Figure 2.
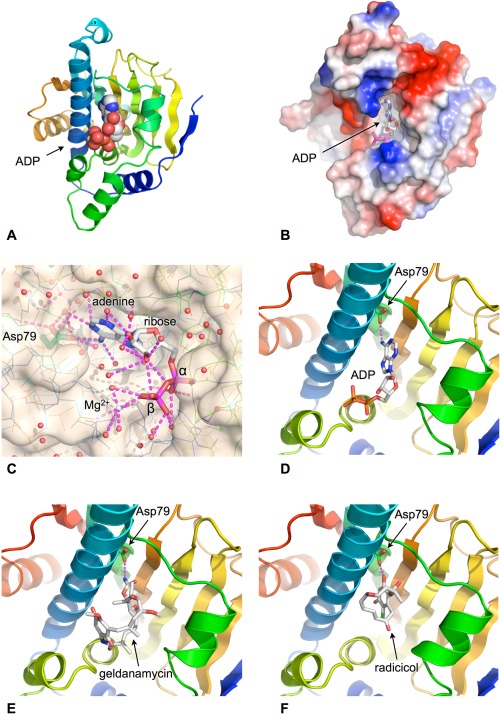
ATP‐binding site. A: ATP/ADP binds into a pocket formed in the helical face of the N‐terminal domain. Cartoon shows secondary structural elements rainbow colored according to reative position within the primary structure of the domain – blue: N‐terminus, red: C‐terminus. B: Molecular surface of HSP90 N‐domain colored according to electrostatic potential blue:+ve, red:‐ve. The adenine base is buried in the binding pocket with the phosphates exposed to solvent. C: Close up of the water‐filled nucleotide‐binding pocket. Polar interactions made by the bound ADP are shown as dashed lines. D: The adenine base makes only a single direct hydrogen bond with the protein, via the side chain of Asp 79. E: The natural product geldanamycin binds to the nucleotide‐binding pocket and is a competitive inhibitor of ATP binding by HSP90. F: The natural product radicicol binds to the nucleotide‐binding pocket and is a competitive inhibitor of ATP binding by HSP90.
