Abstract
Background
Although satisfactory outcomes have been reported following total knee replacement (TKR), full recovery of muscle strength and physical function is rare. We developed a relative activation index (RAI) to compare leg muscle activity from unnormalized surface electromyography (sEMG) between TKR and control subjects.
Methods
Nineteen TKR and 19 control subjects underwent gait analysis and sEMG. RAIs were calculated by dividing the average sEMG for two consecutive subphases of stance defined by the direction of the external sagittal plane moment (flexion or extension).
Results
RAIs and external moments indicate TKR subjects have less initial stance antagonist rectus femoris activity (p=0.004), greater middle stance antagonist biceps femoris activity (p<0.001), and less late stance agonist biceps femoris activity (p<0.001) than control subjects. Individuals with TKR demonstrate increased flexor muscle activation during weight bearing, potentially contributing to altered gait patterns found during stance phase of gait.
Conclusion
The RAI helps detail whether decreased external moments correspond to less agonist or more antagonist muscle activity to determine true muscle activity differences between subject groups. Identifying the mechanisms underlying altered muscle function both pre and post-TKR is critical for developing rehabilitation strategies to address functional deficits and disability found in this patient population.
Keywords: total knee replacement, surface electromyography, gait analysis, muscle co-contraction
Introduction
Subjects with total knee replacement (TKR) often walk with an abnormal sagittal plane external knee joint moment [1]. The sagittal plane external knee moment has been considered a surrogate for knee flexor and extensor muscle activity [2] representing net muscle activity equal and opposite to the action of internal knee structures (e.g. muscles). Thus, abnormal flexion-extension moment patterns are thought to be associated with aberrant flexor and extensor muscle activity [3]. While successful outcomes have been reported following TKR [4], significant functional deficits remain post-surgery [5]. In fact, approximately 25% report a fall within the year following TKR surgery [6]. It has been suggested that these functional deficits may be related to persistent altered muscle function in the lower limb. Unfortunately, without additional muscle activity information, the sagittal plane external knee moment cannot give insight to whether a lower moment means reduced agonist muscle activity or increased antagonist muscle activity, or both.
Normalization of surface electromyography (sEMG) signals is necessary when comparing muscle activity across different subjects and activities. Often a maximum voluntary isometric contraction (MVIC) is collected for normalization of sEMG signals. Subjects with knee pathology, including a total knee replacement (TKR), have difficulties performing a true MVIC because of pain, the perception that they are injuring themselves, and muscle weakness characterized by the specific pathology [7]. This could result in erroneous conclusions if a lower than maximum MVIC results in high normalized muscle activity.
In this study sEMG data was utilized without prior normalization and a relative activity index was developed to evaluate sEMG data within phases of stance defined by the sagittal plane external knee moment. Such an index provides additional information about muscle function not available through separate analysis of external moments or unnormalized sEMG. While researchers have defined co-contraction indices to investigate TKR agonist-antagonist muscle pair activity [8–10], none of these studies used unnormalized EMG data and sagittal plane moments to characterize deviations from normal. Increased co-contraction has been proposed as a compensation for perceived instability yet recent neurophysiological studies have suggested an alternative hypothesis for altered muscle activation patterns found in persons with knee OA [11, 12].
Thus, the objective of this study was to describe a new relative activation index (RAI) for the purpose of characterizing muscle activation patterns during walking in individuals following TKR which will allow analysis of relative antagonist muscle activity without normalizing sEMG signals. We hypothesized that RAIs of TKR subjects would differ from control subjects. Sagittal plane external extension moments, muscle on-off times and co-contraction times were reported to allow for the evaluation of our TKR population behavior within the broader context of the literature.
Material and methods
Nineteen TKR subjects were enrolled in this IRB approved study after informed consent. The TKR subjects were all implanted with a NexGen cruciate retaining TKR (Zimmer, Warsaw, IN, USA) by orthopaedic surgeons from the same practice (Midwest Orthopaedics at Rush, Chicago, IL, USA). Inclusion criteria were an original diagnosis of primary degenerative osteoarthritis, no history of neurological disorders or significant lumbar spine disease, at least 10 months post-operative [13–15], the ability to perform activities that required a considerable amount of knee flexion (walking, sitting, and climbing stairs), activity in the workforce or at home, and participation in regular exercise. Control subjects with no evidence of symptomatic osteoarthritis and a radiographic K/L grade ≤ 2 were chosen from a larger cohort tested in our laboratory with available gait and sEMG data [16, 17].
All subjects underwent gait analysis using an optoelectronic video-based measuring system (Qualisys North America Inc., Charlotte, NC, USA), and a multi-component force plate (Bertec, Columbus, OH, USA) embedded in the level walkway to obtain foot-ground reaction forces. Knee joint flexion angles and three-dimensional external moments were obtained using a published marker set and standard inverse dynamics methods [18–20]. All subjects walked along the level walkway at three different walking speeds: a self-selected normal walking speed, and speeds slightly slower and faster than the self-selected normal walking speed. For TKR subjects, walking trials at the self-selected normal walking speed were analyzed. For control subjects, we analyzed trials with walking speeds that were the closest match to the TKR subjects. No differences were found between TKR and control subjects for walking trial speed (TKR: 1.3± 0.2 m/s, Control: 1.3±0.2 m/s, p=0.811, t-test).
Surface EMG data were collected simultaneously with the kinematic and kinetic data using a TeleMyo transmitter and receiver, model 2400T/2400R (Noraxon U.S.A. Inc., Scottsdale, AZ, USA) for six major leg muscles: vastus medialis, rectus femoris, vastus lateralis, bicep femoris, semimembranosus/semitendinosus, medial gastrocnemius. Self-adhesive pre-gelled dual electrodes (Ag/AgCl electrodes, Noraxon U.S.A. Inc., Scottsdale, AZ, USA) were placed along the muscle fibers over the muscle belly and distal to the motor point region. The skin was cleaned using antimicrobial wipes prior to electrode application to reduce inter-electrode impedance caused by dead skin cells, oil, and moisture [7]. The sEMG signals were pre-amplified (500 gain), band pass filtered between 10 and 500 Hz, and sampled at a rate of 1200 Hz. After data collection, the Noraxon software was used to process the sEMG signals. The sEMG signals were band-pass filtered with a Finite Impulse Response filter (Lancosh type 79 point window filtering, band-pass range from 20 to 450 Hz), rectified, and smoothed using Root Mean Square calculations over a 50 ms window. Muscle “on” and “off” timings were obtained from the processed voltages using custom-written software (Matlab R2012a, The Mathworks, Inc., Natick MA). Following Abbink et al. [21], a threshold was calculated from histograms of the processed voltage amplitudes. The threshold corresponded to the voltage amplitude with a frequency of occurrence three standard deviations above the voltage amplitude with the highest frequency of occurrence. When the voltage amplitude was greater than the threshold the muscle was deemed “on”, and when the voltage amplitude was less than the threshold the muscle was considered “off”.
The variables of interest in this study included (1) knee flexion angle and sagittal plane external knee moments, (2) co-contraction times of agonist-antagonist muscle pairs during stance, and (3) the relative muscle activation (RAI) for subphases of stance. External sagittal plane knee moments analyzed included the first maximum extension moment, the maximum flexion moment, and the second maximum extension moment.
We defined muscle pair co-contraction time as the percentage of stance during which both muscles of an agonist-antagonist pair were active. The agonist-antagonist muscle pairs were vastus lateralis-biceps femoris, vastus medialis-semimembranosus/semitendinosus, vastus medialis-medial gastrocnemius, and semimembranosus/semitendinosus-medial gastrocnemius. These pairs were chosen based on their anatomical position about the knee: either anterior or posterior and either medial or lateral. This defines agonist-antagonist pairs as pairs of muscles that have opposite knee joint function in both the sagittal and frontal planes. The agonist-antagonist muscle co-contraction times were also compared for a quadriceps/hamstring (QUAD-HAM) group where the QUAD group included vastus lateralis, vastus medialis, and rectus femoris muscles, and the HAM group included biceps femoris and semimembranosus/semitendinosus muscles.
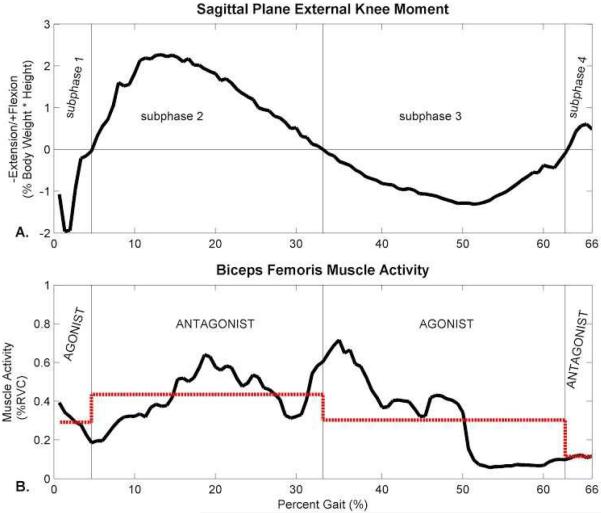
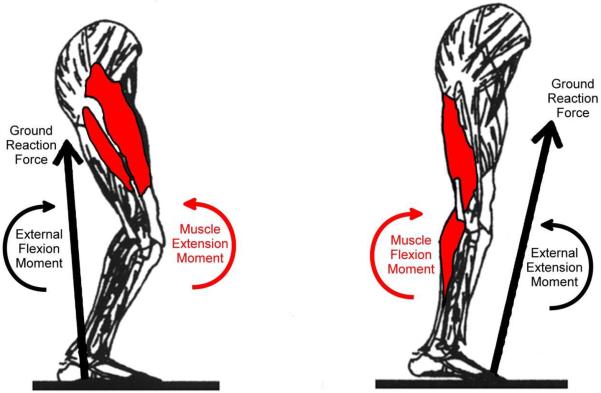
The RAI was defined to quantify antagonist muscle activity from unnormalized sEMG data between two subsequent subphases of stance, as shown in Figure 1. First, subphases of stance were defined by the sign of the external sagittal plane moment (Figure 1A). The sign of the external sagittal plane moment was used to define phases of muscle activity because the external moment reflects net muscle activity equal and opposite to the action of internal knee structures (e.g. muscles, Figure 2). For example, during the first subphase of stance, an external extension (negative) sagittal plane moment is present about the knee. This reflects the net action of knee joint flexors as they create a flexion moment internally about the knee joint equal and opposite to the external extension moment to maintain equilibrium during walking. Next, the sEMG activity was averaged over each subphase of stance (Figure 1B). Finally, the RAI between two consecutive subphases of stance was equal to the average muscle activity of the first subphase divided by the average muscle activity of the second subphase (Equation 1). A RAI less than, equal to, or greater than one means that the average activity of a muscle in the first subphase of stance is less than, equal to, or greater than the average activity of a muscle in the second subphase of stance, respectively. The RAI values can be compared across muscles, activities and subjects, while the average muscle activity values alone cannot.
| (1) |
Figure 1.
Example relative activation index (RAI) calculation. A. Subphases of stance are first defined according to the sign of the external sagittal plane extension moment. B. The RAI between two consecutive subphases of stance is equal to the average sEMG activity during the first subphase divided by the average sEMG activity during the second subphase. Average sEMG activity during each subphase is shown as the dotted line. In this example RAIs equal 0.7 for subphase 1-subphase 2, 1.4 for subphase 2-subphase 3, and 2.6 for subphase 3-subphase 4. EMG data are shown as %RVC (relative voluntary contraction). Such data normalization is optional.
Figure 2.
Moment balance about the knee during gait. The ground reaction force results in an external moment about the knee joint that must be balanced by the action of muscles. When the ground reaction force is posterior to the knee joint (left), it causes an external flexion moment which is balanced by a muscle extension moment created by the net action of the knee extensor muscles. The knee extensors are agonists because they help balance the external moment. When the ground reaction force is anterior to the knee joint (right), it causes an external extension moment which is balanced by a muscle flexion moment created by the net action of the knee flexor muscles. The knee flexors are agonists because they help balance the external moment.
An examination of histograms revealed that many variables were not normally distributed; thus non-parametric tests were used. Mann-Whitney U tests were used to test for differences in knee flexion angles, maximum sagittal plane external knee moments, average sagittal plane external moment during subphases of stance, muscle on-off timings, muscle co-contraction times, and to test the hypothesis that the RAIs were different for the TKRs than the control subjects. Statistical significance was set to an alpha level of 0.05. Nineteen subjects in each group permitted the detection of an effect size of approximately 0.96 with a power level of 0.8.
Results
The TKR group consisted of nine males and ten females. The average time since surgery for the TKR subjects was 38±27 months. Nineteen control subjects (9 males and ten females) were chosen for comparison to the TKR patients. The mean age was 62±7.1 (50–83) years for the TKR subjects and 56±6.9 (46–68) years for the control subjects (p= 0.012, t-test). The mean BMI was 30±6.7 (22–49) kg/m2 for the TKR subjects and 28±4.8 (23–39) kg/m2 for the control subjects (p= 0.275, t-test). Hence, TKR subjects were slightly older, but with a similar BMI than control subjects.
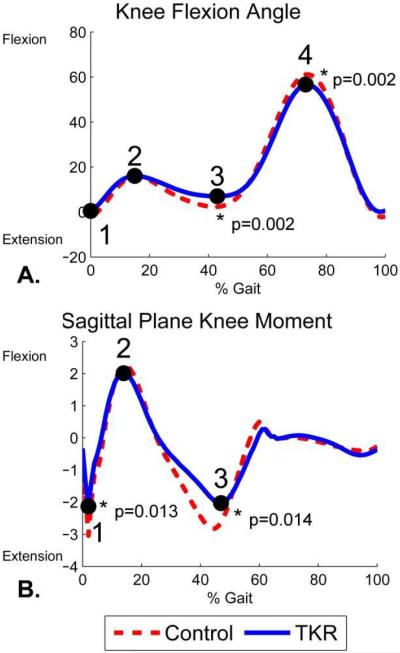
The TKR subjects had less extension during terminal stance (p=0.002) and less flexion during swing (p=0.002) than the controls (Figure 3, Table 1). Among the sagittal plane kinetic variables, there were significant between-group differences only in values of the 1st and 2nd maximum extension moments (Figure 3, Table 1). The maximum extension moments reflect net activity by knee flexors. Both were over 25% lower in TKR subjects compared to controls (1st maximum p=0.013, 2nd maximum p=0.014). The timing (% stance) of the maximum flexion moment and 2nd maximum extension moment occurred significantly later for the TKR subjects compared to controls (maximum flexion p=0.022, 2nd maximum extension p<0.001). The average external sagittal plane knee moments were significantly lower for the TKR subjects than the control subjects in subphases 1, 2, and 3 (p=0.003–0.028, Table 2), but not in subphase 4 during the stance phase.
Figure 3.
Kinematics and kinetics measured with marker-based gait analysis during level walking for 19 TKR and 19 control subjects. Numbers represent locations of gait parameters compared between the TKR and control subjects. A. Flexion angle gait parameters included: 1) heel strike flexion angle, 2) mid stance maximum flexion angle, 3) terminal stance minimum flexion angle, and 4) maximum flexion angle during swing. B. External knee moment gait parameters included: 1) 1st maximum extension moment, 2) 1st maximum flexion moment, and 3) 2nd maximum extension moment.
Table 1.
Flexion angles, external sagittal plane moments, and time of occurrence for gait parameters (Figure 3) for the TKR and the control subjects. Flexion angles are given in degrees, external moments are given in percent body weight times height (%BW*Ht), and timings are given as a percentage of the gait cycle and of the stance phase. P-values correspond to Mann-Whitney U tests.
| TKR | Control | p | |
|---|---|---|---|
| Flexion Angle (°) | |||
| Heel Strike Flexion Angle | 0.4 ± 5.0 | −2.0 ± 3.2 | 0.080 |
| Mid Stance Maximum Flexion Angle | 16 ± 3.9 | 16 ± 5.4 | 0.686 |
| % Stance | 25 ± 3.9 | 26 ± 2.4 | 0.773 |
| Terminal Stance Minimum Flexion Angle | 6.3 ± 5.9 | 1.4 ± 3.4 | 0.002 |
| % Stance | 65 ± 9.6 | 67 ± 7.5 | 0.686 |
| Maximum Flexion Angle during Swing | 57 ± 5.5 | 62 ± 4.7 | 0.002 |
| % Gait Cycle | 74 ± 1.6 | 74 ± 1.7 | 0.817 |
|
| |||
| Sagittal Plane Moment (%BW*Ht) | |||
| 1st Maximum Extension Moment | −2.3 ± 0.9 | −3.1 ± 1.0 | 0.013 |
| % Stance | 3.4 ± 1.1 | 3.1 ± 0.9 | 0.488 |
| Maximum Flexion Moment | 2.1 ± 0.9 | 2.3 ± 1.5 | 0.297 |
| % Stance | 22 ± 2.5 | 24 ± 2.4 | 0.022 |
| 2nd Maximum Extension Moment | −2.1 ± 0.8 | −3.0 ± 1.0 | 0.014 |
| % Stance | 76 ± 2.2 | 73 ± 3.2 | <0.001 |
Table 2.
Average external sagittal plane knee moments during subphases of stance.
| Average moment in Subphase | TKR | Control | p |
|---|---|---|---|
| subphase 1 | −1.0 ± 0.4 | −1.4 ± 0.4 | 0.003 |
| subphase 2 | 1.2 ± 0.5 | 1.6 ± 0.5* | 0.028 |
| subphase 3 | −1.2 ± 0.4 | −1.7 ± 0.6 | 0.017 |
| subphase 4 | 1.1 ± 0.4 | 1.2 ± 0.5 | 0.452 |
n=17 for the control subjects average subphase 2 external flexion moment because two subjects had an extension moment throughout subphase 2 and subphase 3 (in loading response and mid stance).
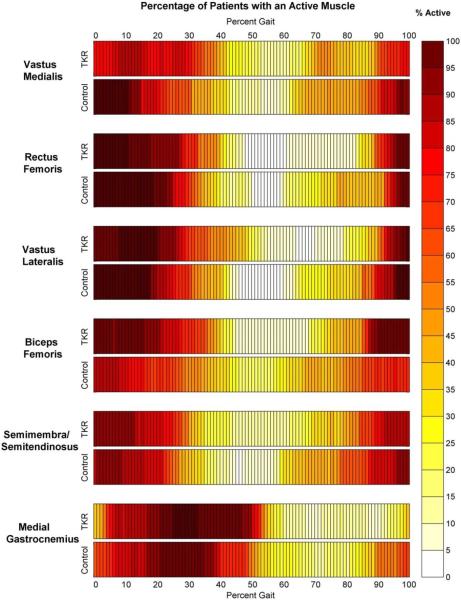
Muscle activity onset and offset appeared very similar between the TKR and control subjects (Figure 4, Table 3). The only statistically significant differences was that the TKR subjects had a later offset of the vastus medialis (p=0.037) than the control subjects. Co-contraction times during stance tended to be longer for TKR subjects (Table 4). For the individual pairing of vastus lateralis and biceps femoris, co-contraction times during stance were significantly longer for the TKRs than the control subjects (p=0.046).
Figure 4.
Percentage of TKR and control subjects with active muscles at each percentage of the gait cycle. Darkest color indicates instances during gait where all 19 of the subjects had muscles considered “on” and white indicates instances during gait where all 19 of the subjects had muscle considered “off”. Muscles were designated as “on” when their sEMG voltages were at least three standard deviations greater than the voltage with the highest frequency of occurrence. The locations of the darkest and lightest areas are different for the TKR and control subjects, indicating differences in the timings of muscle activation.
Table 3.
Muscle onset and offset timings during gait (% gait cycle). P-values correspond to Mann-Whitney U tests.
| Muscle | TKR | Control | p |
|---|---|---|---|
| Vastus Medialis ON | −8.1 ± 19 | −17 ± 13 | 0.181 |
| Vastus Medialis OFF | 38 ± 17 | 28 ± 16 | 0.037 |
| Rectus Femoris ON | −11 ± 8.0 | −14 ± 13 | 0.908 |
| Rectus Femoris OFF | 33 ± 9.4 | 33 ± 8.3 | 0.624 |
| Vastus Lateralis ON | −11 ± 8.7 | −18 ± 12 | 0.116 |
| Vastus Lateralis OFF | 38 ± 12 | 32 ± 9.2 | 0.123 |
| Biceps Femoris ON | −18 ± 10 | −13 ± 20 | 0.583 |
| Biceps Femoris OFF | 34 ± 13 | 34 ± 20 | 0.729 |
| Semitendinosus/Semimembranosus ON | −14 ± 16 | −13 ± 27 | 0.583 |
| Semitendinosus/Semimembranosus OFF | 30 ± 14 | 34 ± 20 | 0.840 |
| Medial Gastrocnemius ON | 3.9 ± 8.1 | −1.9 ± 15 | 0.169 |
| Medial Gastrocnemius OFF | 51 ± 14 | 47 ± 13 | 0.178 |
Table 4.
Percentage of the stance phase that muscle pairs were co-contracted (both on). QUAD – HAM indicates co-contraction between a QUAD group (vastus lateralis, vastus medialis, and rectus femoris muscles) and a HAM group (biceps femoris and semimembranosus/semitendinosus muscles). P-values correspond to Mann-Whitney U tests.
| Muscle Pair | TKR | Control | p |
|---|---|---|---|
| Vastus Lateralis – Biceps Femoris | 49 ± 20 | 36 ± 19 | 0.046 |
| Vastus Medialis – Semitendinosus/Semimembranosus | 35 ± 18 | 35 ± 19 | 0.885 |
| Vastus Medialis – Medial Gastrocnemius | 46 ± 21 | 40 ± 20 | 0.354 |
| Semitendinosus/Semimembranosus – Medial Gastrocnemius | 39 ± 17 | 35 ± 21 | 0.686 |
| QUAD – HAM | 62 ± 17 | 54 ± 13 | 0.085 |
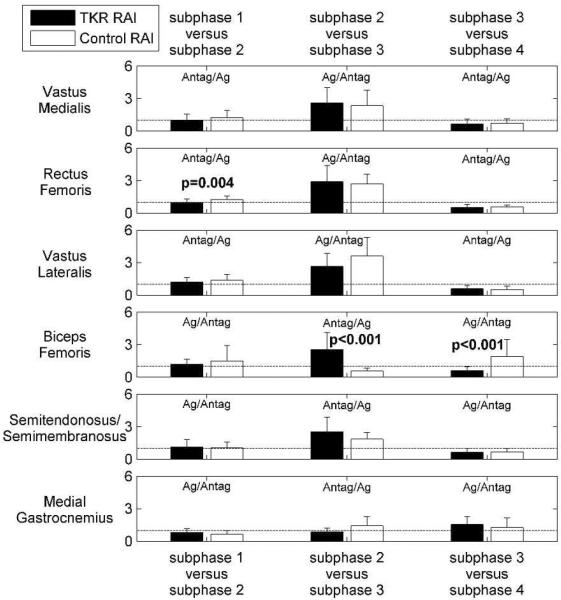
To determine if there were differences in relative muscle activity over subphases of stance within each subject group, RAIs were first compared to “one” using single sample Wilcoxon signed rank tests (Table 5). An RAI of one means that muscle activity in the first subphase was the same as muscle activity in the second subphase. Earlier in stance (subphase 1 versus subphase 2), RAIs for both subject groups were closest to one. The RAIs were statistically different than one for the rectus femoris, vastus lateralis, and medial gastrocnemius muscles of the control subjects (RAIs of 1.2±0.3 p=0.010, 1.4±0.5 p=0.015, and 0.7±0.3 p=0.002, respectively). For the middle of the stance phase (subphase 2 versus subphase 3), RAIs were all different than one (p<0.001–0.003) with the exception of the medial gastrocnemius for both subject groups. RAIs were typically greater than one except for the biceps femoris for the control subjects (RAI 0.6±0.3). For the end of the stance phase (subphase 3 versus subphase 4), RAIs were also different than one (p<0.001–0.018) except for the medial gastrocnemius of the control subjects. RAIs were typically less than one except for the biceps femoris of the control subjects (RAI 1.9±1.6) and the medial gastrocnemius for the TKR subjects (RAI 1.6±0.7).
Table 5.
Relative activation index (RAI) between subphases of stance for 19 TKR and 19 control subjects (mean ± SD). The RAI is equal to the average muscle activation during a subphase of stance divided by the average muscle activation during the next subphase of stance. For RAI comparisons to one, p-values correspond to single sample Wilcoxan signed rank tests. RAI comparisons between TKR and control subjects' p-values correspond to Mann-Whitney U tests.
| Subphase 1 vs. Subphase 2 | Subphase 2 vs. Subphase 3 | Subphase 3 vs. Subphase 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Subject Group | RAI | p RAI vs. 1 | p TKR vs. control | RAI | p RAI vs. 1 | p TKR vs. control | RAI | p RAI vs. 1 | p TKR vs. control |
| Vastus Medialis | TKR | 1.0 ± 0.6 | 0.778 | 0.397 | 2.6 ± 1.4 | <0.001 | 0.38 | 0.7 ± 0.4 | 0.018 | 0.234 |
| Control | 1.2 ± 0.7 | 0.554 | 2.3 ± 1.4 | 0.003 | 0.7 ± 0.4 | 0.008 | ||||
|
| ||||||||||
| Rectus Femoris | TKR | 1.0 ± 0.4 | 0.398 | 0.004 | 2.9 ± 1.5 | <0.001 | 0.876 | 0.5 ± 0.3 | <0.001 | 0.748 |
| Control | 1.2 ± 0.3 | 0.010 | 2.7 ± 0.92 | <0.001 | 0.5 ± 0.2 | <0.001 | ||||
|
| ||||||||||
| Vastus Lateralis | TKR | 1.2 ± 0.4 | 0.053 | 0.415 | 2.6 ± 1.2 | <0.001 | 0.081 | 0.6 ± 0.3 | <0.001 | 0.234 |
| Control | 1.4 ± 0.5 | 0.015 | 3.6 ± 1.7 | <0.001 | 0.5 ± 0.3 | <0.001 | ||||
|
| ||||||||||
| Biceps Femoris | TKR | 1.2 ± 0.5 | 0.117 | 0.300 | 2.5 ± 1.6 | <0.001 | <0.001 | 0.6 ± 0.3 | <0.001 | <0.001 |
| Control | 1.5 ± 1.4 | 0.981 | 0.56 ± 0.3 | <0.001 | 1.9 ± 1.6 | 0.003 | ||||
|
| ||||||||||
| Semitendinosus/Semimembranosus | TKR | 1.1 ± 0.7 | 0.748 | 0.950 | 2.5 ± 1.4 | <0.001 | 0.186 | 0.6 ± 0.4 | 0.002 | 0.435 |
| Control | 1.0 ± 0.5 | 0.981 | 1.9 ± 0.6 | <0.001 | 0.7 ± 0.3 | 0.003 | ||||
|
| ||||||||||
| Medial Gastrocnemius | TKR | 0.8 ± 0.4 | 0.064 | 0.258 | 0.9 ± 0.3 | 0.091 | 0.061 | 1.6 ± 0.7 | 0.006 | 0.123 |
| Control | 0.7 ± 0.3 | 0.002 | 1.4 ± 0.9 | 0.136 | 1.3 ± 0.9 | 0.658 | ||||
The hypothesis that TKR subjects will have different relative muscle activity than healthy controls was partially accepted (Figure 5, Table 5). Earlier in stance (subphase 1 versus subphase 2), RAIs were significantly different between the subject groups for the rectus femoris (p=0.004). For the middle of the stance phase (subphase 2 versus subphase 3), RAIs were significantly different between the subject groups for the biceps femoris (p<0.001). For the end of the stance phase (subphase 3 versus subphase 4), RAIs were again significantly different between the subject groups for the biceps femoris (p<0.001).
Figure 5.
Relative activation index (RAI) between subphases of stance for 19 TKR and 19 control subjects. A RAI value of one (dashed horizontal lines) means that the muscle activity in the earlier subphase is equal to the muscle activity in the later subphase of stance. P-values correspond to comparisons between the TKR and control subjects (Mann-Whitney U-tests).
Discussion
The objective of this study was to quantify antagonist muscle activity with a new muscle activation index that used unnormalized sEMG data. Because external joint moments need to be balanced by muscle activity internally, the necessary minimum agonistic muscle activity can be estimated from external joint moments [18], but the concomitant antagonistic activity remains unknown. The relative activation index (RAI), as introduced here, provides a tool to estimate the additional antagonist muscle contribution from unnormalized sEMG data. This is attractive for TKR subjects because this population has difficulty executing a trustworthy MVIC for normalization of the sEMG signal. The RAI is equal to the average muscle activity (voltage) during a subphase of stance divided by the average muscle activity during the following subphase of stance. In this study, subphases of stance were defined by the external sagittal plane knee moment. A muscle was defined as an agonist when it created an internal moment about the knee in the opposite direction as the external sagittal plane knee moment, thus contributing to knee joint equilibrium. A muscle was defined as an antagonist when it created an internal moment about the knee in the same direction as the external sagittal plane knee moment, and therefore did not contribute to knee joint equilibrium. The RAI is then the ratio of the agonist or antagonist activity for two subphases of stance. This methodology may provide a more accurate interpretation of muscle activation patterns during functional activities such as walking, particularly in individuals with painful conditions such as knee OA, or following lower extremity joint surgery.
There are several limitations to this study. First, we report results of sEMG, which is susceptible to soft tissue impedance and has the potential for cross-talk between muscles. Needle EMG would have eliminated the effect of soft tissue impedance and ensured that there was no cross-talk between muscles, but it is also invasive and limited to a small area within the muscle and does not describe overall activity for an entire muscle. Second, we had a limited set of muscles that we analyzed and did not evaluate, for example, gluteus muscles or the tensor fasciae latae. Third, we do not have MVICs to compare the results of our study to MVIC normalization. We did attempt to collect MVICs but the results were inconsistent for the TKR subjects. Fourth, we only report sagittal plane external moments in this study as they were compared to the RAI results and used in the calculations. We collected three-dimensional external moments but did not find any differences between the TKRs and controls for frontal or transverse plane external moments. Finally, we have a relatively small number of subjects (19) in each group. For pairwise comparisons using a two-tailed Mann–Whitney–Wilcoxon, 19 subjects in each group permitted the detection of an effect size of approximately 0.96 with a power level of 0.8 at a confidence level of 95%. We deemed the detection of group differences in the range of 1 standard deviation adequate to assess physically meaningful differences in EMG activity patterns between TKA and healthy subjects.
Based on previous reports of increased co-contraction during walking in TKR subjects [1, 22–24], we hypothesized that there should be differences in muscle activation patterns between control and TKR subjects. Indeed, agonist-antagonist muscles were co-contracted for a longer time in TKR than control subjects, especially the vastus lateralis and biceps femoris muscle pair. Several other groups also found prolonged muscle activity for TKR subjects during stance [1, 19, 22, 25–27]. These differences were also visible in the RAI for the rectus femoris and biceps femoris muscles. TKR RAIs of about one for subphases 1 and 2 indicate equal rectus femoris activity at the beginning of the gait cycle. In contrast, control subject RAIs greater than one indicate higher antagonist rectus femoris activity during subphase 1 than agonist rectus femoris activity during subphase 2. Knee extensor muscle activity prevents flexion and stabilizes the knee during initial foot contact [28, 29]. Equal activity between the first two subphases of stance for the TKR subjects represents prolonged knee extensor activation. Since the TKR subjects had lower RAIs and lower sagittal plane knee external moments than the control subjects for subphases 1 and 2, more muscle activity of the agonist muscles in subphase 1 (hamstrings, gastrocnemius) and subphase 2 (quadriceps) could allow the TKR subjects to utilize a more normal walking gait, like the control subjects.
Alternatively, increased biceps femoris activation during subphase 2 may occur due to a persistent hyperexcitability of the nociceptive pathways. The nociceptive reflex is a centrally mediated reflex, characterized by excitation of the flexor musculature (e.g., hamstrings) and inhibition of the extensor musculature (e.g., quadriceps). A previous study has identified facilitation of nociceptive reflex activity in individuals with chronic knee OA [11] – an indication of central sensitization of nociceptive pathways. A subsequent study demonstrated that joint compression further facilitated the reflexive hamstring activation several fold [12]. The functional ramifications of these findings have not been explored, however it was suggested that these aberrant responses to noxious and non-noxious input may be an underlying source of altered knee function. While TKR should potentially remove the source of noxious input to the central nervous system (which maintains central sensitization), it has been suggested that centrally mediated pain persists following surgical joint replacement [30]. Subsequent research exploring the potential relationship between central pain mechanisms and functional muscle activation patterns would be beneficial.
The observed TKR muscle activity pattern for the biceps femoris between subphases 2 and 3 was surprisingly different when compared to controls: an RAI of 2.5 indicated that the average antagonist muscle activity during subphase 2 was over twice the average agonist muscle activity during subphase 3. In contrast, the control subjects showed the opposite pattern, activating their biceps femoris only half as much during subphase 2 compared to subphase 3. The pattern again switched for subphase 3 compared to subphase 4. TKRs had lower agonist biceps femoris activity during subphase 3 than antagonist biceps femoris activity in subphase 4 and controls had higher agonist biceps femoris activity in subphase 3 than antagonist activity in subphase 4.
From a biomechanical/energetic perspective it is surprising that biceps femoris activity in TKR subjects tends towards antagonistic action and surpasses agonistic activity during late stance. Previously, increased co-contraction has been discussed and attributed to a mechanism for protection and decreasing pain [31], and a response to feelings of a less stable knee [1]. While increased stabilization is an intuitive explanation for increased co-contraction, it is possible that this phenomenon neither promotes stability nor is protective. Quadriceps dysfunction has been investigated as the cause for co-contraction differences between TKRs and healthy control subjects [8, 32–35], and less attention has been paid to hamstring differences [8].
The number of TJR surgeries has increased in recent years and is projected to increase further with the rising numbers of older adults and persons with obesity [36, 37]. A surprising number of individuals continue to experience functional deficits following this surgery and nearly 40% demonstrate deficits in quadriceps strength [38]. It is clear from review of recent evidence that clinical impairments associated with knee osteoarthritis extend further than articular degeneration at the knee joint. Identifying the mechanisms underlying altered muscle function both pre and post-TKR is critical for developing rehabilitation strategies to address musculoskeletal and non-musculoskeletal functional deficits and disability found in this patient population. Specifically, rehabilitative methods that target aberrant neurophysiological processing, including chronic pain mechanisms, are essential in the treatment protocols for chronic musculoskeletal conditions.
In conclusion, we developed a new metric, the RAI, to evaluate differences in the magnitude of muscle activity during two consecutive subphases of the stance phase of level walking. The RAI uses unnormalized sEMG data and therefore may be used for subjects with joint pathology or other subject populations who cannot reliably perform MVICs. Evaluating the RAIs, external knee moments, and co-contraction times concomitantly allows one to interpret whether external sagittal plane moments correspond to more or less antagonist muscle activity and if there are time differences in the muscle activity. Although, most of the patterns of muscle activation appeared very similar between the TKR and control subjects, TKR subjects had much higher antagonist muscle activity of the biceps femoris muscle than the control subjects, possibly a remnant of a pain related reflex.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH: R03 AR052039 (MAW), R01 AR059843 (MAW), and F32 AR057297 (HJL). The study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. We thank Dr. Jeremy Fields for providing copy editing assistance through resources of the Rush Research Mentoring Program. We also thank Laura Quigley, Sheila Sanders, and Reggie Barden from Midwest Orthopaedics at Rush University Medical Center for assistance with patient recruitment, and Dr. Valentina Ngai, Dr. Diego Orozco, Andrea Swanson, Dr. Laura Thorp, and Robert Trombley for assistance with data collection and analysis. We thank Dr. Carol Courtney for providing valuable assistance with data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech. 2003;18(9):871–6. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP. Dynamics of pathological motion: applied to the anterior cruciate deficient knee. J Biomech. 1990;23(Suppl 1):99–105. doi: 10.1016/0021-9290(90)90044-4. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP. Functional analysis of pre and post-knee surgery: total knee arthroplasty and ACL reconstruction. J Biomech Eng. 1993;115(4B):575–81. doi: 10.1115/1.2895543. [DOI] [PubMed] [Google Scholar]
- 4.da Silva RR, Santos AA, de Sampaio Carvalho J, Junior, Matos MA. Quality of Life after Total Knee Arthroplasty: Systematic Review. Rev Bras Ortop. 2014;49(5):520–7. doi: 10.1016/j.rboe.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther. 2010;40(9):559–67. doi: 10.2519/jospt.2010.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swinkels A, Newman JH, Allain TJ. A prospective observational study of falling before and after knee replacement surgery. Age Ageing. 2009;38(2):175–81. doi: 10.1093/ageing/afn229. [DOI] [PubMed] [Google Scholar]
- 7.Cram JR, Kasman GS, Holtz J. Introduction to surface electromyography. Aspen Publishers; Gaithersburg, Md.: 1998. [Google Scholar]
- 8.Stevens-Lapsley JE, Balter JE, Kohrt WM, Eckhoff DG. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin Orthop Relat Res. 2010;468(9):2460–8. doi: 10.1007/s11999-009-1219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knarr BA, Zeni JA, Jr., Higginson JS. Comparison of electromyography and joint moment as indicators of co-contraction. J Electromyogr Kinesiology. 2012 doi: 10.1016/j.jelekin.2012.02.001. doi: 10.1016/j.jelekin.2012.02.001(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas AC, Judd DL, Davidson BS, Eckhoff DG, Stevens-Lapsley JE. Quadriceps/hamstrings co-activation increases early after total knee arthroplasty. Knee. 2014;21(6):1115–9. doi: 10.1016/j.knee.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened Flexor Withdrawal Responses in Subjects With Knee Osteoarthritis. J Pain. 2009;10(12):1242–9. doi: 10.1016/j.jpain.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Courtney CA, Witte PO, Chmell SJ, Hornby TG. Heightened Flexor Withdrawal Response in Individuals With Knee Osteoarthritis Is Modulated by Joint Compression and Joint Mobilization. J Pain. 2010;11(2):179–85. doi: 10.1016/j.jpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Heiberg KE, Bruun-Olsen V, Mengshoel AM. Pain and recovery of physical functioning nine months after total knee arthroplasty. J Rehabil Med. 2010;42(7):614–9. doi: 10.2340/16501977-0568. [DOI] [PubMed] [Google Scholar]
- 14.Satterly T, Neeley R, Johnson-Wo AK, Bhowmik-Stoker M, Shrader MW, Jacofsky MC, Jacofsky DJ. Role of total knee arthroplasty approaches in gait recovery through 6 months. J Knee Surg. 2013;26(4):257–62. doi: 10.1055/s-0032-1329719. [DOI] [PubMed] [Google Scholar]
- 15.Engh GA, Parks NL, Whitney CE. A prospective randomized study of bicompartmental vs. total knee arthroplasty with functional testing and short term outcome. J Arthroplasty. 2014;29(9):1790–4. doi: 10.1016/j.arth.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57(7):1254–60. doi: 10.1002/art.22991. [DOI] [PubMed] [Google Scholar]
- 17.Thorp LE, Orozco D, Block JA, Sumner DR, Wimmer MA. Activity Levels in Healthy Older Adults: Implications for Joint Arthroplasty. 2012;2012:5. doi: 10.5402/2012/727950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andriacchi TP, Johnson TS, Hurwitz DE, Natarajan RN. Basic Orthopaedic Biomechanics and Mechano-Biology. Lippincott Williams & Wilkins; Philadelphia: Musculoskeletal Dynamics, Locomotion, and Clinical Applications. p. 1; pp. 20051–1. [Google Scholar]
- 19.Andriacchi TP, Hurwitz DE. Gait biomechanics and total knee arthroplasty. J Knee Surg. 1997;10(4):255–60. [PubMed] [Google Scholar]
- 20.De Luca CJ, Erim Z. Common drive in motor units of a synergistic muscle pair. J Neurophysiol. 2002;87(4):2200–4. doi: 10.1152/jn.00793.2001. [DOI] [PubMed] [Google Scholar]
- 21.Abbink JH, Van Der Bilt A, Van Der Glas HW. Detection of onset and termination of muscle activity in surface electromyograms. J Oral Rehabil. 1998;25(5):365–9. doi: 10.1046/j.1365-2842.1998.00242.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SA, McCann PD, Gotlin RS, Ramakrishnan HK, Wootten ME, Insall JN. Comprehensive gait analysis in posterior-stabilized knee arthroplasty. J Arthroplasty. 1996;11(4):359–67. doi: 10.1016/s0883-5403(96)80023-4. [DOI] [PubMed] [Google Scholar]
- 23.Hubley-Kozey CL, Hatfield GL, Wilson JLA, Dunbar MJ. Alterations in neuromuscular patterns between pre and one-year post-total knee arthroplasty. Clin Biomech. 2010;25(10):995–1002. doi: 10.1016/j.clinbiomech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Fallah-Yakhdani HR, Abbasi-Bafghi H, Meijer OG, Bruijn SM, van den Dikkenberg N, Benedetti M, van Dieën JH. Determinants of co-contraction during walking before and after arthroplasty for knee osteoarthritis. Clin Biomech. 2012;27(5):485–94. doi: 10.1016/j.clinbiomech.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti MG, Bonato P, Catani F, D'Alessio T, Knaflitz M, Marcacci M, Simoncini L. Myoelectric activation pattern during gait in total knee replacement: relationship with kinematics, kinetics, and clinical outcome. IEEE Trans Rehabil Eng. 1999;7(2):140–9. doi: 10.1109/86.769404. [DOI] [PubMed] [Google Scholar]
- 26.McClelland JA, Webster KE, Feller JA. Gait analysis of patients following total knee replacement: a systematic review. Knee. 2007;14(4):253–63. doi: 10.1016/j.knee.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech. 2008;23(3):320–8. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peat M, Woodbury MG, Ferkul D. Electromyographic analysis of gait following total knee arthroplasty. Physiother Can. 1984;36(2):68–72. [Google Scholar]
- 29.Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. SLACK; Thorofare, New Jersey: 2010. [Google Scholar]
- 30.Wylde V, Dieppe P, Hewlett S, Learmonth ID. Total knee replacement: is it really an effective procedure for all? Knee. 2007;14(6):417–23. doi: 10.1016/j.knee.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Fisher NM, White SC, Yack HJ, Smolinski RJ, Pendergast DR. Muscle function and gait in patients with knee osteoarthritis before and after muscle rehabilitation. Disabil Rehabil. 1997;19(2):47–55. doi: 10.3109/09638289709166827. [DOI] [PubMed] [Google Scholar]
- 32.Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83(4):359–65. [PubMed] [Google Scholar]
- 33.Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32(8):1533–9. [PubMed] [Google Scholar]
- 34.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87(5):1047–53. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23(5):1083–90. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: Updated projections to 2021. J Bone Jt Surg Am Vol. 2014;96(8):624–30. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 38.Walsh M, Woodhouse LJ, Thomas SG, Finch E. Physical impairment and functional limitations: A comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther. 1998;78(3):248–58. doi: 10.1093/ptj/78.3.248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.