Abstract
The ‘MAlB’ phases are nanolaminated, ternary transition metal borides that consist of a transition metal boride sublattice interleaved by monolayers or bilayers of pure aluminum. However, their synthesis and properties remain largely unexplored. Herein, we synthesized dense, predominantly single-phase samples of one such compound, MoAlB, using a reactive hot pressing method. High-resolution scanning transmission electron microscopy confirmed the presence of two Al layers in between a Mo-B sublattice. Unique among the transition metal borides, MoAlB forms a dense, mostly amorphous, alumina scale when heated in air. Like other alumina formers, the oxidation kinetics follow a cubic time-dependence. At room temperature, its resistivity is low (0.36–0.49 μΩm) and – like a metal – drops linearly with decreasing temperatures. It is also a good thermal conductor (35 Wm−1K−1 at 26 °C). In the 25–1300 °C temperature range, its thermal expansion coefficient is 9.5 × 10−6 K−1. Preliminary results suggest the compound is stable to at least 1400 °C in inert atmospheres. Moderately low Vickers hardness values of 10.6 ± 0.3 GPa, compared to other transition metal borides, and ultimate compressive strengths up to 1940 ± 103 MPa were measured at room temperature. These results are encouraging and warrant further study of this compound for potential use at high temperatures.
Binary transition metal borides and carbides are among the hardest and most refractory materials known. In addition, many of them have a unique combination of mechanical, electronic, and thermal properties that make them technologically important for applications such as wear resistant coatings1, primary battery electrodes2,3, chemical catalysis4,5, and high-temperature structural materials6. However, their use, especially in bulk form, is often limited by high processing costs and, more importantly, poor oxidation resistance when heated in air.
In general, the aforementioned properties also apply to most ternary transition metal borides, carbides, and nitrides. Notable exceptions are the Mn+1AXn (MAX) phases, a family of layered early transition metal carbides and nitrides, where M is an early transition metal, A is a Group IIIA-IVA element, and X is C and/or N, and n = 1, 2 or 3 (Space Group P63/mmc)7. The crystal structure of the MAX phases comprises a ‘Mn+1Xn’ sublattice interleaved with monolayers of the ‘A’ element. This unique, layered structure results in properties that combine those of their MX binary carbide/nitride counterparts and transition metals making some MAX phases (e.g. Ti2AlC, Ti3SiC2) thermal shock resistant, readily machinable, resistant to high-temperature oxidation, mechanically rigid and plastic at high temperatures8,9,10.
Despite the extensive compositional variability possible for the MAX phases, phases where X = B do not exist. However, the M2AlB2-type (space group Cmmm) and MAlB-type (space group Cmcm) ternary transition metal borides – discovered by Jeitschko11,12–are close structural analogs to the MAX phases in that a transition metal boride sublattice is interleaved by one or two Al layers, respectively. To date, studies on these ternary borides have focused mostly on single-crystal growth and on determining their crystal structures. For example, Ade et al. recently synthesized single crystals of several previously reported M2AlB2 (M = Cr, Mn, Fe) and MAlB (M = Mo, W) compounds, and discussed their structural relationship to other transition metal borides and their similarity to the MAX phases13. Isostructural compounds with M-site solid solutions, viz. (Mox,Me1−x)AlB, where Me = Cr, W14 and (Fe2,Me2−x)AlB2, where Me = Cr, Mn, have been also recently synthesized15.
A few studies investigated the electronic and magnetocaloric properties of polycrystalline (Fe2,Me2−x)AlB2 samples16,17. Okada et al. reported that single crystals of (Mox,Cr1−x)AlB and (Mox,W1−x)AlB have low electrical resistivities that vary between 0.65 to 2.45 μΩm and Vickers hardness (HV) values ranging from 10 to 20 GPa. Differential thermal analysis (DTA) on powders revealed that MoAlB and WAlB powders are only stable in air up to 800 °C. Among these, MoAlB is particularly attractive to study in bulk form because of its known thermodynamic stability18, relatively lower hardness (HV = 10.3 GPa) compared to WAlB (HV = 19.3 GPa), and higher electrical conductivity than WAlB.
As noted above, one of the major disadvantages of all transition metal borides, both binary and ternary, known to date, is their propensity for oxidation when heated in air at high temperatures19. Given the structural similarities between the ternary compound MoAlB and the MAX phases, and the relatively high Al content in the former, it was postulated that, like Ti2AlC and Cr2AlC20,21, a protective alumina layer would form upon heating in air. As shown herein, our postulate was correct and heating polycrystalline samples of MoAlB in air, to temperatures as high as 1400 °C, resulted in formation of a passivating, mostly amorphous, alumina layer. The purpose of this paper is to report on the synthesis of MoAlB using a reactive hot pressing technique. In addition to studying its oxidation resistance, we also report on some of its electrical, thermal, and mechanical properties.
Results and Discussion
Synthesis
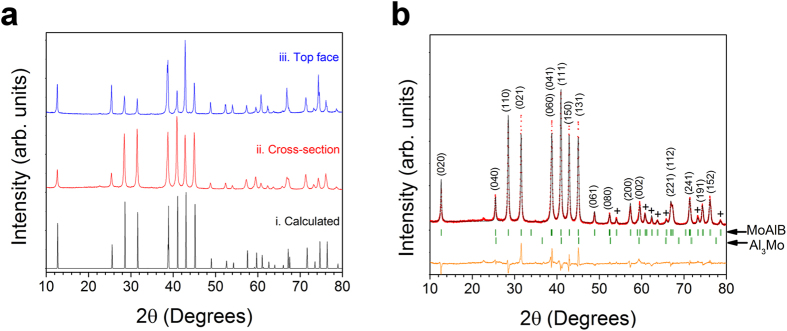
X-ray diffractograms of the hot-pressed sample’s polished cross-section (HP4) and polished top surface are in good agreement with the calculated diffractogram reported by Ade et al.13, as shown in Fig. 1a. However, the experimentally observed peak intensity ratios of the hot-pressed sample differed from those of the calculated diffractogram. The relatively higher intensities of the {0k0} peaks of the top surface compared to those of the cross-section indicate that hot pressing helped to preferentially orient the [010] axis of the grains parallel with the hot pressing direction. XRD also showed the presence of Al3Mo and an unidentified impurity with minor peaks at 22.8o and 43.9o. In contrast to HP4, the HP2 sample (see Methods) had more porosity and Al2O3 impurities, but negligible amounts of intermetallic impurities (Fig. S1a).
Figure 1.
(a) XRD diffractograms of (i) calculated 2θ positions, (ii) hot-pressed cross section, and, (iii) top surface of the hot-pressed sample; (b) Rietveld refinement of the HP4 cross-section’s diffractogram with the observed pattern (red), calculated pattern (black), and difference in observed and calculated intensities (orange). Green dashes show calculated 2θ positions for MoAlB (top row) and Al3Mo (bottom row). Diffraction peaks marked with (+) also belong to MoAlB.
Rietveld refinement on the polished HP4 cross-section’s diffractogram showed the sample to be predominantly single phase MoAlB with impurities of 3 vol.% Al3Mo (Fig. 1b). Lattice constants of a = 3.21 Å, b = 13.98 Å, and c = 3.10 Å were obtained from Rietveld refinement and compared well those reported for MoAlB by Ade et al. and Okada13,22. A χ2 value of 8.9 was obtained for the refinement despite accounting for all major peaks. This high χ2 value is presumably due to a preferred orientation relationship too complex to be determined by 1-dimensional XRD used herein. Indirect evidence for this conjecture is the fact that Rietveld refinement for MoAlB powders, synthesized in the tube furnace, showed a much better fit (χ2 = 3.9). In this case, the powder was predominantly single phase, with Al2O3 (8 vol.%) and unreacted Al (2 vol.%) as impurities (Fig. S2). Not surprisingly, in contrast to the hot-pressed sample, the experimentally observed peak intensity ratios were quite similar to the calculated diffractogram (Fig. S2). When the refined lattice constants of the sample HP4 and the powders are compared with previous results (Table S1), excellent agreement is found.
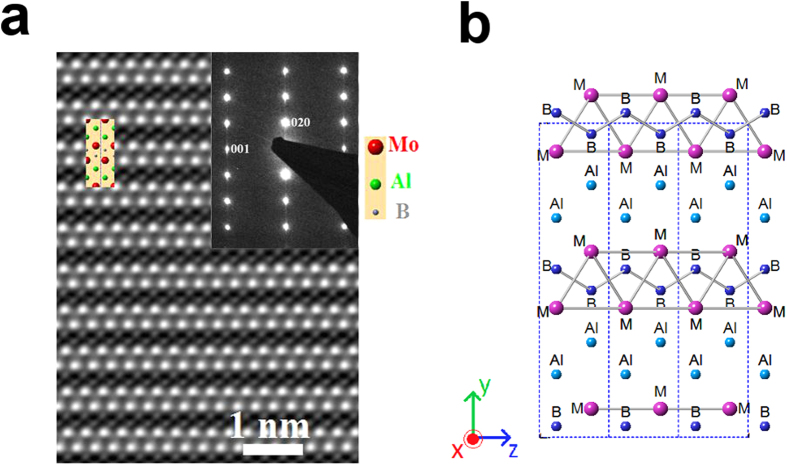
In Fig. 2a, high-resolution scanning transmission electron microscopy (HRSTEM) along the [100] zone axis further confirms that the atomic layering - shown in Fig. 2b - is correct. Selected area electron diffraction (SAED) along the [100] zone axis (inset of Fig. 2a) confirms the orthorhombic symmetry of MoAlB. Interestingly, some grains contain stacking faults in which only one Al layer, instead of two, is sandwiched by the Mo-B layers (Fig. S3). However, the density of such stacking faults is low as judged by the weak diffraction streaks along [010] in the SAED pattern.
Figure 2.
(a) HRSTEM image of MoAlB along the [100] zone axis. Insets shows SAED pattern along the [100] zone axis (top right) and the positions of Mo, Al, and B atoms (top left); (b) Crystal structure of MoAlB viewed on the (100) plane.
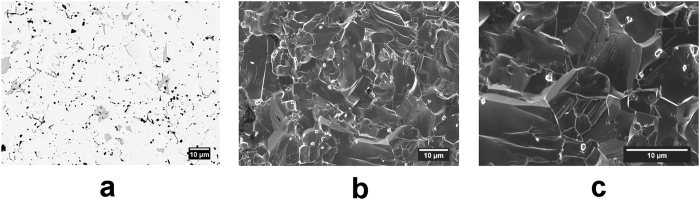
A typical backscattered electron micrograph of the polished HP4 cross-section is shown in (Fig. 3a) where the presence of, at least three phases - a majority phase, and at least two minority phases – are evidenced. The areas of lightest contrast are the majority phase, MoAlB. In agreement with XRD, EDS shows that the impurity phase with intermediate contrast is an Al-Mo impurity with Al:Mo molar ratio of 2.5:1. Image analysis showed that 6 ± 2 vol.% of this impurity phase is present. The areas of darkest contrast, making about 3 ± 0.5 vol.% of the sample, are primarily Al2O3 impurities according to EDS. In contrast to HP4, the HP2 cross-section showed primarily Al2O3 impurities (up to 9 vol.%) and negligible amounts of intermetallic compounds, which is consistent with our XRD results (Fig. S1b).
Figure 3.
(a) Backscattered electron micrograph of the hot-pressed HP4 cross-section; (b) secondary electron micrograph of the fracture surface at low magnification; (c) fracture surface at higher magnification reveals striations and layered structure of grains.
The cross-sectional fracture surface of the HP4 sample (Fig. 3b) shows it to contain mostly elongated, plate-like grains. Upon fracture, the grains were cleaved exposing large facets, which are presumably the {0k0} planes. The same fracture surface, shown at higher magnification in Fig. 3c, reveals sheared areas and other striations characteristic of nanolaminated materials.
Electronic Transport Properties
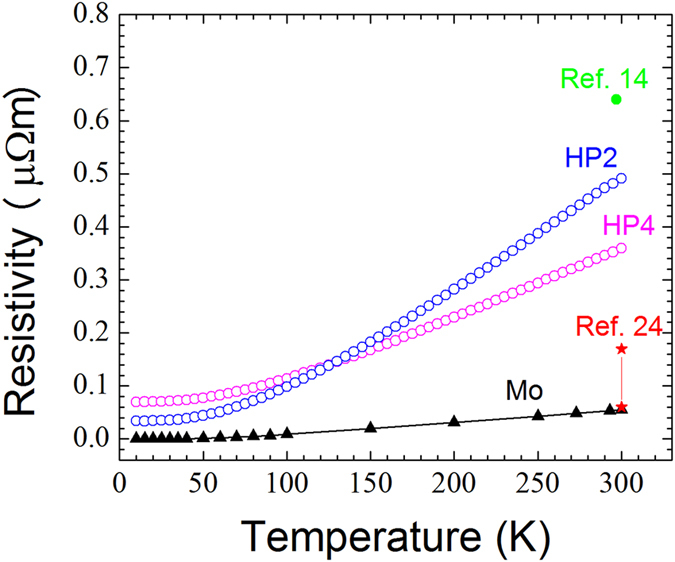
The temperature dependence of the electrical resistivity (ρ) of the HP4 and HP2 samples is compared with that of pure Mo reported by Desai et al.23 in Fig. 4. At 300 K, the resistivity is 0.36 μΩm for HP4 and 0.49 μΩm for HP2. Like a metal, the resistivity of both samples increases linearly with temperature above 100 K. This temperature dependence can be fit to the following equation:
Figure 4.
Resistivity vs. temperature of hot-pressed samples, MoAlB single crystals14,24, and pure Mo metal23.
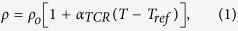 |
where Tref is 300 K, T the absolute temperature, ρo is the resistivity at 300 K, and αTCR is temperature coefficient of resistivity. Least-squares linear fitting in the 100–300 K temperature range results in a αTCR = 0.0042 K−1 for HP2 and αTCR = 0.0035 K−1 for HP4. A larger residual resistivity ratio, defined as ρ300K/ρ10K, for HP2 suggests that this sample is less defective. On the other hand, the larger resistivity above 140 K, and the larger αTCR of HP2, are possibly due to its slightly lower density and the presence of more Al2O3 impurities than HP4. For comparison, the resistivity of pure Mo metal is 8.8 × 10−6 μΩm and 0.055 μΩm at 10 K and 300 K, respectively.
At 300 K, Okada et al. reported a slightly higher value of resistivity (0.64 μΩm) when measured along the b-planes of MoAlB single crystals14. In contrast, the resistivity values reported by Sinel’nikova et al. (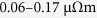 ) are much lower24. The reason for the large differences in reported literature values is unclear at this time. This comment notwithstanding, fabricating fully dense MoAlB samples with fewer impurities should further decrease the resistivity values at all temperatures.
) are much lower24. The reason for the large differences in reported literature values is unclear at this time. This comment notwithstanding, fabricating fully dense MoAlB samples with fewer impurities should further decrease the resistivity values at all temperatures.
Thermal Properties
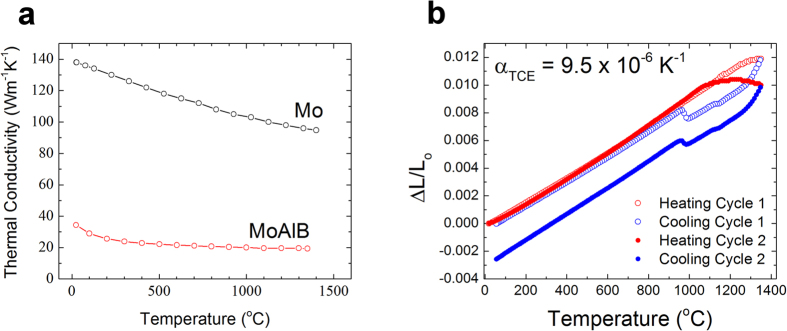
The thermal conductivity (κ) of sample HP4 measured parallel with hot-pressing direction as a function of temperature from 25 to 1350 °C is shown in Fig. 5a. A constant heat capacity of 559.5 J kg−1K−1 was assumed as per the Neumann-Kopp rule to calculate the thermal conductivity. At 26 °C, the thermal conductivity is 35 Wm−1K−1 and steadily decreases to 19.4 Wm−1K−1 at 1350 °C. The thermal conductivity of Mo metal is shown in Fig. 5a for comparison and is roughly 5 times higher than that of MoAlB at all temperatures25.
Figure 5.
(a) Temperature dependence of thermal conductivity of hot-pressed MoAlB (HP4) and Mo metal25; (b) Temperature dependence of normalized thermal expansion measured by dilatometry in an Ar atmosphere. The first two cycles are shown.
Based on the results shown in Fig. 5b, the thermal expansion coefficient, CTE, of this compound was calculated to be 9.5 × 10−6 K−1 up to 1350 °C during heating. Upon cooling a non-linear contraction is observed from 1350 °C to 974 °C, before the expansion folds back onto the heating curve. When the same sample was heated a second time the same CTE was measured, but now the non-linear effect was magnified and a permanent shrinkage of the sample is observed. The origin of this anomaly is unclear at this time, and more work is ongoing to understand it. The value measured herein is higher than that of Mo metal (4.8 × 10−6 K−1) or hot-pressed MoB (6.7 × 10−6 K−1)26. The excellent adhesion of the protective alumina scales that form on MoAlB during high temperature oxidation (see below) can partially be explained by the closeness of its CTE at high temperatures to that of Al2O3, viz. 8.5 × 10−6 K−1 27.
Preliminary differential thermal analysis (DTA) and thermogravimetric analysis (TGA) on dense HP4 samples show no evidence of dissociation or melting up to 1400 °C. More work is ongoing to characterize the thermal stability of MoAlB at even higher temperatures.
Mechanical Properties
The Vickers hardness values (Hv) measured at different loads on the cross-section are shown in Fig. 6. At all loads up to 9.8 N, the hardness was approximately constant at 10.6 ± 0.3 GPa, which agrees well with the hardness measured on the b-planes of MoAlB single crystals (10.3 ± 0.2 GPa) by Okada et al.14. In contrast, Ade et al. found the hardness on different crystal planes to range from 11.4–13.6 GPa13. At this time, we cannot comment on the effect of structural anisotropy on hardness, but it is clear that MoAlB is relatively soft compared to other borides such as MoB (Hv = 23 GPa)19, MoB2 (Hv = 21–27 GPa)28,29, and many other transition metal borides19. As the inset in Fig. 6 shows, no dominant cracks formed at the corners of the indents, even at the highest indentation load of 9.8 N, so MoAlB, like the MAX phases9, may be quite damage tolerant.
Figure 6.
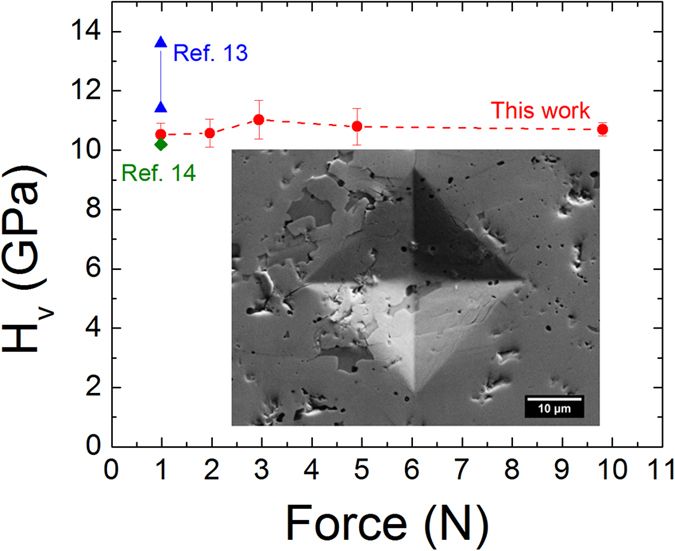
Vickers microindentation hardness as a function of indentation load. Inset shows indent formed under a 9.8 N load. Single crystal hardness values are shown for comparison13,14.
The test cylinders compressed perpendicular to the HP direction had an ultimate compressive strength, UCS, σ+=1940 ± 100 MPa; those compressed parallel with the HP direction had a UCS, σ//=1420 ± 300 MPa. Figure S4 shows the fracture surfaces after loading in the two perpendicular directions in relation the hot pressing direction. Two observations are salient. Firstly, the UCS values measured, especially the one close to 2 GPa, are quite high considering the size of the grains. Secondly, despite the fact that the most likely reason for the differences in strengths is the preferred orientation of the grains described above, these micrographs are not sufficiently different to make that case. More work is obviously needed. In both cases, brittle fracture occurred, and no yielding was observed prior to fracture. In general, cracks initiated at the base of the specimens and propagated upwards to create fracture surfaces often nearly parallel with the loading direction before the samples shattered into a few pieces.
Oxidation Resistance
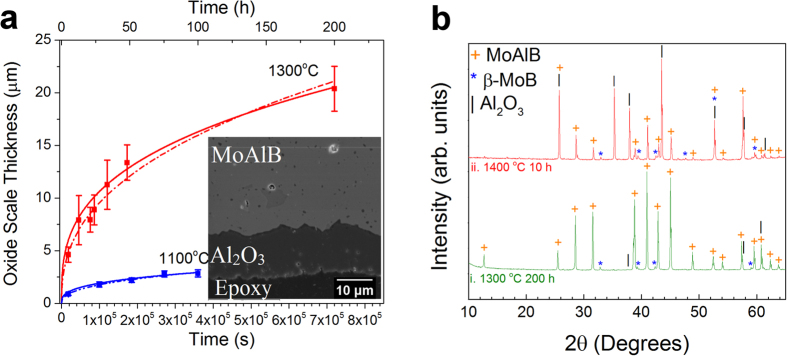
Oxidation at 1100 °C and 1300 °C resulted in the formation of dense, adherent oxide scales on the surface. A sample oxidized for 200 h at 1300 °C showed the presence of a 20 ± 2 μm thick scale, as shown in a cross-sectional micrograph (inset of Fig. 7a). Oxidation for 100 h at 1100 °C resulted in the formation of a 3 ± 0.4 μm thick scale. EDS near MoAlB/oxide interface (Fig. S5), formed after 200 h of oxidation at 1300 °C, revealed large differences in the relative atomic concentrations of Mo, Al, and O on the scale compared to the underlying MoAlB. It is clear that the nearly equal relative atomic concentrations of Mo and Al found at points 1, 4, and 5 correspond to pristine MoAlB. In contrast, the absence of Mo and an O:Al atomic ratio of 1.67 at points 6–10 suggest that Al preferentially diffused out of MoAlB and reacted with O to form an Al2O3 scale.
Figure 7.
(a) Time dependence of the oxide scale thickness from isothermal oxidation testing at 1300 °C (red) and 1100 °C (blue). Solid curves show fits to a cubic law; dashed curves show fits to a power law (see text). Inset show MoAlB/oxide scale interface after 200 h at 1300 °C; (b) XRD after oxidation at (i) 1300 °C for 200 h and, (ii) 1400 °C for 10 h.
XRD of the samples oxidized for 1300 °C for 200 h (Fig. 7bi) shows faint diffraction peaks corresponding to the formation of β−ΜοΒ and Al2O3 (ICSD #01-071-1125). Clear XRD evidence for the formation of Al2O3, however, was only obtained when a sample was oxidized at 1400 °C for 10 h (Fig. 7bii). It follows that the alumina layers formed at temperatures as high as 1300 °C were mostly amorphous and quite resistant to crystallization. For example, when Ti2AlC, another alumina former, is oxidized, clear and sharp alumina peaks are observed in XRD diffraction patterns of samples oxidized in air at 1000 °C for 120 h30. This resistance to crystallization is quite unusual and warrants further work. Notably, when understood, it may be possible to synthesize an amorphous alumina that may flow like glass, an exciting prospect.
Since there was no strong evidence for other phases forming during oxidation, it is reasonable to assume the oxidation reaction is:
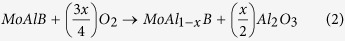 |
In other words, like in several alumina forming MAX phases31, one can conclude that MoAlB can exist with a deficiency of Al. Although the extent of that deficiency is unknown at the testing temperatures, phase equilibria of the Mo-Al-B system at 1000 °C support the idea that MoAlB can exist with a sub-stoichiometric content of Al to accommodate for the Al lost during oxidation18. From the fact that MoB does not precipitate below the alumina layer, it is possible that the B dissolves in, and diffuses out through the protective alumina layer. The same conclusion was reached for the fate of C during the oxidation of Ti2AlC20. However, more work needs to be done to clearly understand the role of Mo and B during high-temperature oxidation, and their role, if any, in preventing the crystallization of the alumina layer.
The oxidation kinetics were determined by measuring the scale thicknesses, x, as a function of time, t, at a fixed temperature. The results - shown in Fig. 7a – were fit to the well-known power law equation:
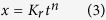 |
where Kr is the oxidation rate constant and n is the power law scale growth exponent. At 1300 °C, a good power law fit (R2 = 0.97) was obtained with n = 0.4. Similarly, at 1100 °C, a good power law fit (R2 = 0.99) was obtained with n = 0.4. Since these growth exponents are close to the value of n = 0.33 typical of alumina32, the data were also fit to the cubic rate law viz.
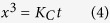 |
where Kc is the cubic rate constant. Figure 7a shows that very good fits were again observed at 1100 °C (R2 = 0.96) and 1300 °C (R2 = 0.99). Since cubic oxidation kinetics are rationalized on the basis of crystal growth, it is not clear why the kinetics observed here are cubic. TEM studies are ongoing to understand the exact oxidation mechanism.
Table 1 compares the Kc values obtained in this work with those of Ti2AlC and Ti3AlC2, which both form dense, crystalline alumina scales when heated in air30,33. For straightforward comparison of these materials, the weight gain rate constants of Ti2AlC and Ti3AlC2 were multiplied by 9.4 × 10−10 m9kg−3 to obtain scale growth rate constants, which is based on the oxidation reaction that causes alumina to form on Ti3AlC2 and Ti2AlC32. MoAlB shows better oxidation resistance than Ti2AlC and Ti3AlC2 at 1100 °C. At 1300 °C, however, the oxidation resistances are comparable.
Table 1.
| 1100 °C | 1300 °C | References | |
|---|---|---|---|
| MoAlB | 7.1 × 10−23 m3/s | 1.2 × 10−20 m3/s | This work |
| Ti2AlC | 1.0–1.8 × 10−21 m3/s | 1.4–4.8 × 10−20 m3/s | 30,33 |
| Ti3AlC2 | 1.6 × 10−21 m3/s | 1.9 × 10−20 m3/s | 30,33 |
Comparison of the cubic oxidation rate constant Kc of MoAlB, Ti2AlC, and Ti3AlC2.
As noted above, similar thermal expansion coefficients for MoAlB (9.5 × 10−6 K−1) and Al2O3 (8.5 × 10−6 K−1) and high thermal conductivity of MoAlB suggest that this compound would be resistant to spallation of the protective oxide. Therefore, further investigations on the oxidation mechanisms and kinetics are warranted once the processing steps have been optimized.
It is interesting to compare the oxidation behavior of MoAlB with that of zirconium diboride (ZrB2), a leading candidate for high-temperature aerospace applications. Opeka et al. performed isothermal oxidation on nominally pure hot-pressed ZrB2 and demonstrated that active oxidation occurs above 1200 °C due to the evaporation of the B2O3 from the mixed ZrO2-B2O3 scale34, while other studies have demonstrated paralinear, or parabolic, oxidation kinetics for ZrB2 in the 1100–1400 °C range for short oxidation times (<5 h)35,36. In Opeka’s study, a 200 μm thick porous ZrO2 scale was observed after only 5 h at 1300 °C compared to the dense 5 μm thick Al2O3 scale that forms on MoAlB under the same conditions in the current study34.
Summary
Reactive hot pressing of MoB and Al powders resulted in predominantly single phase, >94% dense MoAlB samples with ~9 vol.% secondary phases. HRSTEM images confirmed the atomically layered structure of MoAlB for the first time. Static oxidation testing for up to 200 h led to the formation of dense, adherent alumina scales that render MoAlB highly oxidation resistant up to at least 1300 °C.
MoAlB is a metallic conductor with a correspondingly high thermal conductivity. Also, its thermal expansion coefficient (9.5 × 10−6 K−1) makes it compatible with many engineering alloys. The high compressive strength – comparable with that of alumina or silicon carbide - and relatively low hardness of MoAlB certainly warrant further investigation of the damage tolerance of this material at ambient and elevated temperatures. The properties of MoAlB measured herein are summarized in Table 2. Although these preliminary results are not optimized, MoAlB ceramics hold great promise as high temperature materials and coatings.
Table 2.
| Minimum relative density | 94 ± 1% |
| Electrical Resistivity at 300 K, ρ300 | 0.35–0.49 μΩm |
| Temperature Coefficient of Resistivity, αTCR | 0.0035–0.0042 K−1 |
| Decomposition/Melting Temperature | >1400 °C |
| Vickers Microindentation Hardness, HV | 10.7 + 0.3 GPa |
| Thermal Expansion Coefficient, CTE | 9.5 × 10−6 K−1 |
| Thermal Conductivity at 300 K, κ | 35.0 Wm−1K−1 |
| Compressive Strength, σ+ | 1940 + 103 MPa |
| Compressive Strength, σ// | 1418 + 281 MPa |
Physical, thermal, and mechanical properties of hot-pressed MoAlB in this work.
Methods
Processing
Dense, polycrystalline samples of MoAlB were synthesized by hot-pressing reactants together in a vacuum hot press (HP), as reported for several MAX phases37. First, molybdenum boride, MoB (>99%, <38 μm, Alfa Aesar, Ward Hill, MA, USA) and aluminum, Al (99.5%, <44 μm Alfa Aesar, Ward Hill, MA, USA), powders were ball milled in a molar ratio of 1.0 to 1.3 in a plastic container for 24 h. The mixture was loaded into a graphite foil lined cylindrical graphite die, heated to 1200 °C at a rate of 300 °C h−1 and pressed to a peak load corresponding to a stress of 39 MPa during the last 1 h of the temperature ramp. This temperature and pressure were held for 5.8 h (HP4 samples) or 5 h (HP2 samples), after which the hot press was allowed to cool. Unless otherwise stated, all characterization was performed on HP4 samples. The densities of the hot-pressed samples - as determined by Archimedes’ principle in water - were typically at least 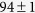 % of theoretical (6.45 g/cm3).
% of theoretical (6.45 g/cm3).
The same reactant mixture was cold-pressed to a load corresponding to 300 MPa and heated to 1000 °C for 15 h in a tube furnace with flowing argon, Ar, gas to obtain loosely sintered compacts. The latter were ground into powder with a drill bit and used for transmission electron microscopy (TEM) and high-resolution scanning transmission electron microscopy (HRSTEM) instead of the hot-pressed samples due to the difficulty of grinding down the latter.
Characterization
X-ray diffraction (XRD) patterns of the hot-pressed samples and MoAlB powders were obtained on a powder diffractometer (SmartLab, Rigaku Corp., Tokyo, Japan) using Cu Kαradiation. The FullProf Software suite was used to perform Rietveld refinement38,39. A scanning electron microscope, SEM (Zeiss Supra 50VP, Carl Zeiss SMT AG, Oberkochen, Germany), equipped with an energy-dispersive X-ray spectroscope (Oxford EDS, Oxfordshire, United Kingdom) was used to characterize the microstructure and the elemental compositions. The MoAlB powders were analyzed with TEM/SAED on a FEI Tecnai G2 TF20 UT (FEI, Hillsboro, Oregon, USA) equipped with a field emission gun operated at a voltage of 200 kV and HRSTEM by the Linköping double Cs corrected FEI Titan3 60–300 (FEI, Hillsboro, Oregon, USA) operated at 300 kV. The TEM specimen was prepared by first mixing the powder with glue, followed by heating, polishing down to 50 μm and then ion milling to make electron transparent.
The electrical resistivity was measured in the 10 K to 300 K temperature range using the 4-probe method in a physical property measurement system (Quantum Design, San Diego, CA, USA). Thin cross-sections (0.5–0.8 mm thick) of the HP2 and HP4 samples were cut with a high speed diamond saw, polished to a smooth finish with 800 grit SiC paper, and washed with ethanol prior to the measurements. The resistivity was measured by placing the electrodes on the cross-sections of these samples.
The thermal conductivity was measured parallel to the hot pressing direction using a laser flash instrument (Netzsch LFA 427, Selb, Upper Franconia, Germany) over the temperature range 25–1350 °C, at a heating rate of 10 °C/min on polished disks (10 mm diameter, 3 mm height) cut by electrical discharge machining (EDM). The coefficient of thermal expansion (CTE) was measured perpendicular to the hot pressing direction in a dilatometer in the 25 °C to 1350 °C temperature range on EDM’d cylinders (20 mm length, 6 mm diameter) under helium gas, using a dual-push-rod dilatometer (Netzsch DIL 402E, Selb, Upper Franconia, Germany). Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed simultaneously in Ar on hot-pressed samples from 25 °C to 1600 °C at a rate of 10 °C min.−1 (Netzsch STA 449F1, Selb, Upper Franconia, Germany).
The Vickers microindentation hardness was measured using a microindenter (LECO-M400 LECO Corp., St. Joseph, MI). Indentation loads between 0.98 and 9.8 N with a 15 s dwell time were used. Indent diagonals were measured using a scanning electron microscope, SEM (Zeiss Supra 50VP, Germany). The hardness values represent the average of at least three indents at each load.
The room temperature ultimate compressive strengths, UCSs, were measured using an Instron 5800R (Instron, Norwood, MA, USA) or MTS servo-controlled hydraulic system (MTS Systems Co., Eden Praire, MN, USA). Test cylinders (5 mm diameter, 13 mm long) were EDM’d lengthwise perpendicular to the HP direction and tested with no further preparation, as per ASTM C1424-10. Sample cylinders (5 mm diameter, 10 mm tall) were also EDM’d lengthwise parallel with the HP direction for comparison. The samples were compressed in displacement-control mode, at a rate of 0.3 mm/s until fracture.
The oxidation resistance was tested at 1100 °C, 1300 °C, and 1400 °C in static air for various times up to 200 h. The samples used were 4 × 4 × 4 mm3 cubes machined via EDM and polished with 1200 grit SiC polishing paper to a mirror-like finish. The oxidized samples were then mounted in epoxy, and again polished down to 1200 grit SiC paper in order to measure the thickness of the oxide scales in the SEM.
Additional Information
How to cite this article: Kota, S. et al. Synthesis and Characterization of an Alumina Forming Nanolaminated Boride: MoAlB. Sci. Rep. 6, 26475; doi: 10.1038/srep26475 (2016).
Supplementary Material
Acknowledgments
We would like to thank Dr. El’ad Caspi, Joseph Halim, and Grady Bentzel for their assistance with Rietveld refinement, Dr. Babak Anasori for guidance on oxidation testing, and Mathias Agne for valuable discussions about thermal properties. We would also thank the Centralized Research Facilities of Drexel University for providing access to XRD and SEM. We would like to thank Brian Wisner and Dr. Antonios Kontsos, of the Department of Mechanical Engineering and Mechanics at Drexel University, for assistance with compression testing. This work was supported by the Leverhulme Trust and the Army Research Office (W911NF-11-1-0525). L.H. and J.L. acknowledge the Knut and Alice Wallenberg Foundation.
Footnotes
Author Contributions S.K. hot-pressed the MoAlB, performed SEM and XRD after sintering, and performed oxidation experiments. E.Z.S. studied the thermal properties, long-term oxidation behavior, and XRD after oxidation. A.L. carried out mechanical properties testing. J.L. did HRSTEM observations of the MoAlB powders. O.E. synthesized the MoAlB powders. A.H. studied the electrical properties of the sintered MoAlB. W.E.L. supervised and discussed all E.Z.S. experimental results. L.H. discussed HRSTEM experiments with J.L. S.J.M. supervised and discussed all A.H. experiments. M.W.B. supervised and discussed all experimental results. M.W.B. and S.K. wrote the main manuscript. All authors revised the manuscript.
References
- Martini C., Palombarini G., Poli G. & Prandstraller D. Sliding and abrasive wear behaviour of boride coatings. Wear 256, 608–613 (2004). [Google Scholar]
- Yu X. W. & Licht S. A novel high capacity, environmentally benign energy storage system: Super-iron boride battery. J. Power Sources 179, 407–411 (2008). [Google Scholar]
- Licht S., Yu X. & Qu D. A novel alkaline redox couple: chemistry of the Fe(6+)/B(2−) super-iron boride battery. Chem. Commun. (Camb). 2, 2753–5 (2007). [DOI] [PubMed] [Google Scholar]
- Vrubel H. & Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chemie - Int. Ed. 51, 12703–12706 (2012). [DOI] [PubMed] [Google Scholar]
- Mahdavi B., Miousse D., Fournier J., Ménard H. & Lessard J. Hydrogen evolution reaction at nickel boride electrodes in aqueos methanolic and ethanolic solutions. Can. J. Chem. 74, 380–388 (1996). [Google Scholar]
- Aylett B. J. Borides & Chemistry Silicides–New and Applications. Br. Polym. J. 18, 359–363 (1986). [Google Scholar]
- Barsoum M. W. The Mn+1AXn Phases: A new Class of Solid; Thermodynamically Stable Nanolaminates. Prog. Solid State Chem. 28, 201–281 (2000). [Google Scholar]
- Barsoum M. W. & El-Raghy T. Synthesis and Characterization of a Remarkable Ceramic: Ti3SiC2. J. Am. Ceram. Soc. 79, 1953–1956 (1996). [Google Scholar]
- Barsoum M. W. & Radovic M. Elastic and Mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 41, 195–227 (2011). [Google Scholar]
- Barsoum M. W., El-Raghy T. & Ali M. Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 31, 1857–1865 (2000). [Google Scholar]
- Jeitschko W. Die Kristallstruktur von MoAlB. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 97, 1472–1476 (1966). [Google Scholar]
- Jeitschko W. The crystal structure of Fe2AlB2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 25, 163–165 (1969). [Google Scholar]
- Ade M. & Hillebrecht H. Ternary Borides Cr2AlB2 , Cr3AlB4 , and Cr4AlB6: The First Members of the Series (CrB2)nCrAl with n = 1, 2, 3 and a Unifying Concept for Ternary Borides as MAB-Phases. Inorg. Chem. 54, 6122–6135 (2015). [DOI] [PubMed] [Google Scholar]
- Okada S. et al. Single Crystal Growth of (MoxCr1−x)AlB and (MoxW1−x)AlB by Metal Al Solutions and Properties of the Crystals. J. Solid State Chem. 133, 36–43 (1997). [Google Scholar]
- Chai P., Stoian S. A., Tan X., Dube P. A. & Shatruk M. Investigation of magnetic properties and electronic structure of layered-structure borides AlT2B2 (T = Fe, Mn, Cr) and AlFe2−xMnx B2. J. Solid State Chem. 224, 52–61 (2014). [Google Scholar]
- Tan X., Chai P., Thompson C. M. & Shatruk M. Magnetocaloric Effect in AlFe2B2: Toward Magnetic Refrigerants from Earth-Abundant Elements. J. Am. Chem. Soc. 135, 9553–9557 (2013). [DOI] [PubMed] [Google Scholar]
- Du Q. et al. Magnetic frustration and magnetocaloric effect in AlFe 2−xMnxB2 (x = 0–0.5) ribbons. J. Phys. D. Appl. Phys. 48, 335001 (2015). [Google Scholar]
- Rieger W., Nowotny H. & Benesovsky F. Uber einige Komlexboride von Ubergangsmetallen. Mh. Chem 96, 844–851 (1965). [Google Scholar]
- Campbell I. E. & Sherwood E. M. in High Temp. Mater. Technol. 1st Edn, Ch. 13, 360–363 (Wiley, 1967). [Google Scholar]
- Tallman D. J., Anasori B. & Barsoum M. W. A Critical Review of the Oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in Air. Mater. Res. Lett. 1, 115–125 (2013). [Google Scholar]
- Sundberg M., Malmqvist G., Magnusson A. & El-Raghy T. Alumina forming high temperature silicides and carbides. Ceram. Int. 30, 1899–1904 (2004). [Google Scholar]
- Okada S. Synthesis, Crystal Structure and Characterizations of the Ternary Borides TMAlB (TM=Mo,W) with UBC Type Structure. Trans. Kokushikan Univ. Fac. Eng. 7–12 (1998). [Google Scholar]
- Desai P. D., Chu T. K., James H. M. & Ho C. Y. Electrical Resistivity of Selected Elements. J. Phys. Chem. Ref. Data 13, 1069 (1984). [Google Scholar]
- Sinel’nikova V. S., Gurin V. N., Pilyankevich A. N., Strashinskaya L. V. & Korsukova M. M. Technology and properties of single crystals of refractory borides. J. Less Common Met. 47, 265–272 (1976). [Google Scholar]
- Ho C. Y., Powell R. W. & Liley P. E. Thermal Conductivity of the Elements. J. Phys. Chem. Ref. Data 1, 279 (1972). [Google Scholar]
- Kosolapova T., Kosolapova T. Y. & Kosolapova T. I. In Handb. High Temp. Compd. Prop. Prod. Appl 1st Edn., Ch. 3, 386–388 (CRC Press, 1990). [Google Scholar]
- Dobrovinskaya E. R., Lytvynov L. A. & Pishchik V. in Sapphire Mater. Manuf. Appl. 1st edn., Ch. 2, 109–110 (Springer Science & Business Media, 2009). [Google Scholar]
- Okada S., Kudou K. & Shishido T. Synthesis and some properties of molybdenum diboride MoB2. Pac. Sci .Rev. 11, 164–171 (2011). [Google Scholar]
- Tao Q. et al. Enhanced Vickers hardness by quasi-3D boron network in MoB2. RSC Adv. 3, 18317 (2013). [Google Scholar]
- Basu S., Obando N., Gowdy A., Karaman I. & Radovic M. Long-Term Oxidation of Ti2AlC in Air and Water Vapor at 1000–1300 °C Temperature Range. J. Electrochem. Soc. 159, C90 (2012). [Google Scholar]
- Cam G., Flower H. M. & West D. R. F. Constitution of Ti–Al–C alloys in temperature range 1250–750 C. Mater. Sci. Technol. 7, 505–511 (1991). [Google Scholar]
- Barsoum M. W. In MAX Phases Prop. Mach. Ternary Carbides Nitrides 1st edn., Ch. 6, 197–202 (Wiley-VCH, 2013).
- Smialek J. L. Oxygen diffusivity in alumina scales grown on Al-MAX phases. Corros. Sci. 91, 281–286 (2015). [Google Scholar]
- Opeka M. M., Talmy I. G., Wuchina E. J., Zaykoski J. a. & Causey S. J. Mechanical, Thermal, and Oxidation Properties of Refractory Hafnium and zirconium Compounds. J. Eur. Ceram. Soc. 19, 2405–2414 (1999). [Google Scholar]
- Basinski Z. et al. High-Temperature Oxidation III. Zirconium and Hafnium Diborides. J. Electrochem. Soc. 113, 905–914 (1964). [Google Scholar]
- Dehdashti M. K., Fahrenholtz W. G. & Hilmas G. E. Effects of temperature and the incorporation of W on the oxidation of ZrB2 ceramics. Corros. Sci. 80, 221–228 (2014). [Google Scholar]
- Amini S., Barsoum M. W. & El-Raghy T. Synthesis and Mechanical Properties of Fully Dense Ti2SC. J. Am. Ceram. Soc. 90, 3953–3958 (2007). [Google Scholar]
- Rietveld H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969). [Google Scholar]
- Rodriguez-Carvajal J. FULLPROF: a program for Rietveld refinement and pattern matching analysis. In Satell. Meet. powder Diffr. XV Congr. IUCr (1990). Available at https://www.ill.eu/sites/fullprof/ (Accessed 23rd September 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.