Abstract
The antitrypanosomal and antifiliarial drug suramin is currently under investigation for treatment of advanced malignancies including prostatic cancer, adrenocortical cancer, and some lymphomas and sarcomas. Here we show that suramin is a potent inhibitor of the nuclear enzyme DNA topoisomerase II. Suramin inhibited purified yeast topoisomerase II with an IC50 of about 5 microM, as measured by decatenation or relaxation assays. Suramin did not stabilize the covalent DNA-topoisomerase II reaction intermediate ("cleavable complex"), whereas other inhibitors of this enzyme, such as amsacrine, etoposide, and the ellipticines, are known to stabilize the intermediate. In contrast, the presence of suramin strongly inhibited the cleavable-complex formation induced by amsacrine or etoposide. Accumulation of the endogenous cleavable complex was also inhibited. Suramin entered the nucleus of DC-3F Chinese hamster fibrosarcoma cells exposed to radiolabeled suramin for 24 hr as shown by both optic and electron microscopy. The suramin present in the nucleus seemed to interact with topoisomerase II, since suramin reduced the number of amsacrine-induced protein-associated DNA strand breaks in DC-3F cells and protected these cells from the cytotoxic action of amsacrine. Cells resistant to 9-hydroxyellipticine, which have been shown to have an altered topoisomerase II activity, are about 7-fold more resistant to suramin than the sensitive parental cells as shown by 72-hr growth inhibition assay. Our results suggest that DNA topoisomerase II is a target of suramin action and that this action may play a role in the cytotoxic activity of suramin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armand J. P., Cvitkovic E. Suramin: a new therapeutic concept. Eur J Cancer. 1990 Apr;26(4):417–419. doi: 10.1016/0277-5379(90)90005-e. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
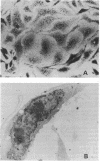
- Bouteille M., Dupuy-Coin A. M., Moyne G. Techniques of localization of proteins and nucleoproteins in the cell nucleus by high resolution autoradiography and cytochemistry. Methods Enzymol. 1975;40:3–41. doi: 10.1016/s0076-6879(75)40003-9. [DOI] [PubMed] [Google Scholar]
- Broder S., Yarchoan R., Collins J. M., Lane H. C., Markham P. D., Klecker R. W., Redfield R. R., Mitsuya H., Hoth D. F., Gelmann E. Effects of suramin on HTLV-III/LAV infection presenting as Kaposi's sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo. Lancet. 1985 Sep 21;2(8456):627–630. doi: 10.1016/s0140-6736(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. DNA is linked to the rat liver DNA nicking-closing enzyme by a phosphodiester bond to tyrosine. J Biol Chem. 1981 May 25;256(10):4805–4809. [PubMed] [Google Scholar]
- Charcosset J. Y., Bendirdjian J. P., Lantieri M. F., Jacquemin-Sablon A. Effects of 9-OH-ellipticine on cell survival, macromolecular syntheses, and cell cycle progression in sensitive and resistant Chinese hamster lung cells. Cancer Res. 1985 Sep;45(9):4229–4236. [PubMed] [Google Scholar]
- Charcosset J. Y., Saucier J. M., Jacquemin-Sablon A. Reduced DNA topoisomerase II activity and drug-stimulated DNA cleavage in 9-hydroxyellipticine resistant cells. Biochem Pharmacol. 1988 Jun 1;37(11):2145–2149. doi: 10.1016/0006-2952(88)90573-4. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Levine A. M., Mildvan D., Kaplan L. D., Wolfe P., Rios A., Groopman J. E., Gill P., Volberding P. A., Poiesz B. J. Suramin therapy in AIDS and related disorders. Report of the US Suramin Working Group. JAMA. 1987 Sep 11;258(10):1347–1351. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Leof E. B., Shipley G. D., Moses H. L. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol. 1987 Jul;132(1):143–148. doi: 10.1002/jcp.1041320120. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 1979 Nov;8(1):9–22. doi: 10.1016/0304-3835(79)90017-x. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Fantini J., Rognoni J. B., Roccabianca M., Pommier G., Marvaldi J. Suramin inhibits cell growth and glycolytic activity and triggers differentiation of human colic adenocarcinoma cell clone HT29-D4. J Biol Chem. 1989 Jun 15;264(17):10282–10286. [PubMed] [Google Scholar]
- Fernandes D. J., Danks M. K., Beck W. T. Decreased nuclear matrix DNA topoisomerase II in human leukemia cells resistant to VM-26 and m-AMSA. Biochemistry. 1990 May 1;29(17):4235–4241. doi: 10.1021/bi00469a028. [DOI] [PubMed] [Google Scholar]
- Fernandes D. J., Smith-Nanni C., Paff M. T., Neff T. A. Effects of antileukemia agents on nuclear matrix-bound DNA replication in CCRF-CEM leukemia cells. Cancer Res. 1988 Apr 1;48(7):1850–1855. [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hawking F. Suramin: with special reference to onchocerciasis. Adv Pharmacol Chemother. 1978;15:289–322. doi: 10.1016/s1054-3589(08)60486-x. [DOI] [PubMed] [Google Scholar]
- Hensey C. E., Boscoboinik D., Azzi A. Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A. FEBS Lett. 1989 Nov 20;258(1):156–158. doi: 10.1016/0014-5793(89)81639-4. [DOI] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kuo M. D. Bovine brain-derived growth factor. Purification and characterization of its interaction with responsive cells. J Biol Chem. 1986 Sep 5;261(25):11600–11607. [PubMed] [Google Scholar]
- Ishihara M., Fedarko N. S., Conrad H. E. Transport of heparan sulfate into the nuclei of hepatocytes. J Biol Chem. 1986 Oct 15;261(29):13575–13580. [PubMed] [Google Scholar]
- Jindal H. K., Anderson C. W., Davis R. G., Vishwanatha J. K. Suramin affects DNA synthesis in HeLa cells by inhibition of DNA polymerases. Cancer Res. 1990 Dec 15;50(24):7754–7757. [PubMed] [Google Scholar]
- Kopp R., Pfeiffer A. Suramin alters phosphoinositide synthesis and inhibits growth factor receptor binding in HT-29 cells. Cancer Res. 1990 Oct 15;50(20):6490–6496. [PubMed] [Google Scholar]
- Larsen A. K., Paoletti J., Belehradek J., Jr, Paoletti C. Uptake, cytofluorescence, and cytotoxicity of oxazolopyridocarbazoles (amino acid-ellipticine conjugates) in murine sarcoma cells. Cancer Res. 1986 Oct;46(10):5236–5240. [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Mahoney C. W., Azzi A., Huang K. P. Effects of suramin, an anti-human immunodeficiency virus reverse transcriptase agent, on protein kinase C. Differential activation and inhibition of protein kinase C isozymes. J Biol Chem. 1990 Apr 5;265(10):5424–5428. [PubMed] [Google Scholar]
- Mitsuya H., Popovic M., Yarchoan R., Matsushita S., Gallo R. C., Broder S. Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science. 1984 Oct 12;226(4671):172–174. doi: 10.1126/science.6091268. [DOI] [PubMed] [Google Scholar]
- Nakajima M., DeChavigny A., Johnson C. E., Hamada J., Stein C. A., Nicolson G. L. Suramin. A potent inhibitor of melanoma heparanase and invasion. J Biol Chem. 1991 May 25;266(15):9661–9666. [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M., Richard M. Suramin blockade of insulinlike growth factor I-stimulated proliferation of human osteosarcoma cells. J Natl Cancer Inst. 1990 Aug 15;82(16):1349–1352. doi: 10.1093/jnci/82.16.1349. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Kohn K. W. Topoisomerase alterations associated with drug resistance in a line of Chinese hamster cells. NCI Monogr. 1987;(4):83–87. [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R. E., Swack J. A., McCurdy A. Altered DNA topoisomerase II activity in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Jun;46(6):3075–3081. [PubMed] [Google Scholar]
- Pommier Y., Schwartz R. E., Zwelling L. A., Kerrigan D., Mattern M. R., Charcosset J. Y., Jacquemin-Sablon A., Kohn K. W. Reduced formation of protein-associated DNA strand breaks in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Feb;46(2):611–616. [PubMed] [Google Scholar]
- Riou G. F., Gutteridge W. E. Comparative study of kinetoplast DNA in culture, blood and intracellular forms of Trypanosoma cruzi. Biochimie. 1978;60(4):365–379. doi: 10.1016/s0300-9084(78)80670-1. [DOI] [PubMed] [Google Scholar]
- Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984 Dec;44(12 Pt 1):5857–5860. [PubMed] [Google Scholar]
- Ruprecht R. M., Lorsch J., Trites D. H. Analysis of suramin plasma levels by ion-pair high-performance liquid chromatography under isocratic conditions. J Chromatogr. 1986 Jun 13;378(2):498–502. doi: 10.1016/s0378-4347(00)80750-1. [DOI] [PubMed] [Google Scholar]
- Salles B., Charcosset J. Y., Jacquemin-Sablon A. Isolation and properties of Chinese hamster lung cells resistant to ellipticine derivatives. Cancer Treat Rep. 1982 Feb;66(2):327–338. [PubMed] [Google Scholar]
- Spigelman Z., Dowers A., Kennedy S., DiSorbo D., O'Brien M., Barr R., McCaffrey R. Antiproliferative effects of suramin on lymphoid cells. Cancer Res. 1987 Sep 1;47(17):4694–4698. [PubMed] [Google Scholar]
- Stein C. A., LaRocca R. V., Thomas R., McAtee N., Myers C. E. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989 Apr;7(4):499–508. doi: 10.1200/JCO.1989.7.4.499. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Tse Y. C., Kirkegaard K., Wang J. C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J Biol Chem. 1980 Jun 25;255(12):5560–5565. [PubMed] [Google Scholar]
- Van Oosterom A. T., De Smedt E. A., Denis L. J., de Bruijn E. A., Mahler C. Suramin for prostatic cancer: a phase I/II study in advanced extensively pretreated disease. Eur J Cancer. 1990 Apr;26(4):422–422. doi: 10.1016/0277-5379(90)90008-h. [DOI] [PubMed] [Google Scholar]
- Waring M. J. The effects of antimicrobial agents on ribonucleic acid polymerase. Mol Pharmacol. 1965 Jul;1(1):1–13. [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Worland S. T., Wang J. C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989 Mar 15;264(8):4412–4416. [PubMed] [Google Scholar]