Abstract
Previous studies have documented use of health care services by oncology patients in the Emergency Department (ED), but little is known about the utilization of health services of patients with acute leukemia after induction therapy. The aim of this study was to examine chief reasons for ED and hospital use by patients newly diagnosed with acute leukemia patients after induction therapy up to one year after discharge. A retrospective, longitudinal study of all visits to the ED or unplanned hospital admissions at a single institution for patients with acute leukemia was conducted. Inclusion criteria were patients ≥18 years of age at time of diagnosis, a confirmed diagnosis of AML or ALL, and received and discharged from induction treatment between 2007–2010. Donabedian’s structure-process-outcome framework guided this study examining health services utilization and assessing patient outcomes. 80 patients met the inclusion criteria; 52 had AML and 28 had ALL; median age was 48 (range: 18–76) and 29% (n=23) were non-Caucasian. 70% (n=56) were discharged from induction in remission. 81% (n=65) had at least 1 ED or hospitalization event, and 44% (n=35) had 2 or more events. Of 137 events in 65 patients, the most common reason was neutropenic fever/infection (55%), bleeding (12%), and GI problems (11%). Mean number of events for ALL was 2.43 compared to 1.33 for AML patients (p=0.02), and 2.23 for <50 years of age compared to 1.20 for those older (p=0.002). 20 patients died within one year of diagnosis. Findings from this study can help inform health services delivery and utilization among patients with acute leukemia after induction therapy. Oncology providers can anticipate discharge needs and enhance follow-up care for those at higher risk for problems needing hospitalization.
Keywords: acute leukemia, emergency department, hospitalization, induction therapy, health care utilization
Introduction
Emergency Departments (EDs) are coping with the increasing symptom needs of patients with cancer. These symptoms may be a life threatening complication requiring further evaluation and treatment, and even hospitalization. Visiting the ED may be appropriate to manage acute cancer related problems such as fever, pain or respiratory distress and do not necessarily represent inappropriate cancer care management or limited community services (Mayer et al, 2011; Vandyk et al, 2012). However, EDs with large volumes of patients and with overcrowding may not be the best environments for cancer patients with neutropenia. Neutropenia increases their risk of developing other bacterial, viral, and fungal infections.
There are few studies available on ED and hospital use by oncology patients. Bozdemir et al (2009) studied all visits to the ED by patients with cancer at one institution in Turkey over a 6 month period and documented 324 visits by 245 patients. Nearly 40% of those patients had more than one visit with pain, nausea and vomiting as two common symptoms. Almost half of those 245 patients died within three months of their ED visit. Mayer, et al (2011) conducted a population-based cross sectional evaluation of 37,760 cancer-related ED visits in North Carolina in 2008 utilizing the North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT) (Hackenwerth et al, 2009). The most common chief complaints were related to pain, respiratory distress, and gastrointestinal (GI) distress. Of all cancer types, the patients most likely to present to the ED were lung cancer patients; a total of 63.2% of visits resulted in hospital admittance compared to 15.1% for non-cancer related visits.
Additional analyses found that of the 37,760 cancer-related ED visits, 283 patients died in the ED with common presenting reasons including shortness of breath and GI complaints (Leak et al, 2012). An earlier study examined cancer deaths in Canada and found that 83.8% of those who died had visited the ED during their last 6 months of life (Barbera, 2010). The presenting symptoms were similar to Leak et al, 2012 including abdominal pain, dyspnea, pneumonia, and fatigue.
Not only do patients enter the ED for treatment or disease related symptoms, they may also be admitted to the hospital for further assessment and management. In 2009, there were 4.7 million cancer-related hospitalizations among adults, and 1.2 million had identified cancer as the chief diagnosis (Anhang, 2012). The mean length of stay for leukemia related hospitalizations (e.g. re-admissions for symptom management) was 15.5 days, with a mean cost per stay at $40,200 (Anhang, 2012). Patients with leukemia had the most expensive cancer hospitalizations compared to all other cancers in 2009. Patients with acute leukemia can become acutely ill within the course of their first treatment, and it is important that we understand their utilization of the ED and hospital after induction therapy so we may best coordinate efforts to prevent or anticipate their needs.
Aims
The aims of this study were to 1) describe chief reasons for ED and hospital use by newly diagnosed acute leukemia patients after induction therapy through one year after discharge and 2) to explore the sociodemographic and disease characteristics of those who utilized the ED or were hospitalized. Donabedian’s structure-process-outcome framework guided this study by examining health services utilization and assessing patient outcomes (Donabedian, 2005). Structure is the setting for which care is provided and delivered (ED or hospital), process is when the patient seeks and follows through with care provided at either the ED, hospital, or both, and outcome is the number of events to the ED and hospital.
Materials and Methods
Patients were identified from the Carolina Data Warehouse for Health (CDW-H), a central repository including clinical, research and administrative data for patients receiving services at the University of North Carolina Hospitals (UNC). The warehouse contains data from various clinical and operations systems within the UNC Health Care System. This enterprise-wide data warehouse was developed in 2004 to meet the dual challenges of enhancement of quality of care and clinical research with our patient populations. The study was reviewed and approved by the University of North Carolina Institutional Review Board.
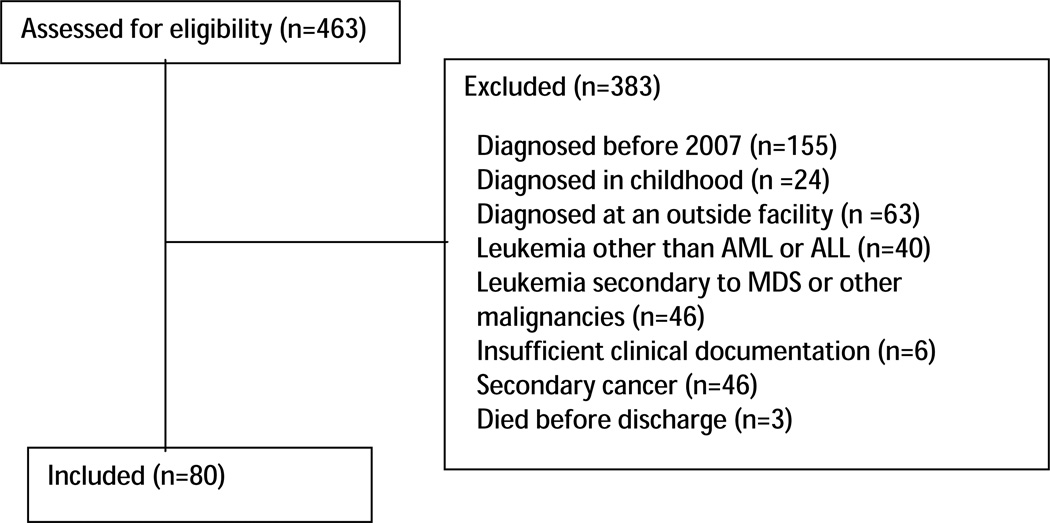
The CDW-H was queried for all patients who received services at UNC from 2007–2010 with an ICD9 code of leukemia (208.00; 208.01; 208.2; 205.0; 205.01; 205.02; 204.00; 204.01; 204.02; 206.00; 206.01; 206.02). The medical records of 463 patients were then reviewed for possible inclusion. Eligible patients were ≥18 years of age at the time of diagnosis, had a confirmed diagnosis of AML or ALL, and received and were discharged from induction treatment at the University of North Carolina Cancer Hospital. Data from 2004 to 2007 are not complete; hence we restricted our sample to those patients diagnosed with acute leukemia in or after 2007. We further narrowed our sample by excluding patients diagnosed before 2007 (n=155) or in childhood (n=24), diagnosed at an outside facility (n=63), with leukemia other than AML or ALL (n=40), leukemia secondary to myelodysplasia or other malignancies (n=46), secondary cancer (n=46), insufficient clinical documentation (n=6), or died before discharge (n=3). (Figure 1).
Figure 1.
Eligibility of Patients Newly Diagnosed with Acute Leukemia between 2007–2010
Data were collected on the 80 eligible patients including demographic/clinical characteristics and for each ED visit and hospitalization after initial induction therapy using the patient’s electronic medical record and searching under both ED Notes (capturing all ED visits) and History and Physical Notes (capturing all hospital admissions). An event is defined as one of the three following occurrences: ED only (the patient came to the ED and was discharged from the ED, hospital only (the patient was admitted directly to the hospital), or ED to hospital (the patient came to the ED and was admitted to the hospital). We were assured that the patients with no events included in this analysis truly had no events outside our institution since there were other notes in their charts indicating continued contact throughout the year with UNC physicians.
Descriptive statistics are provided, including medians and ranges. Differences between patients were compared using Fisher’s Exact tests for categorical variables and Wilcoxon Rank Sum tests for continuous variables. Linear regression was used to model the outcome of number of events, and Cox proportional hazards models were used to model the outcome of time to first event. Patients who died without an event in the first year were censored at time of death (n=2). Patients with no events during the first year were censored at 365 days (n=13). Univariable and multivariable models were used for both ED visit and hospitalization, covariates included in the multivariable models included age, minority, insurance, disease type, and remission. All analyses were conducted using SAS statistical software v9.3 (Cary, NC).
Results
Sample
This retrospective longitudinal study included 80 patients ≥18 years of age at time of diagnosis with a confirmed diagnosis of AML or ALL who received and were discharged from induction treatment at the North Carolina Cancer Hospital between 2007–2010 [Table 1]. Patient data for one year after discharge from initial diagnosis and treatment hospitalization were reviewed for occurrence of emergency department visits and hospitalizations. The sample (n=80) was comprised of 44 females and 36 males (median age 48, range 18–76,). There were 29% (n=23) non-Whites (14 African American/Black, 5 Hispanic, 3 American Indian, 1 Asian) with African Americans comprising 17% of the sample. The majority of patients were married/partnered (72%) and 54% had public insurance [Table 1]. About two-thirds of patients (65%) had AML and 35% had ALL. Most demographic variables were similar between the two groups, however, the patients with AML were significantly older (median age 54 v 39, p=0.01) and less likely to be minorities (20% v 46%, p=0.02). Diagnosis year was evenly distributed across the four years and the most common leukemia subtype for AML was M2 (34%). According to the French-American-British classification of AML, M2, known as acute myeloblastic leukemia with maturation, has a favorable prognostic outcome (ACS, 2014b). Most patients (70%) were in remission after induction treatment.
Table 1.
Patient Characteristics
| Variables | Total n=80 n % |
AML N=52 n % |
ALL N=28 n % |
P value |
|
|---|---|---|---|---|---|
| Age (median, range) |
50 (18– 76) |
54 (21–76) | 39 (18–74) | 0.01 | |
| Gender | Female | 44 (55%) | 28 (54%) | 16 (57%) | 0.82 |
| Male | 36 (45%) | 24 (46%) | 12 (43%) | ||
| Diagnosis Year |
2007 | 23 (29%) | 18 (35%) | 5 (18%) | 0.14 |
| 2008 | 14 (18%) | 11 (21%) | 3 (11%) | ||
| 2009 | 21 (26%) | 12 (23%) | 9 (32%) | ||
| 2010 | 22 (28%) | 11 (21%) | 11(39%) | ||
| Subtype* | M0 | 3 (6%) | |||
| M1 | 7 (15%) | ||||
| M2 | 16 (34%) | ||||
| M4 | 8 (17%) | ||||
| M5 | 10 (21%) | ||||
| M6 | 2 (4%) | ||||
| Race* | White | 56 (71%) | 41 (80%) | 15 (54%) | 0.008 |
| African American/Black |
14 (17%) | 8 (16%) | 6 (21%) | ||
| Hispanic | 5 (6%) | 1 (2%) | 4 (14%) | ||
| American Indian |
3 (4%) | 0 (0%) | 3 (11%) | ||
| Asian | 1 (2%) | 1 (2%) | 0 (0%) | ||
| Minority | Yes | 23 (66%) | 10 (20%) | 13 (46%) | 0.02 |
| No | 56 (71%) | 41 (80%) | 15 (54%) | ||
| Marital Status* |
Married/ Partnered |
53 (72%) | 36 (74%) | 17 (68%) | 0.79 |
| Other | 21 (28%) | 13 (27%) | 8 (32%) | ||
| Insurance | Public | 43 (54%) | 29 (56%) | 14 (50%) | 0.65 |
| Private | 37 (46%) | 23 (44%) | 14 (50%) |
Missing the following variables: subtype=6, race=1 and marital status=6.
ED visits and Hospitalization
Overall, 81% (n=65) of patients had at least one unplanned visit to the ED or hospital within the first year; 93% (n=26) of ALL patients had events compared to 75% (n=39) of AML patients (Table 2). Of the 65 patients who had at least one ED or hospitalization, 30 (38%) had one event, 14 (18%) had two, and 21 (26%) had three or more events. The mean number of events was significantly higher for ALL compared to AML patients (2.43 v 1.33, p=0.005). The time to first event was also compared between groups and showed that the median time to first event was shorter for the ALL compared to AML patients (36.5 days v 115 days, p=0.005).
Table 2.
Clinical and Event Characteristics
| Overall (n=80) n % |
AML (n=52) n % |
ALL (n=28) n % |
||
|---|---|---|---|---|
| Disease Status | ||||
| In Remission | 56 (70%) | 34 (65%) | 22 (79%) | 0.31 |
| Not in remission | 24 (30%) | 18 (35%) | 6 (21%) | |
| Number of Events | ||||
| 0 | 15 (19%) | 13 (25%) | 2 (7%) | 0.05 |
| 1 | 30 (38%) | 21 (40%) | 9 (32%) | |
| 2 | 14 (18%) | 9 (17%) | 5 (18%) | |
| >3 | 21 (27%) | 9 (17%) | 12 (43%) | |
| Time to 1st Event (median, 95% CI) |
72 (47–115) | 115 (63–154) | 36.5 (26–66) | 0.005 |
These 65 patients who had at least one ED or hospitalization had a total of 137 events, 6% of which were only to the ED, 34% entering the hospital through the ED, and 60% only to the hospital. Chief reasons for use of services were classified into 1 of 10 categories based on final discharge diagnoses (Table 3). Categorization of discharge diagnoses was guided by the Mayer et al (2011) paper with categories including neutropenic fever, infection, anemia, cardiovascular, gastrointestinal, respiratory, pain, thrombocytopenia, drug reaction, and other. Neutropenic fever and infection were collapsed into one category and anemia and thrombocytopenia were collapsed into another category due to the overlap in discharge diagnoses descriptors. The “other” category included reasons such as a fall, cholecystitis, and optic neuropathy.
Table 3.
AML v ALL 1st ED or Hospital Reason
| Event Reasons | Overall n % |
AML n % |
ALL n % |
|---|---|---|---|
| Neutropenic fever/infection | 76 (56%) | 43 (62%) | 33 (49%) |
| Anemia/thrombocytopenia | 16 (12%) | 5 (7%) | 11 (16%) |
| Gastrointestinal | 14 (10%) | 2 (3%) | 12 (18%) |
| Respiratory | 10 (7%) | 7 (10%) | 3 (4%) |
| Pain | 7 (5%) | 4 (6%) | 3 (4%) |
| Cardiovascular | 4 (3%) | 2 (3%) | 2 (3%) |
| Drug Reaction | 2 (2%) | 2 (3%) | 0 (0%) |
| Other | 8 (6%) | 4 (6%) | 4 (6%) |
The most common reasons for all visits were neutropenic fever/infection (56%), bleeding (12%), and GI problems (10%). For both AML and ALL patients, the most common event reason was neutropenic fever/infection, but it was higher for AML compared to ALL (62% v 49%, p<0.0001). The reasons for the other AML events were 7% bleeding and 10% respiratory, compared to the ALL patients who had 12% GI issues and 11% bleeding events. [Table 3].
Number of Events
No significant differences were seen in the number of events based on gender, minority, marital status, or being in remission at discharge (Table 4). However, in univariable analysis, the variables that showed associations were: disease status (p= 0.002), insurance (p= 0.06), and age had more events (p= 0.001). The mean number of events for ALL patients was 2.43 compared to 1.33 for AML patients, 2.05 for private insurance compared to 1.42 for those with government insurance, and 2.23 for those under 50 years old compared to 1.20 for those older. In a multivariable model for the outcome of number of events, leukemia type and age retained their significant associations with number of events (p=0.006, 0.017), respectively. On average, patients with ALL had 1 more event than those with AML, and the number of events decreased by 0.24 for each 10 year increase in age.
Table 4.
Linear Regression Model for Number of Events
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | |
| Gender (male) | −.083 | .81 | − | − |
| Minority | −.16 | .68 | −.57 | .11 |
| Not married | .32 | .41 | − | − |
| Government Insurance |
−.64 | .06 | −.47 | .14 |
| ALL | 1.1 | .002 | .99 | .006 |
| In remission | −.17 | .64 | −.45 | .19 |
| Age Diagnosis (10 years) |
−.30 | .001 | −.24 | .017 |
Time to First Event
Time from discharge to first event was estimated for the entire group and found to be 72 days (95%CI: 47–115). In univariable analysis, only age and leukemia type showed significant associations with time to event (p=0.001, 0.01, respectively, Table 5). The median time to event was 44 days for those younger than 50 compared to 115 for those older and 36.5 days for ALL patients compared to 115 for ALL patients. No other characteristics showed differences. In a multivariable model leukemia type and age retained their significant association with time to first event. After controlling for other covariates, the HR for each 10 unit increase in age is 0.76 indicating fewer ED visits and hospitalizations occurred in older patients. From the same multivariable model, the HR for ALL v AML was 1.8 indicating more ED visits and hospitalizations with that type of leukemia.
Table 5.
Cox Proportional Hazard Model for Time to First Event
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| Hazard Ratio | p-value | Hazard Ratio | p-value | |
| Gender (male) | 1.18 | .51 | − | − |
| Minority | 1.08 | .78 | .90 | .72 |
| Not married | 1.25 | .44 | − | − |
| Government Insurance |
0.63 | .07 | .79 | .37 |
| ALL | 2.02 | .01 | 1.8 | .03 |
| In remission | 1.03 | .93 | 0.78 | .41 |
| Age Diagnosis (10 years) |
0.75 | .001 | 0.76 | .004 |
Discussion
This retrospective chart review showed that 81% of patients discharged from initial induction treatment for acute leukemia had an unplanned visit to the ED or hospital within the first year. Younger age and having ALL made patients more likely to have an unplanned visit, even after controlling for gender, minority status, insurance, and remission status. The top reason for these visits were neutropenic fever/ infection, bleeding and GI problems. This is the first study, to our knowledge, to explore reasons for ED and hospital utilization by adults with acute leukemia after induction therapy.
It is not surprising that patients treated for acute leukemia were admitted for neutropenic fever/infection. A fever is often the first clinical sign of infection or a manifestation of the leukemic process and patients are counseled to seek medical attention when first developing a fever (Raab et al, 1960; Flowers et al, 2013). Evidence based guidelines support early, empirical broad-spectrum antibiotic therapy within 1 hour after blood cultures have been obtained when a neutropenic patient presents with a fever of 100.4 or higher (Eastman, 2013). Some of these patients had central line associated bloodstream infection (CLABSI) which led to them being hospitalized due to local or systemic symptoms. Bryant et al (2014) presented a case study on a younger ALL male who entered the ED with febrile neutropenia. This case study provides appropriate, evidence based instructions for care of acute leukemia patients who enter the ED with febrile neutropenia. A quick, but thorough assessment is essential to improve the outcomes for a patient with neutropenic fever or an infection.
Bleeding was the 2nd most common reason (12% of visits) for care that requires immediate medical attention, and, along with thrombosis, is a significant risk factor for early mortality in patients with acute leukemia (Rickles et al 2007). Patients with acute leukemia are at a high risk for bleeding, including hemorrhage, and preventive measures can reduce this risk (Pereira & Phan, 2004). Bleeding can occur for a variety of reasons, one being low platelet count. Oncology providers must educate the patient and caregivers on earlier identification and recognition of bleeding. Creedle et al (2012) support standardized patient education can improve health outcomes for the patient and caregiver.
Gastrointestinal issues such as nausea, vomiting, and dehydration were the 3rd most common reason (10% of visits) for care, particularly patients with ALL. Early recognition of GI issues may decrease the number of ED visits which may be successfully treated as an outpatient. Vandyk et al (2012) systematic review reported fever, infection, pain, and respiratory distress were common symptoms identified when patients with cancer entered the ED. The urgency for all care services was warranted and was deemed clinically important.
Multivariable modeling identified those patients with ALL as using health services more frequently, which was unexpected. Future research exploring why these patient characteristics had higher use of services is needed. These patients may warrant further monitoring by patient navigators, follow-up calls, and more frequent clinic visits. Additionally, increasing patient age was associated with fewer events; for each 10 year increase in age the average number of events decreased by 0.24. This finding may be associated with the ALL sample who were more likely to be younger, sicker, and with a shorter time from diagnosis to their first event. Our findings using Donabedian’s framework supported the idea that when patients with acute leukemia enter the ED or hospital (structure), their diagnoses are accurately made and appropriately treated (process), resulting in an ED discharge or admission to the hospital for further evaluation (outcome).
Our study is limited by having been conducted at a single institution and retrospectively collecting data from medical charts. We were unable to collect data on other health services used by these patients outside of this one setting. We saw that patients were returning for visits with their physician, and thus it is reasonable to assume that the physician would have commented on ED or hospitalization visits in their notes. We did not capture type of induction therapy which may impact frequency and use of services. Despite limitations, this study highlights ED visits and hospitalization use in the acute leukemia population, which spans from young adulthood to older adult ages.
In conclusion, our study builds upon previous preliminary work exploring ED visits by patients with cancer (Mayer et al, 2011). We were able to follow these patients from the time of discharge from induction for a full year after and demonstrated that younger age and having ALL contributed to having more unplanned visits to the ED and hospital. With an 81% unplanned visit to the ED or hospital within the first year, there are patients at risk for services, and others at higher risk.
This information can help providers identify a higher-risk population for symptom management, and also assist oncology providers in anticipating discharge needs and support follow-up care for those at greater risk for ED and hospital utilization after induction treatment for acute leukemia. Future research linking health claims databases might provide a more comprehensive picture of additional services (ie. home health, hospice) utilized by these patients across their acute leukemia trajectory. Future research that focuses on improving the management of the identified symptoms can help to optimize quality of life and care for these patients.
Highlights.
81% of patients had an unplanned visit to the ED or hospital within the first year.
Top reasons for visits were neutropenic fever/ infection, bleeding and gastrointestinal problems.
Those with ALL were younger, sicker, and had shorter time from diagnosis to 1st event compared to those with AML
Acknowledgments
This project was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number 1UL1TR001111. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work is also supported by the National Cancer Institute Cancer Care Quality Grant R25CA116339 (Bryant), Doctoral Scholarship in Cancer Nursing Renewal DSCNR-13-276-03 from the American Cancer Society and the NIH National Institutes of Nursing Research T32 NR013456 (Walton), and the National Institutes of Health Grant KL2TR000084 (Wood). The authors would also like to acknowledge Lauren Gurschick, MSN, AGNP-BC, OCN, CHPN for her assistance with chart abstraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
Contributor Information
Ashley Leak Bryant, School of Nursing, The University of North Carolina at Chapel Hill, Office: 919-966-5329, Fax: 919-843-9900, anleak@email.unc.edu.
Allison M. Deal, Lineberger Comprehensive Cancer Center Biostatistics Core, The University of North Carolina at Chapel Hill, Allison_Deal@med.unc.edu.
AnnMarie Walton, University of Utah College of Nursing, Social Clinical Research Specialist, Lineberger Comprehensive Cancer Center, Adjunct Clinical Instructor, School of Nursing, The University of North Carolina at Chapel Hill, annmarie.walton@utah.edu, awalton@unch.unc.edu.
William Wood, Division of Hematology/Oncology, The University of North Carolina at Chapel Hill, wawood@med.unc.edu.
Hyman Muss, Division of Hematology/Oncology, The University of North Carolina at Chapel Hill, Hyman_muss@med.unc.edu.
Deborah K. Mayer, School of Nursing, The University of North Carolina at Chapel Hill, Chapel Hill, NC, dmayer@unc.edu.
References
- American Cancer Society. How is acute myeloid leukemia classified? 2014b Retrieved August 4, 2014 at http://www.cancer.org/cancer/leukemia-acutemyeloidaml/detailedguide/leukemia-acute-myeloid-myelogenous-classified.
- Cancer Hospitalizations for Adults, 2009. HCUP Statistical Brief #125. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Feb, Anhang Price, R. (RAND), Stranges, E. (Thomson Reuters) and Elixhauser, A. (Agency for Healthcare Quality and Research) http://www.ncbi.nlm.nih.gov/books/NBK92614/ [PubMed] [Google Scholar]
- Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMAALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182:563–568. doi: 10.1503/cmaj.091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini CL, Hackney AC, Garcia R, Groff D, Evans E, Shea T. The effects of an exercise program in leukemia patients. Integrative Cancer Therapies. 2009;8:130–138. doi: 10.1177/1534735409334266. [DOI] [PubMed] [Google Scholar]
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Journal of American Medical Association. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- Bozdemir N, Eray O, Eken C, et al. Demographics, clinical presentations and outcomes of cancer patients admitted to the emergency department. Turk J Med Sci. 2009;39:235–240. [Google Scholar]
- Bryant AL, Walton AM, Albrecht TA. Care of the patient with febrile neutropenia with acute leukemia. Journal of Emergency Nursing. 2013 doi: 10.1016/j.jen.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstien A, Kuhne R, Minder E, Marek A, Goede J, Schanz U, et al. Abdominal pain in a patient with acute lymphoblastic leukaemia. Annals of Hematology. 89:211–212. doi: 10.1007/s00277-009-0789-4. [DOI] [PubMed] [Google Scholar]
- Chang PH, Lai YH, Shun SC, Lin LY, Chen MI, Yang Y, et al. Effects of a walking intervention on fatigue-experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: A randomized controlled trial. Journal of Pain and Symptom Management. 2008;35:524–534. doi: 10.1016/j.jpainsymman.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Cheville AL. Cancer-related fatigue. Physical Medicine Rehabilitation Clinics of North American. 2009;20(2):405–416. doi: 10.1016/j.pmr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the loves of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- Creedle C, Leak A, Deal A, Walton A, Talbert G, Riff B, Hornback A. The Impact of a CarePartner Program on Two Inpatient Oncology Units. Journal of Cancer Education. 2012;27:250–256. doi: 10.1007/s13187-011-0302-3. [DOI] [PubMed] [Google Scholar]
- Donabedian A. Evaluating the quality of medical care. The Millbank Quarterly. 2005;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle C, Park E, Lai B, Weeks J, Ayanian J, Block S. Identifying potential indicators of the quality of end of life cancer care from administrative data. J Clin Oncol. 2003;21(5):1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- Escalante CP, Kallen MA, Valdres RU, Morrow PK, Manzullo EF. Outcomes of a cancer-related fatigue clinic in a comprehensive cancer center. Journal of Pain and Symptom Management. 2010;39(4):691–701. doi: 10.1016/j.jpainsymman.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, et al. Antimicrobial Prophylaxis and Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology Clinical Practice Guideline. 2013;31(6):794–810. doi: 10.1200/JCO.2012.45.8661. [DOI] [PubMed] [Google Scholar]
- Hackenwerth AM, Waller AE, Ising AL, et al. North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT) and the National Hospital Ambulatory Medical Care Survey (NHAMCS): Comparison of emergency department data. Academy of Emergnecy Medicine; [DOI] [PubMed] [Google Scholar]
- Hewitt M, Simone JV, editors. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- Klepin H, Danhauer S, Tooze J, Stott K, Daley K, Vishnevsky T, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. Journal of Geriatric Oncology. 2011;12:11–17. doi: 10.1016/j.jgo.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. Journal of Clinical Oncology. 2007;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- Leak A, Mayer DK, Wyss A, Travers D, Waller A. Why do Cancer Patients Die in the Emergency Department? An analysis of 283 Deaths in NC EDs. American Journal of Hospice and Palliative Medicine. 2013;30(2):178–182. doi: 10.1177/1049909112445306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: National Academies Press; 2006. [Google Scholar]
- Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with Cancer Visit Emergency Departments? Results of a 2008 Population Study in North Carolina. J Clin Oncol. 2011;29(19):2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J, Phan T. Management of Bleeding in Patients with Advanced Cancer. The Oncologist. 2004;9(5):561–570. doi: 10.1634/theoncologist.9-5-561. [DOI] [PubMed] [Google Scholar]
- Raab S, Hoeprich P, Wintrobe M, Cartwright G. The Clinical Significance of Fever in Acute Leukemia. 1960;16:1609–1628. [PubMed] [Google Scholar]
- Rickles FR, Falanga A, Montesinos P, Sanz M, Brenner B, Barbui T. Bleeding and thrombosis in acute leukemia: What does the future of therapy look like? Thrombosis Research. 2007;120(Suppl 2):S99–S106. doi: 10.1016/S0049-3848(07)70137-8. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Wewers D, Heinecke A, Sauerland C, Koch OM, van de Loo J, et al. Fatigue as an important aspect of quality of life in patients with acute myeloid leukemia. Leukemia Research. 2002;26:355–362. doi: 10.1016/s0145-2126(01)00145-x. [DOI] [PubMed] [Google Scholar]
- Shabbir M, O’Neill S, Fisher-Schlombs K, Breunis H, Brandwein J, Timilshina N, et al. A clinical trial of supervised exercise for adult inpatient with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leukemia Research. 2012;36:1255–1261. doi: 10.1016/j.leukres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyk A, Harrison M, Macartney G, Ross-White A, Stacey D. Emergency department visits for symptoms experienced by oncology patients: a systematic review. Supportive Care in Cancer. 2012;29:1589–1599. doi: 10.1007/s00520-012-1459-y. [DOI] [PubMed] [Google Scholar]