Abstract
Introduction
Effectiveness of Alzheimer's disease (AD) treatments is commonly evaluated with coprimary outcomes; cognition with function to ensure clinical meaningfulness of a cognitive effect.
Methods
We reviewed the literature for functional outcomes in mild AD or mild cognitive impairment (MCI) patients (distinct from combined mild-moderate/severe AD) treated with approved AD drugs. Cognitive and functional treatment differences in mild AD patients in solanezumab EXPEDITION/EXPEDITION2 studies were compared across time.
Results
Seven publications provided MCI/mild AD functional outcomes, one of which reported a significant functional treatment effect. Secondary analyses of EXPEDITION studies suggested a smaller functional effect of solanezumab relative to cognition. An increasing effect of solanezumab over 18 months was shown for cognition and function.
Discussion
Function as the sole measure to demonstrate clinical meaningfulness of cognitive effects in mild AD may have limitations. For disease-modifying treatments, point differences on cognitive and functional scales should be qualified with duration of treatment.
Keywords: Solanezumab, Alzheimer's disease, Clinical relevance, Functional rating scales, Cognitive rating scales, Global rating scales, Mild cognitive impairment, Clinical meaningfulness
1. Introduction
Alzheimer's disease (AD) is primarily a disease of cognition, but it can also lead to deficits in function, including activities of daily living and behavioral abnormalities, particularly in more advanced stages of the disease. Current evidence suggests that the pathologic and clinical manifestations of AD exist in a continuum, with the accumulation of amyloid plaque and structural biological changes starting to occur as early as 10–20 years before the emergence of clinical symptoms [1], [2], [3], [4]. For clinical research purposes, the continuum of AD progression must be categorized into narrowed and more homogeneous stages so that disease progression and treatment effects can be observed, measured, and compared using scales with appropriate psychometric properties. These stages include preclinical AD, prodromal AD/mild cognitive impairment (MCI), mild AD dementia, moderate AD dementia, and severe AD dementia.
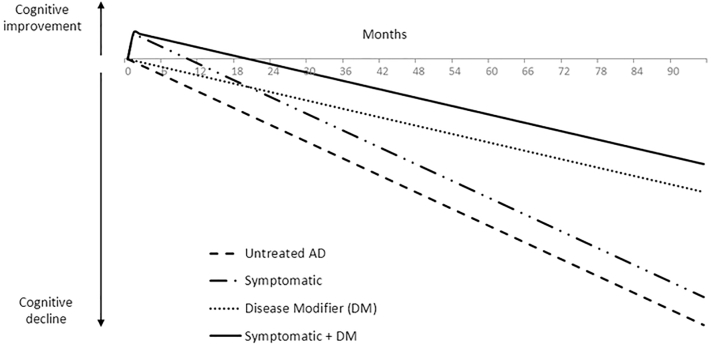
The current AD clinical trial landscape focuses on putative disease-modifying agents in patients with preclinical AD, MCI, or mild AD dementia. Conversely, currently approved AD treatments include the acetylcholinesterase inhibitors donepezil, galantamine, and rivastigmine, and the N-methyl-D-aspartate receptor antagonist, memantine for mild, moderate, and/or severe AD dementia. While providing symptomatic improvement, these treatments have not been shown to slow disease progression through modification of the underlying disease pathology [5], [6]. Potential disease-modifying treatments for AD are intended to slow decline rather than produce transient improvement; conceptually, this appears as a gradually increasing benefit over time rather than the acute improvement seen with symptomatic treatments (Fig. 1). As of yet, none of the putative disease-modifying treatments in development have gained regulatory approval.
Fig. 1.
Hypothetical effect on progression of AD with treatments providing symptomatic improvement, slowing of disease progression, or both, compared with placebo. Slowing of disease progression is assumed to be 50% compared with placebo. Abbreviations: AD, Alzheimer's disease; DM, disease modifier (that is, a treatment that modifies the underlying pathology of disease, thereby slowing its progression).
Historically, the effectiveness of approved AD treatments has been established not only based on an effect on cognition but also with a coprimary outcome based on a global or functional scale. The coprimary measure was intended to ensure that the demonstrated cognitive effects were clinically meaningful to patients and caregivers. The registration studies for donepezil [7], galantamine [8], rivastigmine [9], and tacrine [10] (which was withdrawn from use due to its safety profile [11]) in combined mild-to-moderate AD populations included coprimary cognitive and global measures; the 11-item Alzheimer's Disease Assessment Scale—Cognitive subscale (ADAS-Cog11) [12], [13] was used as the measure of cognition, and the Clinician's Interview-Based Impression of Change plus caregiver or the Clinical Global Impression of Change were used as the global measures to assess the clinical meaningfulness of cognitive effects. Although these global measures were effective for these short-term clinical trials of symptomatic agents, they are not appropriate for long-term disease progression studies (typically 18+ months) as they rely on a clinician's subjective assessment of memory and patient performance in contrast to the baseline. The use of a functional scale, which mitigates the limitations of global scales in long-term trials, as a coprimary measure in registration studies for currently available symptomatic drugs, has been limited to studies of memantine in moderate to severe AD [14].
Although symptomatic treatments were developed for patients with mild-to-moderate or moderate-to-severe dementia, most current AD clinical trials are evaluating putative disease-modifying agents in patients with preclinical AD, MCI, or mild AD dementia; thus, an assessment of the appropriateness of currently existing clinical rating scales, in particular functional scales, to ensure that demonstrated cognitive effects are clinically meaningful in these earlier patient populations is warranted. This article provides a brief literature review of the outcomes of functional measures in mild AD dementia and MCI subpopulations. We also compare results of this literature review with cognitive and functional treatment effect data from secondary efficacy results in the mild AD subpopulation from the solanezumab phase 3 EXPEDITION and EXPEDITION2 studies [15], [16]. Additionally, because clinical meaningfulness is sometimes assessed simply as a minimal point difference between active treatment and placebo on a particular scale [17], [18], [19], we assess point differences and effect sizes in cognitive and functional scales at various times during the EXPEDITION and EXPEDITION2 clinical trials. As predicted in the scientific literature [20] and as shown in Figure 1, for a hypothetical disease-modifying treatment, the point difference between active treatment and placebo and the effect size is not constant but increases with duration of treatment.
2. Methods
2.1. Literature search
To assess the effect of approved symptomatic treatments on activities of daily living (ADLs) in patients with mild AD dementia, we reviewed the literature on clinical trials of currently approved treatments for AD. A Medline search ([“Alzheimer Disease” or “Mild Cognitive Impairment”] and “Activities of Daily Living” and [“donepezil” or “galantamine” or “rivastigmine” or “memantine”]) that included publications to October Week 5 2015 was conducted. Those publications were limited to reports that provided results from randomized, placebo-controlled studies for MCI and/or mild AD patients separately (not combined with moderate or severe AD patients). Treatment effect sizes (treatment difference divided by pooled standard deviation) were calculated for publications that provided the required data for both cognitive and functional outcomes.
2.2. EXPEDITION and EXPEDITION2 secondary outcomes
The primary outcomes for the EXPEDITION and EXPEDITION2 trials for solanezumab have been previously reported [15]. These studies used the ADAS-Cog and the mini mental state examination (MMSE) as cognitive measures and the Alzheimer's Disease Cooperative Study—Activities of Daily Living Inventory (ADCS-ADL) and the Resource Utilization in Dementia (RUD-Lite) as functional measures. Although the coprimary outcomes (ADAS-Cog and ADCS-ADL) in the combined mild-to-moderate AD subjects were not positive, in a secondary analysis, mild AD subjects treated with solanezumab for 18 months indicated a slowing in cognitive decline of approximately 34% and a slowing in functional decline of approximately 18% using the instrumental subscale of the ADCS-ADL (ADCS-iADL) when comparing baseline to endpoint changes [16]. We estimated the treatment point differences and effect sizes (treatment difference divided by pooled standard deviation) on the cognitive and functional scales using mixed-model repeated measures (MMRM) at several time points during the 18-month treatment period in mild AD dementia patients from the EXPEDITION studies and compared them across time to assess the time dependence of the difference between active treatment and placebo.
3. Results
3.1. Literature review
The search criteria yielded 183 publications, and just seven reported functional results specifically for patients with MCI or mild AD (separately from moderate and/or severe AD) from studies of an approved AD drug (Table 1). Of these seven publications, only one reported a statistically significant drug effect on a functional measure: when evaluating three trials of rivastigmine, Potkin et al. [21] reported a statistically significant change in the Progressive Deterioration Scale (PDS), a secondary measure of function, in patients who completed 26 weeks of treatment and who were classified as mild based on a Global Deterioration Scale (GDS) of 3 or less. Although later analyses of the same studies showed statistically significant effects on the ADAS-Cog in mild (MMSE ≥22), moderate (MMSE 16 to 22), and severe (MMSE ≤15) AD groups and statistically significant effects on function, as measured by the PDS in the moderate and severe groups, the difference on function in the mild AD group failed to reach statistical significance in these analyses [22]. In addition to the difference in severity classification methodology, the later analyses also did not limit the analyses to patients who completed 26 weeks of treatment.
Table 1.
Summary of publications reporting analyses of effects of approved AD medications on function in mild AD populations
| Citation | Treatments (N) | Analysis population | Design | Duration | Findings by baseline severity |
|---|---|---|---|---|---|
| Potkin et al. 2002 [21] | Rivastigmine (158), placebo (180) | Mild AD | Pooled analysis of 3 randomized, double-blind, placebo-controlled studies∗ | 6 mo | Statistically significant change in the PDS when classifying subjects as mild based on a GDS of ≤3. |
| Sano et al. 2003 [23] | Galantamine (261), placebo (258) | Mild AD | Pooled analysis of 2 randomized, double-blind, placebo-controlled studies | 6 mo | Differences in caregiver time vs placebo were greater and statistically significant in subjects with moderate dementia (MMSE 11 to 18) relative to those with mild dementia (MMSE 19 to 24, not significant). |
| Galasko et al. 2004 [24] | Galantamine (424), placebo (235) | Mild AD | Randomized, double-blind, placebo-controlled study | 5 mo | Largest and only statistically significant treatment vs placebo difference in ADCS-ADL scores occurred in subjects with more severe dementia at baseline (MMSE 10 to 15). In placebo group, worsening of ADCS-ADL scores was greater in those with more severe dementia. |
| Kurz et al. 2004 [22] | Rivastigmine (365), placebo (288) | Mild AD | Pooled analysis of three randomized, double-blind, placebo-controlled studies∗ | 6 mo | Among mild AD patients (MMSE 22 to 26) at 26 weeks, a statistically significant difference between rivastigmine and placebo on ADAS-Cog was observed, but the difference in PDS was not significant. |
| Petersen et al. 2005 [25] | Donepezil (253), placebo (259), vitamin E (257) | MCI | Randomized, double-blind, placebo-controlled study | 3 y | Statistically significant differences vs placebo in a modified ADAS-Cog were demonstrated with donepezil in the first 18 mo but without significant differences in the ADCS-ADL (modified for use in MCI patients) at any time point. |
| Gauthier et al. 2010 [26] | Donepezil (702), placebo (453) | Mild AD | Pooled analysis of 6 randomized, double-blind, placebo-controlled studies | 6 mo | No statistically significant differences in any instrumental or basic ADL items were found compared with placebo among those with mild disease (MMSE 18 to 26), whereas changes were found in those with moderate (MMSE 10 to 17) and severe (MMSE 5 to 9) disease. |
| Farlow et al. 2011 [27] | Rivastigmine (256), placebo (92) | Mild AD | Randomized, double-blind, placebo-controlled study | 6 mo | Significant changes in ADCS-ADL scores were found for those with moderate (MMSE 16 to18) and moderately severe (MMSE 13 to 15) disease, but no differences vs placebo were found for mild disease (MMSE 19 to 25). |
Abbreviations: AD, Alzheimer Disease; PDS, Progressive Deterioration Scale; GDS, Global Deterioration Scale; MMSE, mini mental state examination; MCI, mild cognitive impairment; ADCS-ADL, Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive subscale; ADL, activity of daily living.
Potkin et al. (2002) and Kurz et al. (2004) reported results from analyses of the same three studies.
Similarly, the other five studies that reported results for MCI or mild AD patients did not demonstrate an effect on function even when an effect on cognition was shown. In a study of galantamine treatment assessing caregiver time, differences versus placebo were statistically significant for patients with moderate dementia (MMSE 11 to 18) and greater than for patients with mild dementia (MMSE 19 to 24, statistical significance not reported) [23]. In another study of galantamine among mild-to-moderate AD patients (MMSE 10 to 22), the largest and only statistically significant treatment-versus-placebo difference in ADCS-ADL scores occurred in patients with more severe dementia (MMSE 10 to 15) at baseline; additionally, in placebo-treated patients, worsening of ADCS-ADL scores was greater in patients with more severe dementia [24]. In a study of donepezil and vitamin E in patients with MCI, statistically significant differences relative to placebo in a modified ADAS-Cog were demonstrated but without significant differences in the ADCS-ADL (modified for use in MCI patients) [25]. Gauthier et al. [26] combined results from six studies of donepezil to assess individual ADL items among patients stratified by disease severity. In an analysis of over 1000 mild (MMSE 18 to 26) patients, no statistically significant differences in any instrumental or basic ADL items were found compared with placebo, although differences were observed in the moderate (MMSE 10 to 17) patients. Farlow et al. [27] compared response of two doses of a rivastigmine patch, one dose of rivastigmine capsule, and placebo. In their analysis, significant changes in ADCS-ADL scores were found for moderate (MMSE 16 to 18) and moderately severe (MMSE 13 to 15) patients, but no differences versus placebo were found for mild (MMSE 19 to 25) patients. Thus, our literature review suggests that in studies of treatments recognized as having clinical benefits, the treatment effect on ADLs in combined mild plus moderate populations has been driven by the moderate AD patients; treatment effect on ADLs has been difficult to demonstrate in patients with mild AD dementia alone.
3.2. Secondary analyses of phase 3 solanezumab studies
For both cognitive and functional scales assessed in the solanezumab EXPEDITION studies, the point differences between active treatment and placebo and effect sizes gradually increased over the 18-month treatment duration (Table 2). Thus, an assessment of the point differential between active treatment and placebo as well as the effect size must be qualified with the time point of the assessment. Effect sizes on the cognitive scales were higher than those on the functional scales at all time points.
Table 2.
Changes in cognitive and functional measures over time; solanezumab EXPEDITION and EXPEDITION2 studies, pooled in the mild population
| LS mean change from baseline∗ |
||||||
|---|---|---|---|---|---|---|
| Week | Placebo | Solanezumab | Difference | P value† | Effect size | |
| Cognitive measures | ||||||
| ADAS-Cog14 | 28 | 0.63 | 0.34 | −0.29 | .465 | .036 |
| 52 | 3.36 | 2.29 | −1.08 | .025 | .119 | |
| 80 | 6.21 | 4.08 | −2.13 | .001 | .191 | |
| MMSE | 28 | −0.30 | −0.18 | 0.12 | .468 | .031 |
| 52 | −1.43 | −1.01 | 0.42 | .051 | .097 | |
| 80 | −2.76 | −1.83 | 0.93 | .001 | .190 | |
| Functional measures | ||||||
| ADCS-iADL | 28 | −1.73 | −1.54 | 0.19 | .608 | .025 |
| 52 | −3.77 | −2.98 | 0.79 | .100 | .088 | |
| 80 | −6.77 | −5.56 | 1.21 | .045 | .116 | |
| RUD-lite iADL | 28 | −0.02 | −0.08 | −0.06 | .716 | .018 |
| 52 | 0.21 | 0.07 | −0.13 | .419 | .038 | |
| 80 | 0.53 | 0.26 | −0.27 | .174 | .073 | |
Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory instrumental subscale; LS, least squares; MMSE, mini mental state examination; RUD-Lite iADL, resource utilization in dementia-lite instrumental activities of daily living item.
Least squares (LS) mean change from baseline to endpoint, mixed-model repeated measures (MMRM).
P value for the comparison of placebo and solanezumab LS mean change, MMRM.
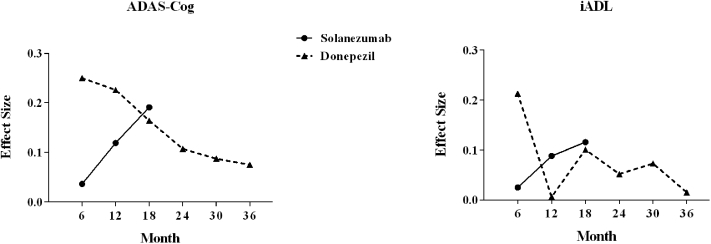
Of the seven articles that reported functional outcomes from studies of an approved AD drug in mild AD and MCI patients, only Petersen et al. [25] also provided cognitive outcomes and the data necessary to calculate effect size. In this reported comparison of donepezil and placebo over 3 years in MCI patients, the effect sizes for the cognitive and functional measures did not increase over time but were at their maximum value at the initial post-baseline time point of 6 months. A comparison of effect sizes over time for the solanezumab EXPEDITION studies and the Petersen donepezil study are provided in Fig. 2.
Fig. 2.
Effect sizes of treatment versus placebo over time. Solanezumab data are from the EXPEDITION and EXPEDITION2 studies in the pooled mild AD population using the ADAS-Cog14 and the ADCS-iADL. Donepezil data are derived from Table 2 of the Petersen et al. [25] donepezil MCI study that used the ADAS-Cog13 and the ADCS-MCI-ADL. Calculations were done assuming the values in Table 2 are mean change plus/minus standard deviation. Abbreviations: AD, Alzheimer disease; ADAS-Cog, Alzheimer's Disease Assessment Scale—Cognitive subscale; ADCS-iADL, Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory; MCI, mild cognitive impairment.
4. Discussion
Changes in global or functional measures have traditionally been used to ensure the clinical relevance of a cognitive treatment effect in AD trials. Global or functional measures were used for assessing clinical meaningfulness in clinical trials of symptomatic treatments in mild-to-moderate or severe patient populations, typically ranging in duration from 3 to 6 months. Given that AD research has evolved to focus more on patients earlier in the disease continuum, testing investigational disease-modifying drugs in studies lasting at least 18 months, the results of this literature review and the secondary outcomes of the solanezumab EXPEDITION studies suggest that corresponding changes in the methodology for demonstrating clinical meaningfulness may be warranted for disease-modifying treatments in mild patient populations.
Alternate outcome measures in trials of MCI patients have been recommended previously [28]; the proposed primary outcome measure was cognition, with ADLs and related measures proposed as secondary outcomes as they are less likely to be impaired. Moreover, in recognition of the shifting focus of clinical research and the challenges associated with accurately measuring functional or global impairments using existing measurement tools, draft guidance from FDA in 2013 [29] on developing treatments for early AD states that among patients on the AD continuum closest to overt dementia (prodromal AD or MCI due to AD), use of a composite endpoint validated in early stage patients that incorporates both cognition and function is appropriate. For the “dementia stage” of AD, including mild, moderate, and severe dementia the draft guidance does, however, reiterate the previously established requirements for the use of coprimary outcomes reflecting both cognitive effect and functional or global effects. The uncertainty in the field regarding use of currently available functional measures was captured at a meeting of the Alzheimer's Association's Research Roundtable in 2013, where the need for improved cognitive and functional outcome measures for patients with preclinical AD and MCI due to AD was addressed. Given the uncertainties in the field, a single “gold standard” test of function could not be determined. A consensus agreement from a panel discussion indicated that scales may be assessed primarily based on broad psychometric properties, and those properties could be used to determine if a particular scale is fit for a particular purpose [30].
As stated previously, of the 183 manuscripts identified in our search for functional outcomes in patients treated with currently approved symptomatic AD treatments, only seven actually reported these results specifically for an MCI or mild AD population, and only one of these seven publications reported an effect of an approved AD treatment on function. These findings suggest that in studies of approved AD treatments generally recognized as having clinical benefits, the treatment effect on ADLs in combined mild and moderate AD patients is apparently driven by the effect in patients with moderate dementia. Consistent with our literature review, the secondary analyses in mild patients participating in the phase 3 solanezumab EXPEDITION and EXPEDITION2 studies demonstrated an effect on cognition with a smaller effect on iADLs.
Although relationships between cognitive and functional changes in AD are not well understood, it has been theorized that cognitive decline precedes functional decline [31]. An analysis of the correlation between raw cognitive and functional scores across the mild and moderate AD patients participating in the EXPEDITION studies demonstrated that correlation increases steadily over time. In mild AD EXPEDITION patients, cognition (measured by ADAS-Cog14) was more highly correlated with iADLs than with bADLs, whereas in moderate AD patients, the correlations with iADLs and bADLs were much more similar [32]. Observational studies have shown that patients with milder disease have slower rates of decline in ADLs [31], and in a study of mild AD patients, Park et al. [33] noted that of patients with no functional impairment at baseline, 56% had no functional loss after 1 year, despite worsening of cognitive scores. Consistent with observational studies, moderate (MMSE 14 to 20) placebo-treated patients in a 24-week study of cerebrolysin showed worsening in Disability Assessment in Dementia (DAD) scale scores, whereas DAD scores in mild (MMSE 21 to 25) placebo-treated patients were essentially unchanged [34]. Likewise, patients who are cognitively impaired but do not meet criteria for dementia have less decline in ADLs than patients with mild AD [35]. Finally, additional analyses using sophisticated statistical techniques are now emerging that suggest cognitive decline precedes and predict functional decline [36], [37]. Taken together and consistent with findings from our literature review, these data suggest that for patients earlier in the continuum of AD, treatment effects on function would be more difficult to demonstrate relative to cognitive effects. Reviews of AD trials broadly have concluded that the treatment effect size for functional outcome measures is small [38], and functional measures tend to be less responsive to changes than cognitive or global measures [39].
Across many disease states, the clinical meaningfulness of a therapeutic effect may be judged by the point difference or effect size on a particular scale. However, with a disease-modifying therapy, point difference and effect size would be expected to increase over time, as observed in the 18-month EXPEDITION studies (Table 2). Thus, for a disease-modifying treatment, a threshold point difference or effect size must also be accompanied by the duration of treatment necessary to achieve that effect. Given a range of clinical trial durations for patients with mild AD dementia, MCI or preclinical AD, and the use of different scales in these studies, a consensus regarding duration of treatment and point differences/effect sizes may be difficult to achieve. Interestingly, data derived from cognitive and functional outcomes of donepezil and placebo-treated MCI patients provided in Petersen et al. [25] suggest effect sizes for this symptomatic drug do not increase over time.
Clinical meaningfulness might be judged by a number of approaches independent of specific point differences on a particular instrument or scale. Evidence of disease modification alone might, in part, support the clinical meaningfulness of a treatment. Such evidence could be supported by biomarker changes, a delayed start analysis [40], or an increasing effect over time during the double-blind portion of the trial. Slope analysis, which has been proposed to demonstrate slowing of decline [41], [42], is another statistical approach that has appeal in its intuitiveness and illustrative value (i.e., Fig. 1). Slope analysis, however, requires an assumption of linear decline in disease, which has been the focus of continued research in the field, especially for studies of long duration [6], [43], [44]. Finally, advanced statistical approaches, including path analysis [37] can be used to show that treatment effects on function are driven by a primary effect on cognition.
In conclusion, the published literature suggests that in mild AD and MCI patients, using currently available, well-recognized clinical scales in studies of treatments generally accepted to have beneficial effects, cognitive treatment effects are more likely to be demonstrated than functional treatment effects. A fundamental question that cannot yet be answered is whether patients in the earlier stages of the AD continuum have fewer functional deficits, or whether currently available scales are inadequate to measure these deficits given ceiling effects. As evidenced by our literature review, very little research has been reported to specifically address this point thus far. When considered in context of the historical requirement that AD treatment effectiveness be established with a coprimary global or functional scale, this conclusion has significant implications for AD treatment research; specifically that reliance on function as the sole measure to demonstrate clinical meaningfulness of cognitive effects should be reconsidered. A comparison of the cognitive and functional effects of solanezumab in secondary analyses of the EXPEDITION studies is consistent with this hypothesis. Use of specific point differentials in trials of disease-modifying drugs to assess clinical meaningfulness is also likely to be problematic given their time dependence and the variety of available scales. Thus, use of a functional scale should be considered as only one of several methods to assess clinical meaningfulness for patients early in the continuum of AD who are given treatments intended to slow disease progression. More data and discussion are needed in this evolving characterization of AD progression and in the assessment of treatment effects.
Research in context.
-
1.
Systematic review: Authors assessed scientific literature and secondary analyses of the 18-month, placebo-controlled periods in solanezumab phase 3 EXPEDITION and EXPEDITION2 studies to clarify utility of functional outcomes in determination of clinical meaningfulness of treatments for mild AD and MCI.
-
2.
Interpretation: Regulatory guidance currently suggests function is the only method to demonstrate clinical meaningfulness of a cognitive effect for putative disease-modifying agents in mild AD; however, this guidance is based on historic studies of symptomatic agents conducted in combined mild-moderate or severe AD populations. To our knowledge, a functional effect has only been demonstrated for mild AD-only or MCI population in one published study of an approved AD drug. Secondary outcomes from the first phase 3 solanezumab study in mild AD patients were consistent with this observation.
-
3.
Future directions: With increasing focus on earlier stages of AD, function as the sole mechanism for determining clinical meaningfulness may have substantial limitations.
Acknowledgments
The authors thank Giedra Campbell (Eli Lilly and Company) for assistance with review of the literature, David Henley and Janice Hitchcock (Eli Lilly and Company) for review of the article, and Laura Ramsey (Eli Lilly and Company) for assistance with manuscript preparation.
References
- 1.Dubois B., Feldman H.H., Jacova C., Cummings J.L., Dekosky S.T., Barberger-Gateau P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider L.S. Treatment of Alzheimer's disease with cholinesterase inhibitors. Clin Geriatr Med. 2001;17:337–358. doi: 10.1016/s0749-0690(05)70072-0. [DOI] [PubMed] [Google Scholar]
- 6.Ito K., Ahadieh S., Corrigan B., French J., Fullerton T., Tensfeldt T. Disease progression meta-analysis model in Alzheimer's disease. Alzheimers Dement. 2010;6:39–53. doi: 10.1016/j.jalz.2009.05.665. [DOI] [PubMed] [Google Scholar]
- 7.Aricept, [package insert]. 2013.
- 8.Razadyne, [package insert]. 2015.
- 9.Exelon, [package insert]. 2015.
- 10.Farlow M., Gracon S.I., Hershey L.A., Lewis K.W., Sadowsky C.H., Dolan-Ureno J. A controlled trial of tacrine in Alzheimer's disease. The Tacrine Study Group. JAMA. 1992;268:2523–2529. [PubMed] [Google Scholar]
- 11.Mehta M., Adem A., Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:728983. doi: 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 13.Mohs R.C., Knopman D., Petersen R.C., Ferris S.H., Ernesto C., Grundman M. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–S21. [PubMed] [Google Scholar]
- 14.Namenda, [package insert]. 2013.
- 15.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 16.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 17.Vellas B., Andrieu S., Sampaio C., Coley N., Wilcock G., European Task Force Group Endpoints for trials in Alzheimer's disease: a European task force consensus. Lancet Neurol. 2008;7:436–450. doi: 10.1016/S1474-4422(08)70087-5. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K., Fay S., Gorman M. The ADAS-cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25:191–201. doi: 10.1002/gps.2319. [DOI] [PubMed] [Google Scholar]
- 19.Schrag A., Schott J.M., Alzheimer's Disease Neuroimaging Initiative What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry. 2012;83:171–173. doi: 10.1136/jnnp-2011-300881. [DOI] [PubMed] [Google Scholar]
- 20.Leber P. Slowing the progression of Alzheimer disease: methodologic issues. Alzheimer Dis Assoc Disord. 1997;11(Suppl 5):S10–S21. discussion S37–9. [PubMed] [Google Scholar]
- 21.Potkin S.G., Anand R., Hartman R., Veach J., Grossberg G. Impact of Alzheimer's Disease and rivastigmine treatment on activities of daily living over the course of mild to moderately severe disease. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:713–720. doi: 10.1016/s0278-5846(02)00212-9. [DOI] [PubMed] [Google Scholar]
- 22.Kurz A., Farlow M., Quarg P., Spiegel R. Disease stage in Alzheimer disease and treatment effects of rivastigmine. Alzheimer Dis Assoc Disord. 2004;18:123–128. doi: 10.1097/01.wad.0000127445.00442.a1. [DOI] [PubMed] [Google Scholar]
- 23.Sano M., Wilcock G.K., van Baelen B., Kavanagh S. The effects of galantamine treatment on caregiver time in Alzheimer's disease. Int J Geriatr Psychiatry. 2003;18:942–950. doi: 10.1002/gps.1000. [DOI] [PubMed] [Google Scholar]
- 24.Galasko D., Kershaw P.R., Schneider L., Zhu Y., Tariot P.N. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52:1070–1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- 25.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier S., Lopez O.L., Waldemar G., Jones R.W., Cummings J., Zhang R. Effects of donepezil on activities of daily living: integrated analysis of patient data from studies in mild, moderate and severe Alzheimer's disease. Int Psychogeriatr. 2010;22:973–983. doi: 10.1017/S1041610210000888. [DOI] [PubMed] [Google Scholar]
- 27.Farlow M.R., Grossberg G.T., Meng X., Olin J., Somogyi M. Rivastigmine transdermal patch and capsule in Alzheimer's disease: influence of disease stage on response to therapy. Int J Geriatr Psychiatry. 2011;26:1236–1243. doi: 10.1002/gps.2669. [DOI] [PubMed] [Google Scholar]
- 28.Schneider L.S. The potential and limits for clinical trials for early Alzheimer's disease and some recommendations. J Nutr Health Aging. 2010;14:295–298. doi: 10.1007/s12603-010-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozauer N., Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 30.Snyder P.J., Kahle-Wrobleski K., Brannan S., Miller D.S., Schindler R.J., DeSanti S. Assessing cognition and function in Alzheimer's disease clinical trials: do we have the right tools? Alzheimers Dement. 2014;10:853–860. doi: 10.1016/j.jalz.2014.07.158. [DOI] [PubMed] [Google Scholar]
- 31.Cortes F., Nourhashemi F., Guerin O., Cantet C., Gillette-Guyonnet S., Andrieu S. Prognosis of Alzheimer's disease today: a two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008;4:22–29. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Liu-Seifert H., Siemers E., Selzler K., Sundell K., Aisen P., Cummings J. Correlation between cognition and function across the spectrum of Alzheimer's disease. J Prev Alzheimers Dis. 2014;1:2140296. doi: 10.14283/jpad.2016.99. [DOI] [PubMed] [Google Scholar]
- 33.Park K.W., Pavlik V.N., Rountree S.D., Darby E.J., Doody R.S. Is functional decline necessary for a diagnosis of Alzheimer's disease? Dement Geriatr Cogn Disord. 2007;24:375–379. doi: 10.1159/000109268. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez X.A., Cacabelos R., Sampedro C., Aleixandre M., Linares C., Granizo E. Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer's disease: results of a randomized, double-blind, controlled trial investigating three dosages of Cerebrolysin. Eur J Neurol. 2011;18:59–68. doi: 10.1111/j.1468-1331.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsiung G.Y., Alipour S., Jacova C., Grand J., Gauthier S., Black S.E. Transition from cognitively impaired not demented to Alzheimer's disease: an analysis of changes in functional abilities in a dementia clinic cohort. Dement Geriatr Cogn Disord. 2008;25:483–490. doi: 10.1159/000126499. [DOI] [PubMed] [Google Scholar]
- 36.Zahodne L.B., Manly J.J., MacKay-Brandt A., Stern Y. Cognitive declines precede and predict functional declines in aging and Alzheimer's disease. PLoS One. 2013;8:e73645. doi: 10.1371/journal.pone.0073645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu-Seifert H., Siemers E., Sundell K., Price K., Han B., Selzler K. Cognitive and functional decline and their relationship in patients with mild Alzheimer's dementia. J Alzheimers Dis. 2015;43:949–955. doi: 10.3233/JAD-140792. [DOI] [PubMed] [Google Scholar]
- 38.Hansen R.A., Gartlehner G., Lohr K.N., Kaufer D.I. Functional outcomes of drug treatment in Alzheimer's disease: A systematic review and meta-analysis. Drugs Aging. 2007;24:155–167. doi: 10.2165/00002512-200724020-00007. [DOI] [PubMed] [Google Scholar]
- 39.Rockwood K. The measuring, meaning and importance of activities of daily living (ADLs) as an outcome. Int Psychogeriatr. 2007;19:467–482. doi: 10.1017/S1041610207004966. [DOI] [PubMed] [Google Scholar]
- 40.Liu-Seifert H., Andersen S.W., Lipkovich I., Holdridge K.C., Siemers E. A novel approach to delayed-start analyses for demonstrating disease-modifying effects in Alzheimer's disease. PLoS One. 2015;10:e0119632. doi: 10.1371/journal.pone.0119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olanow C.W., Rascol O., Hauser R., Feigin P.D., Jankovic J., Lang A. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R.Y., Leon A.C., Chuang-Stein C., Romano S.J. A new proposal for randomized start design to investigate disease-modifying therapies for Alzheimer disease. Clin Trials. 2011;8:5–14. doi: 10.1177/1740774510392255. [DOI] [PubMed] [Google Scholar]
- 43.Ito K., Corrigan B., Zhao Q., French J., Miller R., Soares H. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement. 2011;7:151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Sperling R.A., Jack C.R., Jr., Aisen P.S. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]