Abstract
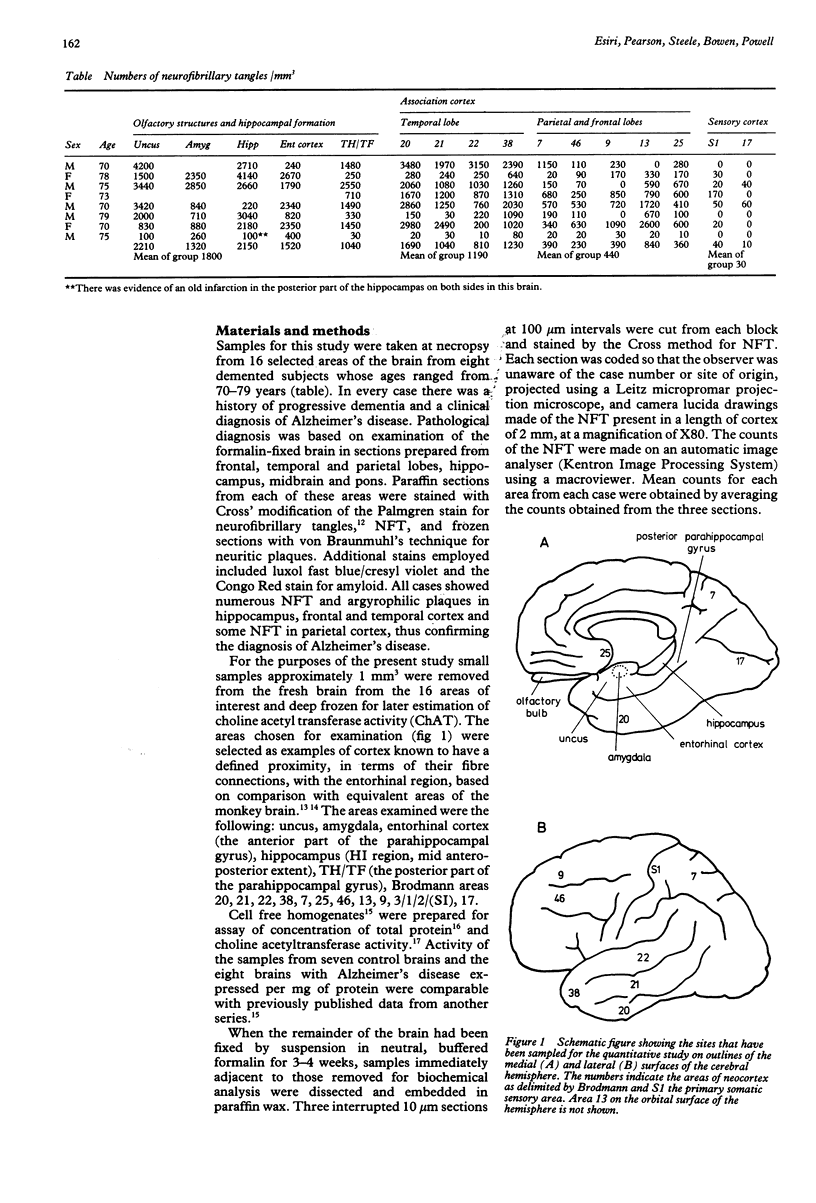
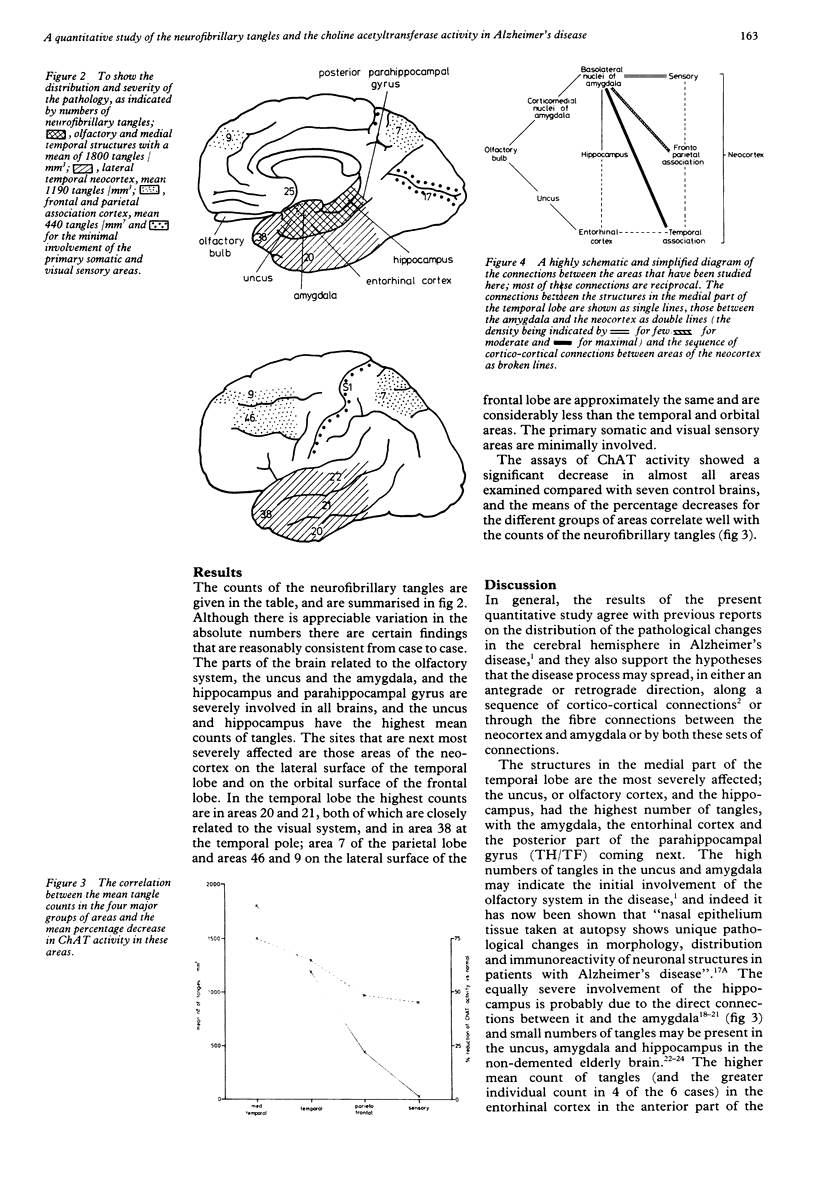
A quantitative study has been made of the number of neurofibrillary tangles and of the choline acetyltransferase activity in several sites in the cerebral hemispheres of eight patients who had had Alzheimer's disease. The neurofibrillary tangles were maximal in structures in the medial temporal lobe (uncus, amygdala, hippocampus and parahippocampal gyrus), severe in the neocortex on the lateral surface of the temporal lobe, moderate in the "association cortex" of the parietal and frontal lobes and minimal in primary somatic and visual sensory areas. There was a significant decrease in choline acetyltransferase activity in almost all areas, and the means of the percentage decreases for the different groups of areas correlate well with the counts of the neurofibrillary tangles. These results support the hypothesis that the pathological process in Alzheimer's disease may spread along a sequence of corticocortical connections between the main sensory areas and the hippocampal formation. The disease process may also spread along the reciprocal connections between the amygdala and the neocortex because the numbers of tangles in different areas of the neocortex closely parallel the density of their connections and the amygdala.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggleton J. P. A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res. 1985;57(2):390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Aggleton J. P. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res. 1986;64(3):515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., Burton M. J., Passingham R. E. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 1980 May 26;190(2):347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Cowan W. M. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980 Feb 15;189(4):573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Price J. L. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984 Dec 20;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Cross R. B. Demonstration of neurofibrillary tangles in paraffin sections: a quick and simple method using a modification of Palmgren's method. Med Lab Sci. 1982 Jan;39(1):67–69. [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Herzog A. G., Van Hoesen G. W. Temporal neocortical afferent connections to the amygdala in the rhesus monkey. Brain Res. 1976 Oct 8;115(1):57–69. doi: 10.1016/0006-8993(76)90822-2. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kosel K. C., Van Hoesen G. W., Rosene D. L. Non-hippocampal cortical projections from the entorhinal cortex in the rat and rhesus monkey. Brain Res. 1982 Jul 29;244(2):201–213. doi: 10.1016/0006-8993(82)90079-8. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978 Mar 15;178(2):255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977 Apr 15;172(4):723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mann D. M., Esiri M. M. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J Neurol Sci. 1989 Feb;89(2-3):169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Marcyniuk B., Yates P. O., Neary D., Snowden J. S. The progression of the pathological changes of Alzheimer's disease in frontal and temporal neocortex examined both at biopsy and at autopsy. Neuropathol Appl Neurobiol. 1988 May-Jun;14(3):177–195. doi: 10.1111/j.1365-2990.1988.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Tucker C. M., Yates P. O. The topographic distribution of senile plaques and neurofibrillary tangles in the brains of non-demented persons of different ages. Neuropathol Appl Neurobiol. 1987 Mar-Apr;13(2):123–139. doi: 10.1111/j.1365-2990.1987.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982 Feb 10;205(1):30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Palmer A. M., Procter A. W., Stratmann G. C., Bowen D. M. Excitatory amino acid-releasing and cholinergic neurones in Alzheimer's disease. Neurosci Lett. 1986 May 15;66(2):199–204. doi: 10.1016/0304-3940(86)90190-4. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T. P., Cowan W. M., Raisman G. The central olfactory connexions. J Anat. 1965 Oct;99(Pt 4):791–813. [PMC free article] [PubMed] [Google Scholar]
- Procter A. W., Lowe S. L., Palmer A. M., Francis P. T., Esiri M. M., Stratmann G. C., Najlerahim A., Patel A. J., Hunt A., Bowen D. M. Topographical distribution of neurochemical changes in Alzheimer's disease. J Neurol Sci. 1988 Apr;84(2-3):125–140. doi: 10.1016/0022-510x(88)90118-9. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Hiorns R. W., Powell T. P. The basic uniformity in structure of the neocortex. Brain. 1980 Jun;103(2):221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Rosene D. L., Van Hoesen G. W. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977 Oct 21;198(4314):315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Selemon L. D., Goldman-Rakic P. S. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988 Nov;8(11):4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo B. R., Rudel R., Kosik K. S., Lee V. M., Neff S., Adelman L., Kauer J. S. Pathological changes in olfactory neurons in patients with Alzheimer's disease. Nature. 1989 Feb 23;337(6209):736–739. doi: 10.1038/337736a0. [DOI] [PubMed] [Google Scholar]
- Turner B. H., Mishkin M., Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. J Comp Neurol. 1980 Jun 15;191(4):515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Ulrich J. Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol. 1985 Mar;17(3):273–277. doi: 10.1002/ana.410170309. [DOI] [PubMed] [Google Scholar]
- Ulrich J. Senile plaques and neurofibrillary tangles of the Alzheimer type in nondemented individuals at presenile age. Gerontology. 1982;28(2):86–90. doi: 10.1159/000212515. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G., Pandya D. N., Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975 Sep 12;95(1):25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G., Pandya D. N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975 Sep 12;95(1):1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Wilcock G. K., Esiri M. M., Bowen D. M., Smith C. C. Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982 Dec;57(2-3):407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]


