., participant responds with the orientation of the orange or blue bar when probed with the magenta bar). Finally, error may result from guessing, in which case there will be no systematic relationship between the orientations of the memoranda and the participant's response (bottom).
A common method is to present participants with a series of items, for example, colored bars with different orientations (Fig. 1A), and, following a delay, ask them to reproduce the exact quality of one of these items, for example, the appropriate orientation.21 Using adjustment tasks, it is possible to obtain a measure of recall precision (i.e., the resolution with which items are maintained and later reported from WM). Studies using recall precision as the primary measure have also examined memory for spatial location, orientation, and motion direction, with items presented either simultaneously or consecutively in a sequence.21, 22, 23 Similar investigations have now also been performed in the auditory domain, using pitch or vowel sounds on a continuous, analog report space, demonstrating that this distributed resource model is not just applicable to visual WM.24, 25
These behavioral studies have all reported a gradual decline in recall precision with increasing number of items retained in memory, even with an increase of set size from one to two objects (i.e., below putative item capacity limits in “slot” models, which have claimed a hard limit of three or four items for visual WM). The key concept here is that, although WM is indeed limited, there is no item limit; it is simply that with increasing number of stored items, less resource can be devoted to each item, and hence recall precision falls. This decline in WM precision with increasing set size has been explained on the basis of noisier representations of items stored within a limited resource or neuronal pool.6 It has been modeled as decrease in the gain of neural activity in inverse proportion to the number of retained items.26 However, such an interpretation has been contested, with some researchers favoring a modified version of the slot model––so‐called “slots plus averaging”––to account for them.27
Data from a recent functional imaging study that employed multivoxel pattern analysis (MVPA) in occipital, parietal, and frontal cortex revealed coarsening of the fidelity of representations of stored items with increasing set size.28 Even the addition of one object to another held in memory has a significant effect on the quality of the representation of each stored item (Fig. 2). Furthermore, electrophysiological work in monkeys using recall precision as the report measure suggests that local field potentials during maintenance correlate with more precise memory representations.29 These findings would be consistent with the flexible but limited resource framework and, again, might also be reconciled within a modified slot model (see Ref. 27).
Figure 2.
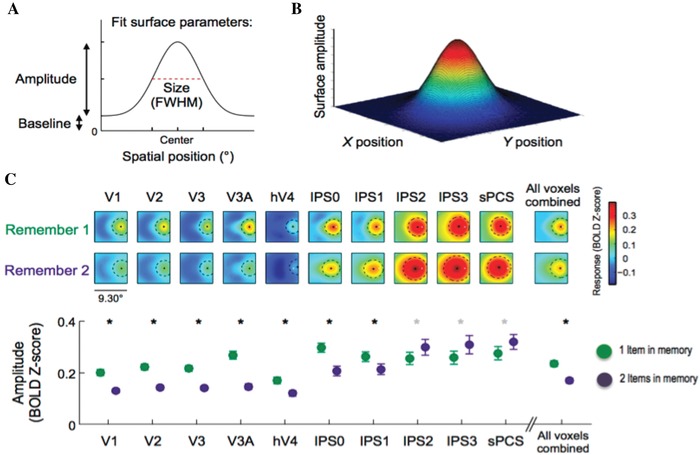
Cortical representations of one or two items held in working memory. (A and B) Two‐ and three‐dimensional representations of remembered locations were reconstructed using MVPA (multivoxel pattern analysis) from an fMRI study with a surface fitted to the average reconstruction. (C) Spatial representations in memory became coarser from V1 (primary visual cortex) through to parietal regions within the intraparietal sulcus (IPS0–3) and frontal eye fields (sPCS, superior precentral sulcus). Crucially, there was a fall in the amplitude of the representation from holding one to two items in memory across many early visual areas and some parietal regions. Adapted from Ref. 28.
Items compete with each other for WM resources
Delayed adjustment tasks introduced to probe recall precision also provide a means to dissect out sources of error contributing to performance, which may be a necessary prerequisite for understanding how resources are distributed between stored items. In such tasks, error in recall can potentially arise from three distinct sources, and statistical techniques, such as maximum likelihood estimation, have enabled researchers to examine how WM recall goes awry under different experimental manipulations (Fig. 1C).
First, error can be due to variability in memory for the probed feature (i.e., how well the orientation of the probed bar is stored and maintained (Fig. 1C)). Second, error can arise from misreporting features of nonprobed items that were presented in the original memory array, instead of reporting the features that belonged to the probed item. Such errors have been referred to as misbinding errors, because participants fail to correctly bind the color of the probed item with its orientation and instead reproduce the orientation of another item held in memory (Fig. 1C). In other words, a participant's response might be systematically corrupted by other objects encoded into WM.30 Finally, participants may make random errors because, on some trials, they are simply guessing (e.g., they failed to encode or retrieve the probed item).
Across many different types of experiment, it has now been established that, as the number of stored items increases, so the variability in memory for the encoded feature increases, but importantly, in addition, the proportion of trials where participants respond by reporting a feature belonging to one of the other items in memory––misbinding––also increases.21, 22, 30, 31 Pathological rates of misbinding turn out to be associated with focal lesions of the medial temporal lobe,32 which we discuss later in the section on the relationship to LTM and the hippocampus.
Top‐down goals and the frontal cortex can flexibly bias resource allocation
Although items can compete with each other for resources, it is rarely the case that all objects within an array compete with each other on a level playing field. The existence of top‐down goals and expectations will lead to certain items being prioritized over others. Within the framework of the resource model of WM, it has been shown that, if goals are altered by cuing an item to be most task relevant (Fig. 1B), a greater share of memory resources will be allocated to it––inferred from improved recall precision. But, crucially, this comes at a cost to other items held in memory, as would be predicted if WM resource is a highly limited resource.21, 24, 31, 33
Findings from early lesion, electrophysiological recording, and neuroimaging studies34, 35, 36 suggested that the lateral frontal cortex is essential, if not synonymous, with both executive control over WM and storage of memoranda. While these studies undoubtedly served to underscore the importance of the prefrontal cortex, the cortical real estate involved in WM has significantly expanded in recent years. Instead of acting as both the store and organizer of WM, the prefrontal cortex is now widely characterized as responsible for biasing processing in specialized, stimulus‐specific posterior cortical areas37 or as responsible for resource allocation.29, 38
In WM research, commonly used stimuli to probe brain responses include faces and scenes that are processed in distinctly different regions: the fusiform face area and the parahippocampal place area, respectively.39, 40, 41 Techniques such as MVPA have allowed researchers to probe the neural representation by examining patterns of activation across voxels. Using this method, WM representations have been decoded from patterns of activity in the visual cortex, across the life span of retained items.42, 43 Furthermore, activity in face‐sensitive and scene‐sensitive areas of the cortex varies according to whether information had to be attended to or ignored.44 Several subsequent studies have reported associations between measures of connectivity between frontal and posterior sensory regions and susceptibility to distraction in WM.45, 46, 47 Moreover, incentivizing the need to exert top‐down control through providing financial bonuses or losses not only enhanced performance but also augmented the blood oxygen level–dependent (BOLD) signal and connectivity measures.48, 49
Transcranial magnetic stimulation (TMS) over the right inferior frontal region both disrupts electrophysiological markers of top‐down control in posterior cortex and significantly impairs WM performance.50 Similarly, utilizing a faces‐versus‐scenes design, it has been shown that TMS over the right dorsolateral prefrontal cortex (DLPFC) modulated activity in regions associated with processing the relevant category, but did not alter activity in those regions processing the irrelevant category.51 In addition, the dopamine agonist bromocriptine increased the disruptive effect of congruent (face) versus incongruent (scene) distractors, accompanied by reduced connectivity between frontal regions and the fusiform face area.52 Cumulatively, these findings provide strong evidence for the idea that the prefrontal cortex is responsible for providing top‐down control. However, some recent findings53 suggest that caution should be exercised in assuming that there are separate systems for exerting top‐down control and maintaining feature‐specific information.
Expectations and temporal coordination of resources in WM
Expectations and assumptions permeate all of our daily activities. Neural resources devoted to a certain task or stimulus may depend on what came before and what is expected to happen next. The last few years have witnessed a huge growth in studies of the role of predictions and expectation in shaping our mental lives.54, 55, 56, 57 Research on WM has not been isolated from this trend. A number of investigations have examined how neural or psychological resources can be flexibly shifted and deployed to meet behavioral demands according to predictions or expectations and hence maximize utilization of WM resources. This view of a flexible set of regions underpinning WM is not new. It has long been proposed that a coalition of brain structures may emerge to perform a specific task, rather than the task resulting from neurons dedicated to perform only one.58
The impact of expectations and predictions in shaping WM can readily be appreciated when clues about which items are going to be probed are provided. For example, interference and competition between items, in both encoding and maintenance, can be overcome by directing attention to one or more of the memoranda. Cueing an item (via its location or color) before encoding (precueing) results in improved precision of recall for that item, but, crucially, with a concurrent cost to recall of other objects in the memory array21 (Fig. 1B and C). Importantly, the increase in memory resolution with cueing can be explained both by a decrease in variability in storage of the maintained feature and an increased probability of misbinding errors, suggesting that the bound representation of the cued item is made more resistant to interference from other, task‐irrelevant items at encoding (Fig. 1B and C).
Cues are effective not only before encoding but also if provided long after stimuli have disappeared, thus demonstrating that resources can be shifted between items already stored in WM. Studies using such retrospective retro‐cues 59 have shown that an item can be protected from interference throughout the delay interval.60 Recently, precueing and retro‐cueing were found to be associated with both similar and distinct neural mechanisms.61 Although both cues had similar effects on oscillations in the alpha band, they produced differences in event‐related potentials (ERPs). Precueing produced changes in ERPs associated with anticipatory attention. In contrast, retro‐cueing was associated with early selection ERPs, such as increased N1 and N2, which are likely to reflect selection of items within WM.62 Thus, increased information about which items will be probed can aid dynamic shifts of memory resource and thereby decrease competition between items at encoding, as well as protect the temporal stability of cued objects.
Recent findings also show that cues may lead to an item being held in different representational states (e.g., see Ref. 63). Specifically, an object might be held in a more prioritized state by directing attention toward it, placing it inside the so‐called focus of attention (FoA) and thereby allowing the prioritized item to be accessed with higher accuracy and/or fidelity. Moreover, imaging studies using the MVPA technique have demonstrated that prioritized items can be decoded more accurately compared to other memoranda with trial‐by‐trial fluctuations in neural dynamics predictive of performance.64, 65 Importantly, the remaining items, although considered to be in different representational states, are still retrievable and have a continued influence on WM processing.
In contrast to classical models of WM in which retention is achieved through persistent neuronal firing, it has been argued that the neural mechanisms supporting retention might be achieved through highly dynamic population coding.66, 67 Causal evidence for different representational states in WM was provided in a recent study22 that demonstrated that recall precision for items in WM is differentially affected by TMS over the sensory cortex (Fig. 3). Items inside the FoA were most vulnerable to stimulation of the visual cortex. Importantly, nonprivileged items were not only retrievable from memory, but were recalled with higher precision following TMS. These findings suggest that the strength of the privileged item representation was weakened after TMS to the visual cortex, effectively reducing interference from this object on nonprivileged memoranda, thereby resulting in an increase in their recall precision. Investigations of tactile WM similarly suggest that an item in the FoA may be held within the primary sensory cortex.68
Figure 3.
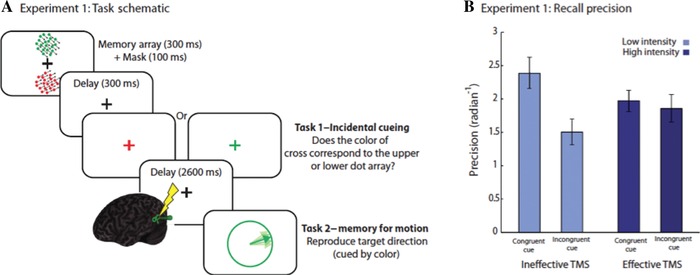
Manipulation of representational states in working memory. (A) Schematic of a task manipulating states of items in WM for motion direction. Participants had to remember the direction of two random dot kinematograms (RDK): one red and the other green. During the delay, they were “incidentally cued” by being asked to make a judgment orthogonal to the memory task: the location of one of the items had to be reported, bringing it into the focus of attention or privileged state. TMS was then applied to the visual area MT before recall for one of the RDKs being probed. If this was the same color as the incidental cue (congruent condition), recall was better than if it was not (incongruent). (B) Recall precision following TMS was reduced for the item in the privileged state, while performance for the unprivileged items actually improved.22
In addition to work on the FoA within WM, a great deal of interest has been directed recently toward neurophysiological mechanisms associated with gating the entry of items into WM under different expectations (see Ref. 37 for a review). Neuronal oscillations in the alpha band (∼8–12 Hz) during the delay period increase in a load‐dependent fashion.69 These increases are frequently interpreted in terms of suppressing irrelevant information70 or functional inhibition.71 For example, the power of alpha oscillations increases in irrelevant areas of the cortex and decreases in relevant regions.72 Thus, within many of these accounts, alpha oscillations are conceptualized as the neural fingerprint of resource allocation––either due to enhanced processing of relevant information or diminished processing of irrelevant information. The control of these alpha oscillations is likely to come from frontal regions,73 consistent with the aforementioned idea that the frontal cortex is involved in controlling resource allocation.29, 38
In line with prior work on attention,74, 75 alpha oscillations have been reported to increase in expectation of the appearance of highly distracting, temporally predictable information during a WM task.76 Expectation of a strong versus weak distractor led to an increase in alpha power, and the strength of this modulation predicted recall performance (Fig. 4). Similarly, the phase of the alpha oscillations was also modulated in anticipation of strong distractors, with the magnitude of this modulation again related to behavioral performance. These phase adjustments were localized to frontal and posterior regions. Finally, as an empirical demonstration of the role of temporal predictions in facilitating WM, jittering the onset of the distractor (to negate the possibility for phase adjustments) resulted in impaired performance.76
Figure 4.
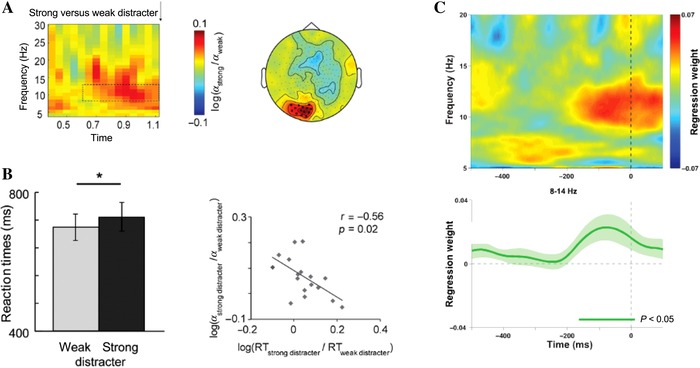
Alpha power and working memory performance. (A) Expectation of a strong versus weak distractor led to an increase in power in the alpha band (8–12 Hz). (B) This was associated with increased reaction times, with a significant correlation between changes in alpha power and reaction time according to distractor presence. Thus, individuals who increased their alpha power the most in anticipation of the distractor were also those who showed the least impairment.76 (C) A related study reported an increase in alpha power lateralized before a distractor appearance and correlating with WM performance for the preceding item. Alpha was higher in contralateral areas, which is generally taken to imply inhibition of the relevant area of cortex.77
In a different study that investigated the role of alpha oscillations in shaping recall, both alpha power and phase before a stimulus were related to recall precision.77 This finding provides a link between indices of neural oscillations in the alpha band and the fidelity of an item's representation in WM. However, although there was a relationship between alpha power changes and distractor processing as reported in Ref. 76, the relationship between phase adjustments and distractor was not found. The discrepancy might be due to differences in the predictability of the distractor in each study (blocked versus randomized distractor trials), which may have precluded the possibility of any phase adjustments.
The basal ganglia can guide why and how information is selected
Many investigators have utilized notions of relevant and irrelevant information without specifying the neural or psychological origin of this distinction or how these types of information can become distinct in the brain. Several researchers have argued that the basal ganglia's specific contribution to WM is in gating information.78, 79, 80 For example, an influential study reported that the globus pallidus was preferentially recruited when items have to be filtered out from WM during encoding.80 Increased BOLD signal in preparation for filtering was positively correlated with both WM capacity and physiological evidence for allowing distractors to enter memory. Furthermore, lesions of the basal ganglia––but not the frontal cortex––are associated with impaired filtering of items into WM, whereas frontal lesions lead to reduced WM capacity.81 Taken together, these findings strongly suggest that the basal ganglia gate access to WM representations.
Although the ventral parts of the basal ganglia, such as the nucleus accumbens, are usually associated with reward processing,82, 83 these regions may also be important in acquiring the attentional sets that are subsequently exploited within WM.84 As such, the basal ganglia might have a role in WM similar to that in reinforcement learning:79, 85 computing signals used to reinforce or discourage their antecedent behaviors. For example, information about the relevance or irrelevance of features in the environment is most readily gleaned from their correlation with reward, which can be used to guide WM control processes.79, 86 Indeed, using face‐versus‐scene designs, it has been shown that reward is capable of sculpting attention, tuning it toward certain features in the environment while tuning out other features.87, 88, 89
A mnemonic role for reward‐related signals in the basal ganglia is supported by findings that report that the magnitude of these responses during the presentation of memoranda is directly related to retention of relevant information across time.90, 91 The striatum may support such a mechanism through credit assignment,92 in which the occurrence of a reward is paired with the appropriate stimulus. This property of the ventral striatum was elegantly demonstrated by the finding that reward‐related signals boost activity in stimulus‐specific areas of cortex.92 For example, reward receipt after viewing a face increased activity in an area of the fusiform face region. Thus, these reward‐related striatal signals might engender the top‐down control signals that emanate from the frontal cortex to bias processing in the posterior cortices, a property that other nodes in the basal ganglia have been found to possess.93, 94
Computation models have predicted how reward‐related signaling in the basal ganglia might gate information.79 One recent study examined how reward‐related processing modulates the efficacy with which memoranda can be prevented from entering WM or updates the contents of memory by displacing previously stored items.95 Ignoring and updating recruited distinct constellations of cortical and subcortical regions (Fig. 5). While the dorsal striatum was preferentially active during update compared to ignore trials, the DLPFC was relatively more active for ignore versus update trials. Importantly, a greater BOLD response in the ventral striatum––and the DLPFC––for wins, compared to losses, was associated with enhanced distractor resistance but impaired updating. An additional connectivity analysis revealed that BOLD signal in the ventral striatum was positively coupled to regions of cortex associated with distractor resistance. This provides a mechanism through which striatal responses to salient events can affect WM processing at the cortical level to bias gating in WM. Thus, the basal ganglia seem to play an important modulatory role in the control of control.79
Figure 5.
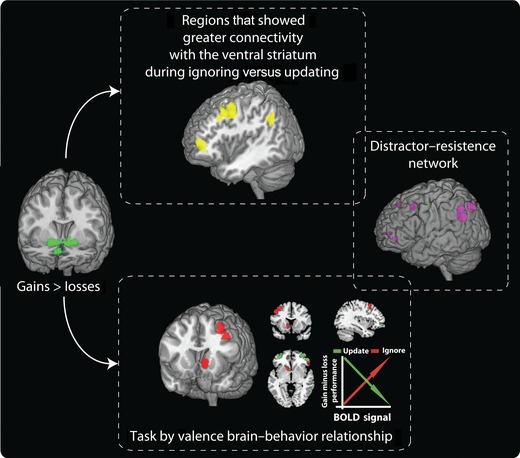
Effects of reward on ignoring versus updating information in working memory. The receipt of an unexpected financial gain relative to a loss during the delay period led to increased BOLD signal in the ventral striatum (left image). The extent to which ignoring and updating items in WM were differentially affected by reward receipt was related to the magnitude of BOLD signal changes in the ventral striatum, left DLPFC, and left frontopolar cortex (bottom image). In addition, there was increased connectivity between the ventral striatum and the left DLPFC and frontopolar cortex when participants had to ignore information compared to when they had to update WM. Regions coupled to the ventral striatum during ignore events that showed a brain–behavior relationship overlapped with a distractor–resistance network (right images). Results from Ref. 95.
The hippocampus, binding, and interactions between WM and LTM
The traditional view of brain regions involved in memory has been that medial temporal lobe (MTL) regions, such as the hippocampus, have an exclusive role in LTM, while WM is supported by the frontoparietal cortex. Recently, however, several lines of evidence have suggested that MTL regions might also be involved in retention of information over short periods of time, although whether the functions of these regions contribute to the short‐term retention of information in WM remains highly controversial and intensely debated (for a spectrum of perspectives, see Refs. 96, 97, 98, 99, 100, 101, 102, 103).
One possible role for MTL regions such as the hippocampus in WM is to support binding of information. In a recent functional magnetic resonance imaging (fMRI) study, successful maintenance of object–spatial binding was associated with the integrity of the pattern of information across both encoding and delay periods only in the anterior hippocampus.104 Participants learned to associate a household object with a particular location on a grid. The imaging results suggested that, while the perirhinal cortex (PRC) coded for objects and the parahippocampal and posterior hippocampus coded for locations, the anterior hippocampus crucially coded for the conjunction––feature binding––of an item and location.
Another important source of evidence highlighting the role of the MTL specifically in maintenance of feature binding in WM comes from lesion studies.32, 96, 97, 98, 99, 105, 106 Recently, it was demonstrated that binding of objects to locations is impaired in patients with focal damage to the MTL.32 In this investigation, patients were presented with a set of colored shapes (fractals) and asked to keep in mind both their identity and location. Following a short delay, they first identified the item they had previously seen in memory (in a two‐alternative forced choice task) and then dragged it on the touchscreen to its remembered location (location recall precision) (Fig. 6). Although patients with focal MTL lesions performed similarly to healthy controls on memory for object identity, they were significantly worse at remembering the location of items. Importantly, further analysis demonstrated that this deficit could be entirely explained by a specific increase in misbinding errors, here manifested by misbinding object location and identity. Thus, MTL patients showed an increased tendency to drag a remembered object to the location of one of the other items they held in WM. These errors have been termed swap errors because object locations were effectively swapped in memory.
Figure 6.
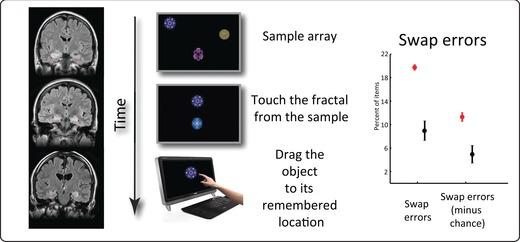
Misbinding in working memory following medial temporal lesions. Patients with focal damage to the MTL were tested on a “What was where?” touchscreen task. While their memory for object identity was good, their recall precision for object location was significantly impaired. However, all of their deficits could be explained by misbinding or swap errors: they were more likely to report the location of the probed item as being that of one of the other (nonprobed) objects held in memory. Adapted from Ref. 32.
Such findings are in line with reports of binding errors observed in patients with Alzheimer's disease (AD) and more recently in asymptomatic carriers of a familial AD gene.107 Similarly, individuals with mutations in the lysosomal enzyme glucocerebrosidase, who are known to have pathological changes to their MTLs, showed increased misbinding errors, whereas, by contrast, patients with Parkinson's disease have a signature of increased random responses in recall.108
Although it is now widely recognized that the hippocampus contributes to the short‐term retention of information, not all investigators agree that these functions should be called WM. Instead, some have argued that performance is impaired in hippocampal patients only when the WM capacity is breached.100, 101 According to this view, patients perform just as well as healthy controls below a capacity limit of three items. However, when more items have to be stored, LTM needs to be used. However, if the hippocampal LTM system is disrupted, performance will fall with memory loads of four or more, because LTM can no longer be accessed. Thus, the apparent impairment in WM observed with higher memory loads in hippocampal patients is argued to be due to impaired LTM. However, such an explanation would predict that patients would make random errors when storing items above the WM capacity limit, because none of the features would be stored for some items. Instead, they make systematic misbinding or swap errors, which suggests that, although features are stored in hippocampal patients, their bindings are fragile.32 Moreover, deficits in binding can be shown for loads at or below the putative capacity limit of three items.32, 96, 99, 105, 106
Baddeley and colleagues studied a developmental amnesic with selective bilateral hippocampal volume loss.109 They were unable to demonstrate any deficit of binding, either in the visual or verbal domain. It is possible that binding deficits require much more extensive MTL disruption or that compensatory mechanisms might have allowed relatively normal binding in this case. But the authors also discuss the potential flaws in some of the studies that have reported binding deficits in WM, including the fact that many investigations have used spatial location as a feature. Given the potentially important role of the hippocampus in spatial memory, they argue that the apparent binding deficit might actually be due to impaired spatial memory. Consistent with this view, one imaging study in humans has shown right hippocampal activation for object–location associations, but not for object–color binding or for single items.110 This type of objection might indeed be applied to many of the lesion studies that report WM binding deficits, but not to all.32 Clearly, this is a highly controversial area that remains to be fully resolved. Meanwhile, some authors have used the findings reported here to argue that the crucial role of the hippocampus is high‐resolution binding, regardless of the maintenance delay––short or long.111
Another line of research has suggested that MTL regions might play a role in aspects of WM other than binding. For example, in tasks using sequential presentation of items, recognition of the last item is accompanied by less hippocampal activation compared to recognition of items presented earlier in the sequence,112 suggesting a specific role for the hippocampus in maintenance of items outside the FoA. In other words, the hippocampus might play a role in storing nonprivileged items in WM, as supported by state‐dependent models of WM.13, 113 Other subregions within the MTL––specifically the entorhinal cortex (EC), parahippocampal cortex (PHC), and PRC––might also play active roles in the maintenance of items in WM, as suggested by some computational models that argue that sustained activity in these regions might facilitate coding of novel items into LTM (reviewed in Ref. 114). Intriguingly, a high‐resolution fMRI study reported load‐dependent modulation of activity with greater signal change in the hippocampus during encoding, but greater activity in the EC, PRC, and PHC during WM maintenance.115 Thus, there might be separate contributions of different MTL subregions to WM processes.
Together, these findings point to overlapping constraints as well as neural correlates between LTM and WM. However, the exact relationship between WM and LTM representations and their influences on each other, as well as other cognitive processes such as perception, remains unclear. Behaviorally, some recent research has reported that recall precision in WM and LTM might be very similar, pointing toward a common limit constraining both of these processes.2 Furthermore, competition for neural recourses in WM can weaken their representations in LTM,116 highlighting the close relationship between both systems. In a similar vein, dopaminergic modulation of WM was found to be mirrored in the effect the drug had on the long‐term recall of that information.117 This relationship between WM and LTM is likely to be the focus of intense research in forthcoming years.
Conclusions
In this brief review, we have attempted to cover some of the key developing trends in the field. It should be apparent that, far from being “done and dusted,” our understanding of WM mechanisms is in a state of evolution. Even fundamental issues, such as whether WM is quantal and limited in capacity to a small set of items, have been challenged in recent years. The development of paradigms that probe the quality of memory––rather than simply asking whether something is remembered or not––has proven to be highly significant. Delayed‐adjustment tasks that use a continuous, analog response space to measure recall precision have, in particular, posed some serious questions. Indeed, findings from behavioral studies have led to an alternative proposal about the architecture of WM. Several investigators now support the proposal that it might be best captured by a flexible resource that is highly limited, but without a fixed hard limit on the number of items that can be stored. Such a view would be consistent with decreased recall fidelity with increasingly noisier representations of retained items as set size increases within a limited neuronal pool. However, there is, as yet, no consensus, and a lively debate on how to arbitrate between the two hypotheses is in progress.
The questioning of the established view of WM has already begun to have an impact on the design of imaging and neurophysiological studies aimed at examining mechanisms underpinning encoding, storage, and dynamic shifts of priorities with altered expectations and predictions. The new methods have also provided tools to examine in more detail the proposal that some items––often only one––might be held in a privileged state or FoA in WM that has a distinct neural substrate. They have also demonstrated that it is possible to shift resources dynamically between stored objects in a flexible manner, bringing the fields of attention and WM research even closer.
These studies are being performed against a background of investigations that makes it increasingly apparent that it is difficult to frame WM as being supported simply by frontal or frontoparietal brain regions. A substantial body of work now makes it clear that early sensory regions, parts of the basal ganglia and, controversially, even the hippocampus––long considered to be a brain area that is not involved in WM––might play key and differing roles, depending upon task requirements. Some of this work challenges the notion of distinct differences between WM and LTM, or at least questions where that division lies. Finally, there is an emerging area of research that has begun to examine the role of cortical oscillations in modulating WM activity across the brain.
This is clearly an exciting time to be involved in WM research and to reconsider what exactly we know about this key cognitive process. Many research groups are investigating the impact of training WM on wider cognitive abilities and in patients with brain disorders who have WM deficits. A better understanding of the fundamental architecture and the brain mechanisms underlying WM would have the potential to improve such endeavors.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
This work was funded by a Wellcome Trust Prinicipal Research Fellowship to MH.
References
- 1. Albers, A.M. , Kok P., Toni I., et al 2013. Shared representations for working memory and mental imagery in early visual cortex. Curr. Biol. 23: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 2. Brady, T.F. , Konkle T., Gill J., et al 2013. Visual long‐term memory has the same limit on fidelity as visual working memory. Psychol. Sci. 24: 981–990. [DOI] [PubMed] [Google Scholar]
- 3. Daneman, M. & Carpenter P.A.. 1980. Individual differences in working memory and reading. J. Verbal Learn. Verbal Behav. 19: 450–466. [Google Scholar]
- 4. Turner, M.L. & Engle R.W.. 1989. Is working memory capacity task dependent? J. Mem. Lang. 28: 127–154. [Google Scholar]
- 5. Gathercole, S.E. , Brown L. & Pickering S.J.. 2003. Working memory assessments at school entry as longitudinal predictors of National Curriculum attainment levels. Educ. Child Psychol. 20: 109–122. [Google Scholar]
- 6. Bays, P.M. 2015. Spikes not slots: noise in neural populations limits working memory. Trends Cogn. Sci. 19: 431–438. [DOI] [PubMed] [Google Scholar]
- 7. D'Esposito, M. & Postle B.R.. 2015. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 66: 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ester, E.F. , Vogel E.K. & Awh E.. 2012. “Discrete resource limits in attention and working memory.” In Cognitive Neuroscience of Attention, 2nd Edit. M. Posner, Ed.: 99–112. New York: Guilford. [Google Scholar]
- 9. Postle, B.R. 2015. The cognitive neuroscience of visual short‐term memory. Curr. Opin. Behav. Sci. 1: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baddeley, A. 2012. Working memory: theories, models, and controversies. Annu. Rev. Psychol. 63: 1–29. [DOI] [PubMed] [Google Scholar]
- 11. Barrouillet, P. & Camos V.. 2014. Working Memory: Loss and Reconstruction. Psychology Press. [Google Scholar]
- 12. Oberauer, K. & Hein L.. 2012. Attention to information in working memory. Curr. Direct. Psychol. Sci. 21: 164–169. [Google Scholar]
- 13. Cowan, N. 2011. The focus of attention as observed in visual working memory tasks: making sense of competing claims. Neuropsychologia 49: 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bays, P.M. & Husain M.. 2008. Dynamic shifts of limited working memory resources in human vision. Science 321: 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma, W.J. , Husain M. & Bays P.M.. 2014. Changing concepts of working memory. Nat. Neurosci. 17: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilken, P. & Ma W.J.. 2004. A detection theory account of change detection. J. Vis. 4: 1120–1135. [DOI] [PubMed] [Google Scholar]
- 17. Awh, E. , Barton B. & Vogel E.K.. 2007. Visual working memory represents a fixed number of items regardless of complexity. Psychol. Sci. 18: 622–628. [DOI] [PubMed] [Google Scholar]
- 18. Luck, S.J. & Vogel E.K.. 1997. The capacity of visual working memory for features and conjunctions. Nature 390: 279–281. [DOI] [PubMed] [Google Scholar]
- 19. Todd, J.J. & Marois R.. 2004. Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature 428: 751–754. [DOI] [PubMed] [Google Scholar]
- 20. Vogel, E.K. , Woodman G.F. & Luck S.J.. 2001. Storage of features, conjunctions, and objects in visual working memory. J. Exp. Psychol. Hum. Percept. Perform. 27: 92–114. [DOI] [PubMed] [Google Scholar]
- 21. Gorgoraptis, N. , Catalao R.F.G., Bays P.M. & Husain M.. Dynamic updating of working memory resources for visual objects. J. Neurosci. 31: 8502–8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zokaei, N. , Manohar S., Husain M. & Feredoes E.. 2014. Causal evidence for a privileged working memory state in early visual cortex. J. Neurosci. 34: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zokaei, N. , Ning S., Manohar S., et al 2014. Flexibility of representational states in working memory. Front. Hum. Neurosci. 8: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph, S. , Iverson P., Manohar S., et al 2015. Precision of working memory for speech sounds. Q. J. Exp. Psychol. 68: 2022–2040. [DOI] [PubMed] [Google Scholar]
- 25. Kumar, S. , Joseph S., Pearson B., et al 2013. Resource allocation and prioritization in auditory working memory. Cogn. Neurosci. 4: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bays, P.M. 2014. Noise in neural populations accounts for errors in working memory. J. Neurosci. 34: 3632–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang, W. & Luck S.J.. 2008. Discrete fixed‐resolution representations in visual working memory. Nature 453: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sprague, T.C. , Ester E.F. & Serences J.T.. 2014. Reconstructions of information in visual spatial working memory degrade with memory load. Curr. Biol. 24: 2174–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lara, A.H. & Wallis J.D.. 2014. Executive control processes underlying multi‐item working memory. Nat. Neurosci. 17: 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bays, P.M. , Catalao R.F.G. & Husain M.. 2009. The precision of visual working memory is set by allocation of a shared resource. J. Vis. 9:7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bays, P.M. , Wu E.Y. & Husain M.. 2011. Storage and binding of object features in visual working memory. Neuropsychologia 49: 1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pertzov, Y. , Miller T.D., Gorgoraptis N., et al 2013. Binding deficits in memory following medial temporal lobe damage in patients with voltage‐gated potassium channel complex antibody‐associated limbic encephalitis. Brain 136: 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zokaei, N. , Gorgoraptis N., Bahrami B., et al 2011. Precision of working memory for visual motion sequences and transparent motion surfaces. J. Vis. 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Funahashi, S. , Bruce C.J. & Goldman‐Rakic P.S.. 1993. Dorsolateral prefrontal lesions and oculomotor delayed‐response performance: evidence for mnemonic “scotomas.” J. Neurosci. 13: 1479–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fuster, J.M. & Alexander G.E., 1971. Neuron activity related to short‐term memory. Science 173: 652–654. [DOI] [PubMed] [Google Scholar]
- 36. McCarthy, G. , Puce A., Constable T., et al 1996. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb. Cortex 6: 600–611. [DOI] [PubMed] [Google Scholar]
- 37. Gazzaley, A. & Nobre A.C.. 2012. Top‐down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 16: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadohisa, M. , Petrov P., Stokes M., et al 2013. Dynamic construction of a coherent attentional state in a prefrontal cell population. Neuron 80: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ranganath, C. , DeGutis J. & D'Esposito M.. 2004. Category‐specific modulation of inferior temporal activity during working memory encoding and maintenance. Cogn. Brain Res. 20: 37–45. [DOI] [PubMed] [Google Scholar]
- 40. Sala, J.B. , Rämä P. & Courtney S.M.. 2003. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia 41: 341–356. [DOI] [PubMed] [Google Scholar]
- 41. Yovel, G. & Kanwisher N.. 2004. Face perception: domain specific, not process specific. Neuron 44: 889–898. [DOI] [PubMed] [Google Scholar]
- 42. Curtis, C.E. & D'Esposito M.. 2003. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 7: 415–423. [DOI] [PubMed] [Google Scholar]
- 43. Sreenivasan, K.K. , Curtis C.E. & D'Esposito M.. 2014. Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 18: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gazzaley, A. , Cooney J.W., McEvoy K., et al 2005. Top‐down enhancement and suppression of the magnitude and speed of neural activity. J. Cogn. Neurosci. 17: 507–517. [DOI] [PubMed] [Google Scholar]
- 45. Clapp, W.C. , Rubens M.T. & Gazzaley A.. 2009. Mechanisms of working memory disruption by external interference. Cereb. Cortex 20: 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gazzaley, A. , Rissman J., Cooney J., et al 2007. Functional interactions between prefrontal and visual association cortex contribute to top‐down modulation of visual processing. Cereb. Cortex 17: i125–i135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon, J.H. , Curtis C.E. & D'Esposito M.. 2006. Differential effects of distraction during working memory on delay‐period activity in the prefrontal cortex and the visual association cortex. Neuroimage 29: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 48. Krawczyk, D.C. & D'Esposito M.. 2013. Modulation of working memory function by motivation through loss‐aversion. Hum. Brain Mapp. 34: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krawczyk, D.C. , Gazzaley A. & D'Esposito M.. 2007. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 1141: 168–177. [DOI] [PubMed] [Google Scholar]
- 50. Zanto, T.P. , Rubens M.T., Thangavel A. & Gazzaley A.. 2011. Causal role of the prefrontal cortex in top‐down modulation of visual processing and working memory. Nat. Neurosci. 14: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feredoes, E. , Heinen K., Weiskopf N., et al 2011. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci. U.S.A. 108: 17510–17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bloemendaal, M. , van Schouwenburg M.R., Miyakawa A., et al 2015. Dopaminergic modulation of distracter‐resistance and prefrontal delay period signal. Psychopharmacology (Berl) 232: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 53. Ester, E.F. , Sprague T.C. & Serences J.T.. 2015. Parietal and frontal cortex encode stimulus‐specific mnemonic representations during visual working memory. Neuron 87: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. den Ouden, H.E. , Kok P. & de Lange F.P.. 2012. How prediction errors shape perception, attention, and motivation. Front. Psychol. 3: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Friston, K . 2009. The free‐energy principle: a rough guide to the brain? Trends Cogn. Sci. 13: 293–301. [DOI] [PubMed] [Google Scholar]
- 56. Rohenkohl, G. , Cravo A.M., Wyart V. & Nobre A.C.. 2012. Temporal expectation improves the quality of sensory information. J. Neurosci. 32: 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schiffer, A.‐M. , Waszak F. & Yeung N.. 2015. The role of prediction and outcomes in adaptive cognitive control. J. Physiol. Paris 109: 38–52. [DOI] [PubMed] [Google Scholar]
- 58. Postle, B.R . 2006. Working memory as an emergent property of the mind and brain. Neuroscience 139: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Griffin, I.C. & Nobre A.C.. 2013. Orienting attention to locations in internal representations. J. Cogn. Neurosci. 15: 1176–1194. [DOI] [PubMed] [Google Scholar]
- 60. Pertzov, Y. , Bays P.M., Joseph S. & Husain M.. 2013. Rapid forgetting prevented by retrospective attention cues. J. Exp. Psychol. Hum. Percept. Perform. 39: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Myers, N.E. , Walther L., Wallis G., et al 2015. Temporal dynamics of attention during encoding versus maintenance of working memory: complementary views from event‐related potentials and alpha‐band oscillations. J. Cogn. Neurosci. 27: 492–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuo, B.‐C. , Rao A., Lepsien J. & Nobre A.C.. 2009. Searching for targets within the spatial layout of visual short‐term memory. J. Neurosci. 29: 8032–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Souza, A.S. , Rerko L. & Oberauer K.. 2015. Refreshing memory traces: thinking of an item improves retrieval from visual working memory. Ann. N.Y. Acad. Sci. 1339: 20–31. [DOI] [PubMed] [Google Scholar]
- 64. Lewis‐Peacock, J.A. , Drysdale A.T., Oberauer K. & Postle B.R.. 2012. Neural evidence for a distinction between short‐term memory and the focus of attention. J. Cogn. Neurosci. 24: 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lewis‐Peacock, J.A. , Drysdale A.T. & Postle B.R.. 2015. Neural evidence for the flexible control of mental representations. Cereb. Cortex 25: 3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stokes, M.G. , Kusunoki M., Sigala N., et al 2013. Dynamic coding for cognitive control in prefrontal cortex. Neuron 78: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stokes, M.G. 2015. “Activity‐silent” working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn. Sci. 19: 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Katus, T. & Eimer M.. 2015. Lateralized delay period activity marks the focus of spatial attention in working memory: evidence from somatosensory event‐related brain potentials. J. Neurosci. 35: 6689–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jensen, O. , Gelfand J., Kounios J. & Lisman J.E.. 2002. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short‐term memory task. Cereb. Cortex 12: 877–882. [DOI] [PubMed] [Google Scholar]
- 70. Klimesch, W. , Sauseng P. & Hanslmayr S.. 2007. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 53: 63–88. [DOI] [PubMed] [Google Scholar]
- 71. Jensen, O. & Mazaheri A.. 2010. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zumer, J.M. , Scheeringa R., Schoffelen J.‐M., et al 2014. Occipital alpha activity during stimulus processing gates the information flow to object‐selective cortex. PLoS Biol 12: e1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marshall, T.R. , O'Shea J., Jensen O. & Bergmann T.O.. 2015. Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipitoparietal cortex. J. Neurosci. 35: 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kelly, S.P. , Lalor E.C., Reilly R.B. & Foxe J.J.. 2006. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J. Neurophysiol. 95: 3844–3851. [DOI] [PubMed] [Google Scholar]
- 75. Worden, M.S. , Foxe J.J., Wang N. & Simpson G.V.. 2000. Anticipatory biasing of visuospatial attention indexed by retinotopically specific‐band electroencephalography increases over occipital cortex. J. Neurosci. 20: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bonnefond, M. & Jensen O.. 2012. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol. 22: 1969–1974. [DOI] [PubMed] [Google Scholar]
- 77. Myers, N.E. , Stokes M.G., Walther L. & Nobre A.C.. 2014. Oscillatory brain state predicts variability in working memory. J. Neurosci. 34: 7735–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gruber, A.J. , Dayan P., Gutkin B.S. & Solla S.A.. 2006. Dopamine modulation in the basal ganglia locks the gate to working memory. J. Comput. Neurosci. 20: 153–166. [DOI] [PubMed] [Google Scholar]
- 79. Hazy, T.E. , Frank M.J. & O'Reilly R.C.. 2007. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos. Trans. R. Soc. B Biol. Sci. 362: 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McNab, F. & Klingberg T.. 2008. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 11: 103–107. [DOI] [PubMed] [Google Scholar]
- 81. Baier, B. , Karnath H.‐O., Dieterich M., et al 2010. Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J. Neurosci. 30: 9788–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Delgado, M.R. , Nystrom L.E., Fissell C., et al 2000. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 84: 3072–3077. [DOI] [PubMed] [Google Scholar]
- 83. Sescousse, G. , Caldú X., Segura B. & Dreher J.‐C.. 2013. Processing of primary and secondary rewards: a quantitative meta‐analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 37: 681–696. [DOI] [PubMed] [Google Scholar]
- 84. Astle, D.E. , Nobre A.C. & Scerif G.. 2009. Applying an attentional set to perceived and remembered features. PLoS One 4: e7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chatham, C.H. & Badre D.. 2013. Working memory management and predicted utility. Front. Behav. Neurosci. 7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suri, R.E. & Schultz W.. 1999. A neural network model with dopamine‐like reinforcement signal that learns a spatial delayed response task. Neuroscience 91: 871–890. [DOI] [PubMed] [Google Scholar]
- 87. Fallon, S.J. , Williams‐Gray C.H., Barker R.A., et al 2013. Prefrontal dopamine levels determine the balance between cognitive stability and flexibility. Cereb. Cortex 23: 361–369. [DOI] [PubMed] [Google Scholar]
- 88. Hampshire, A. , Gruszka A., Fallon S.J. & Owen A.M.. 2008. Inefficiency in self‐organized attentional switching in the normal aging population is associated with decreased activity in the ventrolateral prefrontal cortex. J. Cogn. Neurosci. 20: 1670–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hampshire, A. & Owen A.M.. 2006. Fractionating attentional control using event‐related fMRI. Cereb. Cortex 16: 1679–1689. [DOI] [PubMed] [Google Scholar]
- 90. Adcock, R.A. , Thangavel A. & Whitfield‐Gabrieli S., et al 2006. Reward‐motivated learning: mesolimbic activation precedes memory formation. Neuron 50: 507–517. [DOI] [PubMed] [Google Scholar]
- 91. Wittmann, B.C. , Schott B.H., Guderian S., et al 2005. Reward‐related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus‐dependent long‐term memory formation. Neuron 45: 459–467. [DOI] [PubMed] [Google Scholar]
- 92. Schiffer, A.‐M. , Muller T., Yeung N. & Waszak F.. 2014. Reward activates stimulus‐specific and task‐dependent representations in visual association cortices. J. Neurosci. 34: 15610–15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Schouwenburg, M.R. , den Ouden H.E. & Cools R.. 2015. Selective attentional enhancement and inhibition of fronto‐posterior connectivity by the basal ganglia during attention switching. Cereb. Cortex 25: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 94. van Schouwenburg, M.R. , den Ouden H.E. & Cools R.. 2010. The human basal ganglia modulate frontal‐posterior connectivity during attention shifting. J. Neurosci. 30: 9910–9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fallon, S.J. & Cools R.. 2014. Reward acts on the pFC to enhance distractor resistance of working memory representations. J. Cogn. Neurosci. 26: 2812–2826. [DOI] [PubMed] [Google Scholar]
- 96. Olson, I.R. , Page K., Moore K.S., et al 2006. Working memory for conjunctions relies on the medial temporal lobe. J. Neurosci. 26: 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hannula, D.E. , Tranel D. & Cohen N.J.. 2006. The long and the short of it: relational memory impairments in amnesia, even at short lags. J. Neurosci. 26: 8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hartley, T. , Bird C.M., Chan D., et al 2007. The hippocampus is required for short‐term topographical memory in humans. Hippocampus 17: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Finke, C. , Bruehl H., Düzel E., et al 2013. Neural correlates of short‐term memory reorganization in humans with hippocampal damage. J. Neurosci. 33: 11061–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shrager, Y. , Levy D.A., Hopkins R.O. & Squire L.R.. 2008. Working memory and the organization of brain systems. J. Neurosci. 28: 4818–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jeneson, A. & Squire L.R.. 2012. Working memory, long‐term memory, and medial temporal lobe function. Learn. Mem. 19: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Graham, K.S. , Barense M.D. & Lee A.C.. 2010. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia 48: 831–853. [DOI] [PubMed] [Google Scholar]
- 103. Nadel, L. & Hardt O.. 2011. Update on memory systems and processes. Neuropsychopharmacology 36: 251–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Libby, L.A. , Hannula D.E. & Ranganath C.. 2014. Medial temporal lobe coding of item and spatial information during relational binding in working memory. J. Neurosci. 34: 14233–14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Watson, P.D. , Voss J.L., Warren D.E., et al 2013. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus 23: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lavenex, P.A.B. , Colombo F., Lambert F.R. & Lavenex P.. 2014. The human hippocampus beyond the cognitive map: evidence from a densely amnesic patient. Front. Hum. Neurosci. 8: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Parra, M.A. , Saarimäki H., Bastin M.E., et al 2015. Memory binding and white matter integrity in familial Alzheimer's disease. Brain 138: 1355–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zokaei, N. , McNeill A., Proukakis C., et al 2014. Visual short‐term memory deficits associated with GBA mutation and Parkinson's disease. Brain 137: 2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Baddeley, A. , Allen R. & Vargha‐Khadem F.. 2010. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia 48: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 110. Piekema, C. , Kessels R.P., Mars R.B., et al 2006. The right hippocampus participates in short‐term memory maintenance of object–location associations. Neuroimage 33: 374–382. [DOI] [PubMed] [Google Scholar]
- 111. Yonelinas, A.P . 2013. The hippocampus supports high‐resolution binding in the service of perception, working memory and long‐term memory. Behav. Brain Res. 254: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Öztekin, I. , Davachi L. & McElree B.. 2010. Are representations in working memory distinct from representations in long‐term memory? Neural evidence in support of a single store. Psychol. Sci. 21: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Oberauer, K . 2009. Design for a working memory. Psychol. Learn. Motiv. 51: 45–100. [Google Scholar]
- 114. Lisman, J.E. & Jensen O.. 2013. The theta–gamma neural code. Neuron 77: 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schon, K. , Newmark R.E., Ross R.S. & Stern C.E.. 2015. A working memory buffer in parahippocampal regions: evidence from a load effect during the delay period. Cereb. Cortex [Epub ahead of print]. doi: 10.1093/cercor/bhv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lewis‐Peacock, J.A. & Norman K.A.. 2014. Competition between items in working memory leads to forgetting. Nat. Commun. 5: 5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Eckart, C. , Fuentemilla L., Bauch E.M. & Bunzeck N.. 2014. Dopaminergic stimulation facilitates working memory and differentially affects prefrontal low theta oscillations. Neuroimage 94: 185–192. [DOI] [PubMed] [Google Scholar]


