Abstract
Introduction
Mononuclear phagocytes play a critical role during Alzheimer's disease (AD) pathogenesis due to their contribution to innate immune responses and amyloid beta (Aβ) clearance mechanisms.
Methods
Blood-derived monocytes (BDMs) and monocyte-derived macrophages (MDMs) were isolated from blood of AD, mild cognitive impairment (MCI) patients, and age-matched healthy controls for molecular and phenotypic comparisons.
Results
The chemokine/chemokine receptor CCL2/CCR2 axis was impaired in BDMs from AD and MCI patients, causing a deficit in cell migration. Changes were also observed in MDM-mediated phagocytosis of Aβ fibrils, correlating with alterations in the expression and processing of the triggering receptor expressed on myeloid cells 2 (TREM2). Finally, immune-related microRNAs (miRNAs), including miR-155, -154, -200b, -27b, and -128, were found to be differentially expressed in these cells.
Discussion
This work provides evidence that chemotaxis and phagocytosis, two crucial innate immune functions, are impaired in AD and MCI patients. Correlations with miRNA levels suggest an epigenetic contribution to systemic immune dysfunction in AD.
Keywords: Alzheimer's disease, Monocytes, Macrophages, Chemotaxis, Phagocytosis, CCR2, TREM2, miRNAs
1. Background
Blood-derived monocytes (BDMs) have been shown to have a beneficial role in Alzheimer's disease (AD) mouse models, associated with a higher ability to clear amyloid beta (Aβ) deposits in the brain [1], [2], [3], when compared with resident microglia. This contribution depends on the expression of chemokine receptors, such as CCR2, which mediate BDM migration and infiltration into the brain parenchyma [4], [5] and phagocytosis-related proteins, such as the new AD risk protein, triggering receptor expressed on myeloid cells 2 (TREM2) [6]. However, in humans, the role of BDMs and monocyte-derived macrophages (MDMs; which directly differentiate from BDMs within tissues) in AD is poorly explored. In vitro, macrophages of AD patients usually show minimal surface uptake and poor internalization of Aβ [7], although the ability of their blood monocyte precursors to infiltrate the AD brain is yet poorly studied.
Both CCR2 and TREM2 have been directly implicated in AD pathology in different mouse models. Deficiency in CCR2 in the Tg2576 and APPSwe/PS1 mice exacerbated amyloidosis [4], [8], whereas transplantation of CCR2-competent cells into APPSwe/PS1/CCR2−/− restored cognitive functions [5]. Moreover, two very recent studies implicated TREM2 in Aβ clearance in vivo, with contradictory results. Although 5XFAD mice deficient in TREM2 showed increased accumulation of Aβ and a decrease in the number of microglia around plaques [9], in the APPPS1 mouse model, the absence of TREM2 resulted in a reduction of Aβ load [10]. In contrast with previous reports that restricted TREM2 expression to microglia cells [11], the study in the APPPS1 model hypothesizes that the TREM2+ cells found to surround Aβ plaques are blood-derived macrophages. Despite these evidences, it is unknown whether, in the context of human disease, these cells are able to migrate to the AD brain and phagocyte Aβ and how their function is regulated at the molecular level.
We have recently reported that overexpression of miR-155 occurs both in M1-activated microglia [12] and in the brain of 3xTg AD mice [13] and is critical for the establishment of a chronic inflammatory phenotype. In this study, we decided to further explore the ability of microRNAs (miRNAs) to control immune-specific phenotypes, which hints at a potential use as early biomarkers of neuroinflammation [14]. For this purpose, we compared the expression of immune-related miRNAs in AD and mild cognitive impairment (MCI) patients, with that in healthy age-matched control subjects. Deregulation of miRNA expression and functional impairments in chemotaxis and phagocytosis observed in AD and MCI patients were correlated with the levels of emergent proteins in the realm of AD research. This work suggests that the deregulation of specific immune-related miRNAs in AD patients may contribute to the observed dysfunctions in chemotaxis and phagocytosis.
2. Methods
2.1. Patient selection
Subjects (n = 124) were recruited at the Neurology Department, Coimbra University Hospital, 36 age-matched healthy controls (controls), 52 MCI patients (MCI), and 36 AD patients (AD), and were representative of the Portuguese Caucasian population. Patients' diagnostic investigation comprehended a standard clinical evaluation, routine laboratory tests and imaging studies (computed tomography [CT] or MRI), SPECT, and APOE allele genotyping. TREM2 genotyping for R47H mutation in exon 2 (associated with higher AD risk [15]) was performed in all AD and MCI patients where TREM2 expression was assessed. Positron emission tomography, cerebrospinal fluid analysis, and genetic studies were more restricted, although considered in younger patients.
A comprehensive cognitive-functional-psychological assessment battery was carried out by a team of neuropsychologists, following a standard protocol, and comprising several tests and scales: (1) cognitive instruments as the mini-mental state examination (MMSE) [16], the Montreal Cognitive Assessment (MoCA) [17], the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) [18], and a comprehensive neuropsychological battery validated for the Portuguese population (Battery of Lisbon for the Assessment of Dementia; [19]) were used to explore memory and other cognitive domains; (2) the clinical dementia rating (CDR; [20]) was used for global staging; and (3) the geriatric depression scale (GDS-30; [21]) was used to exclude major depression. All MCI patients were classified at the global CDR staging of 0.5 (no functional impairment) and were selected according to Albert's [22] and Petersen's criteria [23]. The standard criteria for the diagnosis of AD patients were the Diagnostic and Statistical Manual of Mental Disorders–fourth edition and the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders [24]. The AD group included patients with mild disease (CDR = 1) or moderate to severe (CDR = 2 and 3). The control group comprised 36 cognitively healthy adults belonging to the local community (recruited among the patients' spouses, hospital or university staff, or their relatives), that were age, education, and gender matched to the patients. Controls had normal MMSE scores (>24) and were fully autonomous in daily life activities (CDR) according to the information obtained through a general practitioner, and/or an informant. Moreover, to be eligible for this study, subjects (patients and controls) should be in a stable condition, without acute significant events or recent/undergoing changes in medication. Exclusion criteria were (1) significant motor, visual or auditory deficits which could influence the neuropsychological performance; (2) neurologic/psychiatric conditions other than MCI or AD; (3) CT or MRI demonstration of significant vascular burden [25]; (4) diagnosis of diabetes, chronic inflammatory, neoplastic diseases, or prescription of anti-inflammatory drugs; (5) major depression indicated by a GDS score of 20 or more points; (6) active smokers; and (7) patients experiencing uncontrolled hypertension. Informed consent was obtained from all participants, and the study was conducted in accordance with the tenets of the Declaration of Helsinki with the approval of the local ethics committee. Subjects' information/classification was only disclosed in the end of the study.
2.2. Isolation of BDMs
For each study subject, a total of 20 mL of blood was collected in sterile EDTA-coated tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation. BDM isolation was performed using magnetic separation, employing CD14 MicroBeads (Miltenyi Biotec, Germany) as described in the Supplementary Material. The purity of the CD14+ monocytes was determined by flow cytometry, using a monoclonal anti-CD14-FITC antibody (Sigma, USA), and was shown to be more than 96%.
2.3. Flow cytometry analysis
CCR2 and CXCR4 surface expression was analyzed by flow cytometry using anti-CCR2-PE and -CXCR4-PE antibodies (R&D Systems, USA) and the respective isotype controls, as described in detail in the Supplementary Material. Each sample was analyzed in a FACScalibur flow cytometer (BD Biosciences, USA) with the CellQuest Pro software (BD Biosciences).
2.4. Chemotaxis assay
Cell migration experiments were performed using the ChemoTx® Disposable Chemotaxis System (Neuro Probe, Inc., USA), as previously described [26] and detailed in the Supplementary Material. Immediately after isolation, BDMs were labeled with calcein-AM (Sigma) and seeded onto a 5-μm pore-membrane. Thirty microliters of chemoattractant were placed in each well of a 96-well multiwell plate fitted under the membrane: 10 nM CCL2, 12.5 nM CXCL12 (PeproTech, USA), and 5 μM Aβ fibrils. After 4 h at 37°C, the calcein fluorescence in each well was determined in a SpectraMax fluorimeter (λex = 494 nm; λem = 517 nm; Molecular Devices, USA).
2.5. Preparation of Aβ1–42 fibrils and Aβ1–42-FAM fibrils
Aβ1–42 fibrils were prepared as detailed in the Supplementary Materials and as previously described [27]. After incubation at 37°C for 7 days, the mixture of Aβ species was centrifuged for 10 minutes at 15,000 g, room temperature, the supernatant was discarded and the pellet, enriched in Aβ fibrils, was resuspended in HAM's F12 buffer (pH 7.5) at a final concentration of 50 μM. For the preparation of Aβ1–42-FAM fibrils, 0.1 mg of FAM-labeled Aβ1–42 peptide (American Peptide Co., USA) was dissolved in the same buffer containing 4% dimethyl sulfoxide (DMSO), at a concentration of 50 μM and then aged for 24 hours at 37°C in the dark.
2.6. Primary cultures of MDMs and phagocytosis assay
To promote BDM differentiation into MDMs, BDMs were plated in RPMI-1640 without fetal bovine serum (FBS) for 12 hours, and further exposed to 10% FBS and 50 ng/mL M-CSF (Sigma) for 7 days. MDMs were stimulated with 5 μM Aβ-FAM fibrils: Aβ fibrils 1:5 in RPMI-1640 10% FBS for 24 hours. LysoTracker® Red DND-99 (Life Technologies, USA) was used to stain lysosomes, whereas the nuclei were labeled with Hoechst 33342, as described in detail in the Supplementary Material. Confocal images were acquired in a point scanning confocal microscope Zeiss LSM 510 Meta (Zeiss, Germany), with a ×60 oil objective, using the LSM 510 META software.
2.7. RNA extraction and quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
For a selected number of subjects, a Pick-&-Mix microRNA PCR Panel (Exiqon, Germany), incorporating 90 miRNA and control primers, was performed. Results were analyzed using the GenEx qPCR analysis software (Exiqon). The expression of individual miRNAs, found to be differently expressed between groups, was confirmed by specific qRT-PCR assays (Exiqon). Messenger RNA (mRNA) levels of CCR2, CXCR4 and TREM2 genes were also quantified using qRT-PCR as described in detail in the Supplementary Material. All reactions were performed in duplicate in a StepOnePlus™ device (Applied Biosystems, USA).
2.8. Enzyme-linked immunosorbent assay (ELISA) for soluble TREM2 (sTREM2)
Quantification of sTREM2 in plasma samples was performed by ELISA, as previously described [15] and detailed in the Supplementary Material. The optical density in each well was determined at 450 nm, using a SpectraMax Gemini EM fluorimeter (Molecular Devices).
2.9. Statistical analysis
All data are expressed as ± standard error of the mean (SEM) and were analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison post hoc test, to compare normally distributed variables, except for CCR2 and CXCR4 surface expression which were analyzed with two-way ANOVA followed by Bonferroni's post hoc test. To analyze non-Gaussian data distributions (including sTREM2 quantification) and control the effect of potential confounders, such as gender and age, we log-transformed variables (such as sTREM2 concentration) to achieve normal distributions. Differences were considered statistically significant for P values <.05, .01, and .001, as described in the figure legends. All tests were two-tailed, and the data were analyzed using the GraphPad Prism 5 software.
3. Results
3.1. The presence of chemokine receptors is reduced at the cell surface of BDMs from AD and MCI patients
We analyzed the expression levels of CCR2 and CXCR4 in CD14+ BDMs isolated from patients and control subjects. A total of 124 subjects divided in three groups were involved in this study: 36 healthy age-matched subjects (controls), 36 AD patients, and 52 MCI patients. Table 1 summarizes the clinical data available for each one of the patient groups. The three groups presented approximate age and gender distributions.
Table 1.
Characteristics of patient and control study populations
| Variable | Controls | AD | MCI |
|---|---|---|---|
| Number of patients (n) | 36 | 36 | 52 |
| Gender (F/M) (%) | 58/42 | 64/36 | 46/54 |
| Age (years) | 70.3 ± 6.6 | 74.9 ± 8.8 | 73.6 ± 8.9 |
| MMSE score | >24 | 15 ± 7.6 | 26.9 ± 2.9∗∗∗ |
| MoCA score | 9.8 ± 5.8 | 18.3 ± 4.7∗∗∗ | |
| ADAS-cog score | — | 20.9 ± 7.6 | 9.6 ± 4.6∗∗∗ |
| Stage of disease (CDR) (%) | |||
| Mild | — | 63.9 | — |
| Moderate | — | 27.8 | — |
| Severe | — | 8.3 | — |
| APOE genotype ε4, % | — | 50 | 37 |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; MMSE, mini-mental state examination; MoCA, Montreal Cognitive Assessment; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive; CDR, clinical dementia rating; SD, standard deviation; ANOVA, analysis of variance.
NOTE. Data are expressed as mean ± SD, except for gender (expressed in percentage of females–F [%] and males–M [%]), stage of disease (CDR; expressed in percentage of total), and APOE genotype ɛ3/ɛ4 (expressed in percentage of ε4 carries). For MMSE and MoCA, higher scores correspond to better performance and for the ADAS-cog, higher scores indicate greater impairment. MoCA and ADAS-cog were only performed in patients with CDR ≤1. One-way ANOVA was used to compare age between groups, followed by Tukey's post hoc test. Two-tailed t test was used to compare MMSE, MoCA, and ADAS-cog scores, and Fisher's exact test was used for APOE genotype. ***P < .001 versus AD.
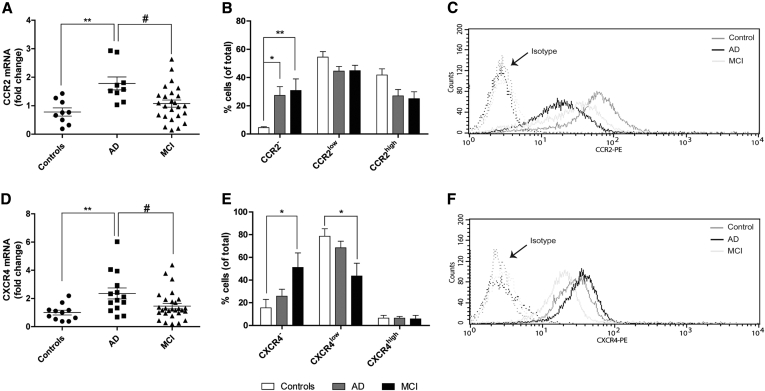
We found that the mRNA levels of CCR2 (Fig. 1A) and CXCR4 (Fig. 1D) were increased by two-fold in BDMs from AD patients, with respect to both controls and MCI patients. However, such increase did not translate into an increase of the presence of CCR2 and CXCR4 receptors at the cell surface. Flow cytometry studies, performed in live cells, revealed that the percentage of CCR2- BDMs was increased in both AD and MCI patients, with respect to controls, which was associated with a decrease in CCR2high populations (Fig. 1B). A reduction in the CXCR4low population was also observed in MCI patients, which presented a concomitant increase in CXCR4– cells (Fig. 1E). Fig. 1C and F shows representative histograms illustrating the distribution of CCR2 and CXCR4 surface signals in the BDM cell population. These results lead us to hypothesize that AD patients present an impairment in CCR2 mRNA translation. Moreover, although bearing CCR2 and CXCR4 mRNA levels similar to controls, MCI patients also presented a reduction in protein levels.
Fig. 1.
Presence of surface chemokine receptors CCR2 and CXCR4 is reduced in BDM of AD and MCI patients. The mRNA levels of chemokine receptors CCR2 (A) and CXCR4 (D) were quantified by qRT-PCR in CD14+ BDMs from controls, AD and MCI patients. Results are expressed as mRNA fold change with respect to the mean of controls and are representative of at least n = 9 per group (one-way ANOVA; **P < .01). *P < .05 and **P < .01 with respect to controls and #P < .05 with respect to AD patients. CD14+ BDM surface expression of CCR2 and CXCR4 proteins was analyzed by flow cytometry. Cell populations with negative, low, and high expression of CCR2 (B) and CXCR4 (E) were quantified by histogram analysis. Results are expressed as the percentage of total cells and are representative of at least n = 7 per group. *P < .05 and **P < .01 with respect to control subjects (two-way ANOVA followed by Bonferroni's post hoc test). (C and F) Representative histograms of a control subject, an AD, and an MCI patient for CCR2 and CXCR4 surface expression. Abbreviations: BDM, blood-derived monocyte; AD, Alzheimer's disease; MCI, mild cognitive impairment; mRNA, messenger RNA; ANOVA, analysis of variance.
3.2. CCL2-driven chemotaxis is impaired in BDM of AD and MCI patients
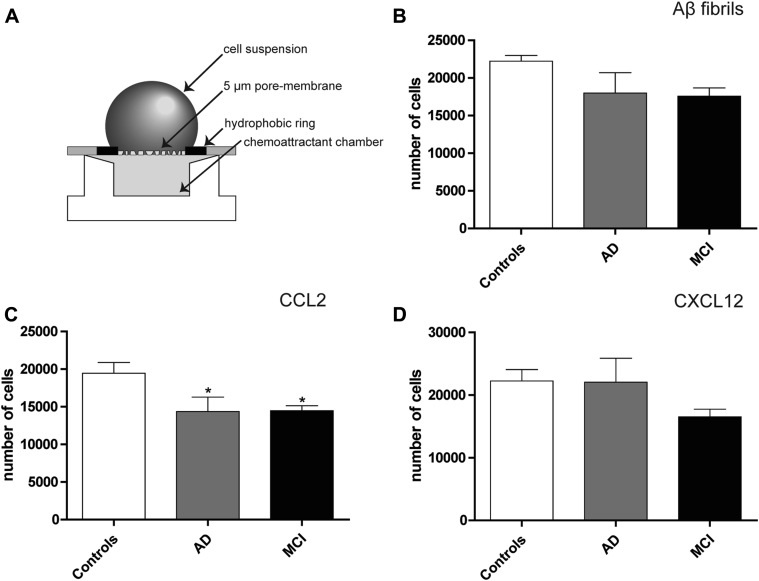
To investigate if the lower availability of CCR2 and CXCR4 at the cell surface of AD and MCI BDMs presented functional consequences, influencing the ability of these cells to migrate in response to chemokine gradients, we performed a chemotaxis assay in the presence of CCL2 and CXCL12, the specific ligands for CCR2 and CXCR4. Immediately after isolation, BDMs were stimulated for 4 hours with 10 nM CCL2 [28] and 12.5 nM CXCL12 in neuroprobe chemotaxis chambers (Fig. 2A). As expected, BDMs isolated from AD and MCI patients presented a significant reduction in cell migration in the presence of CCL2, with respect to BDMs from controls (Fig. 2C). A similar tendency was observed in MCI after exposure to CXCL12 (Fig. 2D), albeit without statistical significance. These results directly correlated with the increased number of BDMs negative for CCR2 and CXCR4 observed in AD and MCI (Fig. 1B and E).
Fig. 2.
CCL2-driven chemotaxis is impaired in AD and MCI BDMs. BDM migration was evaluated using the (A) ChemoTx® Disposable Chemotaxis System (Neuro Probe). CD14+ BDMs were labeled with 10 μM of calcein-AM and plated in a chemotaxis membrane. BDMs were stimulated with 5 μM Aβ fibrils (B), 10 nM CCL2 (C), or 12.5 nM CXCL12 (D), placed directly in the chemoattractant chamber. After 4 hours, the calcein signal was measured by fluorimetry in the wells beneath the membrane. Results are expressed as the number of cells found in the chemoattractant chamber after a 4-hour stimulation and are representative of at least n = 9 per group (one-way ANOVA; *P < .05, P = .08, and P = .1 for CCL2, Aβ, and CCL12, respectively). *P < .05 with respect to control subjects. Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; BDMs, blood-derived monocytes; Aβ, amyloid beta; ANOVA, analysis of variance.
Although previous in vitro studies have suggested that Aβ soluble monomers do not act as a direct chemoattractant for immune cells [29], it is believed that monocytes can potentially bind to vascular Aβ deposits in brain vessels [2]. Therefore, we investigated if other forms of the Aβ peptide, such as Aβ fibrils, could act as chemoattractants, by performing a similar chemotaxis experiment using Aβ fibrils. A tendency for reduced cell migration for both MCI and AD patients was observed (Fig. 2B), suggesting that BDMs from these patients might be less sensitive to chemotaxis signals than controls.
3.3. Phagocytosis of Aβ fibrils by MDMs is compromised in both AD and MCI patients
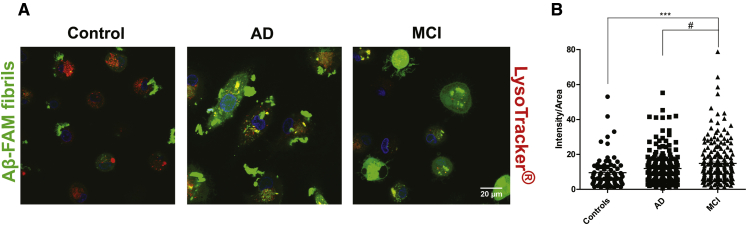
To evaluate phagocytosis efficiency of Aβ fibrils by MDMs, we isolated CD14+ BDMs from AD, MCI patients, and controls and promoted their differentiation into MDMs, which were incubated with Aβ-FAM fibrils for 24 hours before assessing Aβ binding and internalization by confocal microscopy. LysoTracker® was used to label lysosomes and other acidic compartments and thus determine Aβ co-localization with these organelles. MDMs from controls were able to bind, internalize, and degrade Aβ fibrils very efficiently because no big Aβ aggregates were visible inside the cells after the 24-hour incubation period (Fig. 3A, left image). On the other hand, MDMs from AD patients showed poor Aβ fibril internalization and presented big Aβ aggregates associated with the cell membrane (Fig. 3A, middle image). Moreover, in these patients, intracellular Aβ aggregates failed to co-localize with acidic endocytic vesicles. In contrast to AD patients, MDMs from MCI showed a massive uptake of Aβ fibrils. Approximately 50% of MCI cells were able to degrade Aβ, whereas the other half presented accumulation of intracellular Aβ (Fig. 3A, right image). These observations translated into a significant increase in intracellular fluorescent signal in MCI, with respect to controls and AD patients (Fig. 3B). Similar results were observed in a parallel experiment where internalized unlabeled Aβ was detected by immunocytochemistry (data not shown).
Fig. 3.
Internalization of Aβ fibrils is compromised in AD MDMs, whereas MCI MDMs present high Aβ fibril uptake. CD14+ BDMs were plated in 8-well ibidi plates and differentiated into MDMs. MDMs were incubated with 5 μM Aβ fibrils (1:5 Aβ-FAM fibrils: Aβ fibrils) for 24 hours, followed by incubation with 100 nM LysoTracker® for 30 minutes at 37°C. Phagocytosis of Aβ-FAM fibrils was evaluated by confocal microscopy (A) using a Zeiss LSM 510 Meta with a 63x oil objective. The fluorescence intensity inside the cells (B) was quantified using the ImageJ software. For each subject (at least n = 7 per group), six images were taken and, in each image, the intensity of fluorescence/area of four cells was quantified (one-way ANOVA; ***P < .001). ***P < .001 with respect to control subjects and #P < .05 with respect to AD patients. Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; MCI, mild cognitive impairment; BDMs, blood-derived monocytes; MDMs, monocyte-derived macrophages; ANOVA, analysis of variance.
3.4. TREM2 expression is increased in MCI patients
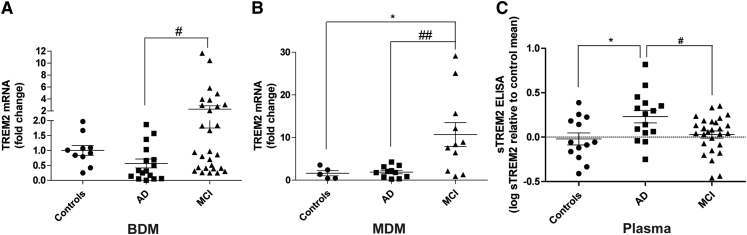
Rare variants of TREM2 and other new immune-related genes have been recently associated with higher risk of developing AD [6], [30], [31], [32]. In the particular case of TREM2, reported mutations, including R47H, have been associated with an impairment in TREM2 processing by secretases and concomitant shedding of TREM2 ectodomain (sTREM2) [15], [33]. Although the R47H TREM2 mutation was absent in our cohort, the fact that TREM2 has been implicated in the regulation of phagocytic activity [15] led us to explore the expression profile of TREM2 in BDMs and MDMs from AD and MCI patients. We observed a high increase in TREM2 mRNA expression, in both BDMs and MDMs from MCI patients, with respect to AD patients (Fig. 4A and B), and TREM2 expression was only upregulated in MCI compared with controls in MDMs (Fig. 4B). Interestingly, sTREM2 levels were elevated in the plasma of AD patients (Fig. 4C) with respect to both controls and MCI, suggesting that TREM2 shedding is not directly proportional to TREM2 mRNA expression. Although a slight decrease in TREM2 mRNA levels was detected in BDMs from AD patients (Fig. 4A), which could potentially correlate with the observed decrease in Aβ phagocytosis, this effect was not present in AD MDMs (Fig. 4B). Nevertheless, the increase in TREM2 expression presented by MCI patients (Fig. 4B) can help explain the high Aβ uptake observed in these patients.
Fig. 4.
Deregulation of TREM2 mRNA and sTREM2 levels in AD and MCI patients. TREM2 mRNA expression was quantified by qRT-PCR in (A) CD14+ BDMs and (B) MDMs of controls, AD, and MCI patients. Results are expressed as mRNA fold change with respect to the mean of controls and are representative of at least n = 10 (A) and n = 5 (B) per group (one-way ANOVA; *P <.05 and **P <.01, for TREM2 expression in BDMs and MDMs, respectively). *P < .05 with respect to control subjects and #P < .05, ##P < .01 with respect to AD patients. The soluble form of TREM2 (sTREM2) was quantified in plasma samples by ELISA (C). Results are expressed as log sTREM2 with respect to the mean of controls and are representative of at least n = 13 per group (one-way ANOVA **P < .01). *P < .05 with respect to control subjects, and #P < .05 with respect to AD patients. Abbreviations: TREM2, triggering receptor expressed on myeloid cells 2; AD, Alzheimer's disease; MCI, mild cognitive impairment; BDMs, blood-derived monocytes; mRNA, messenger RNA; MDMs, monocyte-derived macrophages; ELISA, enzyme-linked immunosorbent assay; ANOVA, analysis of variance.
3.5. Immune-related miRNAs are differentially expressed in AD and MCI patients
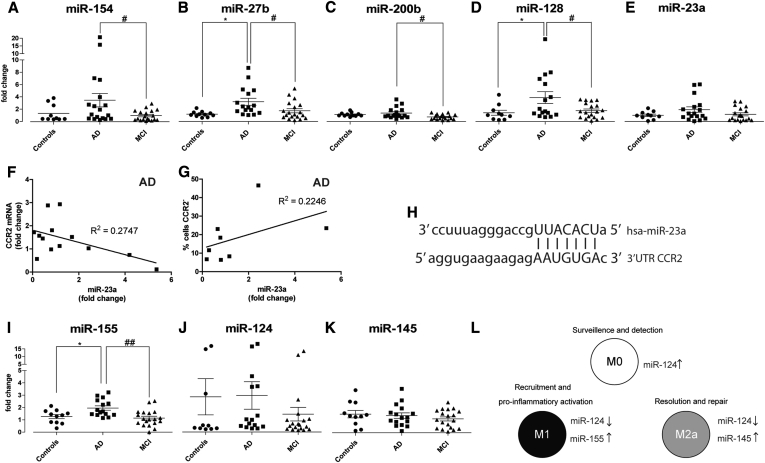
Considering the important role of miRNAs in the control of immune responses, miRNA deregulation may help explain, from a mechanistic point of view, changes in cell-specific phenotypes. In this regard, we investigated the expression of immune-related miRNAs that, according to miRWalk [34], are predicted to bind and regulate the transduction of proteins implicated in chemotaxis and phagocytosis. This database uses up to eight different mathematical algorithms to search for miRNA putative binding sites in all known mRNAs, mitochondrial genes, and 10-kb upstream gene flanking regions. Based on this information, we designed PCR arrays to quantify the levels of 90 miRNAs (Supplementary Table 1) in BDMs from 12 AD, MCI patients, and controls. This study allowed to identify five miRNAs which were differentially expressed in the three experimental groups: miR-154, -27b, -200b, -128, and -23a. These findings were confirmed by single qRT-PCR assays in a larger cohort, using specific primer sets (Fig. 5A–E). Interestingly, all five miRNAs were overexpressed in BDMs from AD patients, with respect to either controls, MCI patients or both, which clearly shows that AD patients present several deficiencies in miRNA networks associated with inflammation and innate immune response. Because miR-154 and miR-23a share CCR2 as a predictive target, whereas miR-27b has been shown to regulate the CXCL12/CXCR4 axis [35], the observed increase in these miRNAs may contribute to the impairments observed in cell migration.
Fig. 5.
Immune-related miRNAs are differentially expressed in AD and MCI patients. Immune-related miRNAs (A–E) were quantified in CD14+ BDMs of controls, AD, and MCI patients by qRT-PCR, using individual qRT-PCR assays for each miRNA. Correlations between the levels of miR-23a (fold change with respect to the mean of controls) in AD patients and (F) mRNA CCR2 fold change or (G) the percentage of cells negative for CCR2 surface expression are shown. (H) Illustration of the miR-23a binding site in the 3′UTR of CCR2 (source: miRNA.org). (I–K) M1-, M0-, and M2a-related miRNAs were quantified by qRT-PCR in BDMs. Results are expressed as miRNA fold change with respect to the mean of controls and are representative of at least n = 10 per group (one-way ANOVA; *P < .05 for miR-154, -200b, and -128 expression and **P < .01 for miR-27b and -155 expression; P = .166 for miR-23a expression, and ns for miR-124 and -145). *P < .05 with respect to controls and #P < .05 and ##P < .01 with respect to AD patients. (L) Illustration of M1, M0, and M2a signature miRNAs. Abbreviations: miRNAs, microRNAs; AD, Alzheimer's disease; MCI, mild cognitive impairment; BDMs, blood-derived monocytes; mRNA, messenger RNA; ANOVA, analysis of variance.
MiR-23a expression was found to inversely correlate with CCR2 mRNA levels (Fig. 5F) and directly correlate with the percentage of cells negative for CCR2 (Fig. 5G). Because no differences in miRNA expression were found between controls and MCI patients, we can anticipate that all five miRNAs have potential, as conversion biomarkers, to distinguish MCI from AD.
Evaluation of the expression of three miRNAs, miR-155, miR-145, and miR-124, which serve as signature markers for the M1, M2a, and M0 activation phenotypes, respectively (Fig. 5L) [36], did not reveal differences in miR-124 and miR-145 expression profiles (Fig. 5J and K) but showcased an increase in miR-155 levels in AD patients with respect to both controls and MCI (Fig. 5I), suggesting a tendency toward the M1 phenotype.
4. Discussion
In this study, we provide new evidence of the deregulation of specific immune-related miRNAs in BDMs from AD and MCI patients, which may have impact in disease pathogenesis. According to several miRNA prediction algorithms, at least two of the identified miRNAs, miR-23a and miR-154, are able to bind and potentially regulate the 3′ untranslated region (3′UTR) of the chemokine receptor CCR2. The elevated levels of both miR-154 and miR-23a in AD patients (Fig. 5A and E) can help explain the observed reduction of CCR2 surface levels because an overexpression of miRNAs able to bind to the 3′UTR of CCR2 mRNA might block its translation into protein. We hypothesized that the low CCR2 cell surface expression observed in BDMs from AD and MCI patients is directly responsible for the impairment in CCL2-driven chemotaxis detected in these patients (Fig. 2C). Although studies in mouse models indicate that the CCR2+ BDM population is the only subset of cells able to efficiently restrict Aβ deposition [37], our results show that, in the context of human disease, CCR2 protein levels are decreased in AD and MCI BDMs, which may reduce cell ability to migrate to the brain and clear Aβ deposits. Despite the heterogeneity of the MCI group, which reflects the status of AD preclinical stage, the decrease in CCR2 expression at the cell surface in MCI patients is prominent and can potentially reflect the progression to definitive dementia. Moreover, because chemokine receptors exert a scavenging role by binding and removing chemokines from circulation and tissues [38], the reduction of CCR2+ BDMs can contribute to the increased levels of CCL2 observed in the serum of AD patients [39]. Therefore, although a global increase in CCR2 expression at the cell surface has been reported in AD PBMCs [40], [41], we provide the missing link regarding CCR2 expression specifically in BDMs, which does not corroborate the benefic role observed for monocytes in AD mouse models.
Concerning the less studied CXCR4/CXCL12 axis, at least one study has reported, in parallel with increased cognitive deficits, downregulation of CXCL12 and CXCR4 in Tg2576 mice [42], which is in line with our findings of a decrease in CXCR4 surface levels in MCI patients, as well as reduced migration toward CXCL12 (Fig. 1D and E; Fig. 2D).
Deficiencies in microglia-mediated Aβ phagocytosis have also been reported in AD [43], suggesting that microglia lose their ability to clear Aβ deposits with age and disease. The similar ontology between monocytes, macrophages, and microglia [44] and the previously mentioned observations gave strength to the hypothesis that circulating monocytes could fulfill the role of microglia, providing an alternative pathway for Aβ clearance [2]. However, for this process to occur efficiently, both in vascular deposition sites and the brain parenchyma, blood monocytes would have to migrate, infiltrate the brain microenvironment, and differentiate into efficient phagocytes. Our results illustrate an impairment in Aβ phagocytosis by AD MDMs, which was associated with a decrease in Aβ internalization (Fig. 3A) and corroborates previous published results [7]. Therefore, although in AD mouse models their similar counterparts have been shown to contribute to the decrease of Aβ load in the brain, in AD patients, their ability to clear Aβ is clearly compromised. Despite the high Aβ internalization observed in MCI patients (Fig. 3A and B), their cells also fail to efficiently degrade the peptide, presenting intracellular accumulation of Aβ but no co-localization with lysosomes or other acidic vesicles (Fig. 3A). This suggests that the observed impairment in Aβ clearance may be multifactorial and is not restricted to full-blown AD.
Several of the recently identified AD risk genes have been directly related with innate immune responses, actin dynamics, and clathrin-mediated endocytosis, suggesting that they may play a role in Aβ clearance. In this context, Kleinberger et al. [15] have recently showed that primary microglia isolated from Trem2 knockout mice present reduced phagocytic ability, whereas inhibition of TREM2 processing by ADAM10, which resulted in reduced sTREM2 shedding, was associated with an increase in phagocytosis in BV2 microglia cells [15], probably through the increase of functional TREM2 at the cell surface.
In what concerns the human disease, only one study reported elevated TREM2 mRNA levels in peripheral blood of AD patients with respect to controls [45], although this study did not include the MCI stage. Moreover, in this study, TREM2 expression was quantified in RNA extracted from blood and, therefore, cannot be directly associated with the mononuclear phagocyte population and with the phagocytosis phenotype. Because the presence of functional TREM2 at the cell surface was previously associated with increased phagocytic activity [15], our results led us to hypothesize that the upregulation of TREM2 mRNA expression observed in MDMs from MCI patients compared with both controls and AD patients (Fig. 4B) may help explain the high Aβ uptake (Fig. 3B) by MDMs. In addition, the elevated levels of TREM2 mRNA in BDMs from MCI patients (Fig. 4A) can constitute evidence of an early dysfunction in the precursors of the effective phagocytes. Interestingly, given the variability in the MCI group, we detected a subgroup within MCI patients that presented similar TREM2 mRNA levels to those observed in AD BDMs (Fig. 4A). It would be interesting to perform a translational evaluation in these patients, to clarify if the shift in TREM2 expression reflects disease progression, because lower levels of TREM2 mRNA in BDMs from MCI patients (Fig. 4A) can suggest a more pronounced AD preclinical stage, and this idea could be explored in the clinic to help disease staging. We also observed that sTREM2 levels were upregulated in the plasma of AD patients, with respect to both MCI and controls (Fig. 4C), which may indicate a reduction in the availability of functional TREM2 and help explaining the limited Aβ internalization by AD MDMs.
MiR-200b and miR-128, which were also found to be upregulated in BDMs from AD patients (Fig. 5C and D), can also be implicated in the observed impairment in Aβ phagocytosis. MiR-128 inhibition was shown to improve Aβ degradation in monocytes from AD patients, whereas its upregulation led to a decrease in the expression of lysosomal enzymes [46]. Regarding miR-200b, several databases predict its binding to the 3′UTR of beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase (MGAT3). According to Fiala et al. [47], downregulation of MGAT3 may be related to defective Aβ phagocytosis, suggesting a link between miR-200b upregulation, MGAT3 downregulation, and decreased Aβ clearance.
Interestingly, we also found that eight of the new AD susceptibility genes (CR1, BIN1, PICALM, SORL1, MS4A4A, CD33, CD2AP, and CLU) [30], [31], [32] are predicted targets of at least one miRNA shown to be differentially expressed (Supplementary Table 2) and can potentially be regulated by miR-154, -27b, -200b, -128, and -155. This further strengthens the idea that miRNA deregulation can be a major player in sporadic AD. Moreover, the expression of the five miRNAs found to be upregulated in AD is significantly different from MCI patients (Fig. 5A–D,I), suggesting that measuring these miRNAs in the preclinical AD stage could benefit the clinicians, helping in the staging of dementia.
We have previously studied the role of miR-155 in different neuroinflammatory contexts and observed that overexpression of this miRNA in microglia exposed to LPS [12] or Aβ fibrils [13] is associated with the classical M1 activation phenotype. In the present study, only miR-155 was differentially expressed between the three experimental groups. Although no significant differences were found in the expression of interleukin 6 and tumor necrosis factor-α (Supplementary Fig. 1A and B), miR-155 upregulation in AD patients (Fig. 5I) supports the current belief that BDMs from AD patients tend toward an M1 phenotype. This finding further correlates with the observed decrease in Aβ phagocytosis, which has also been associated with the M1 activation state.
Overall, this work point toward a systemic dysfunction of the innate immune response mechanisms in AD, encompassing two essential immune cell functions, chemotaxis and phagocytosis. Nevertheless, the availability of BDMs renders these cells promising cellular targets for AD treatment, and efforts should be made to fully disclosure the events responsible for the observed impairments. Considering the potential of miRNAs as biomarkers and molecular targets of disease, we believe that this study also contributes to shed light into the molecular mechanisms behind BDM and MDM dysfunction and opens new miRNA-based avenues for diagnosis and therapeutics in AD.
Research in context.
1.Systematic review: Systemic inflammation is believed to play an important role in Alzheimer's disease (AD), but is still an unexplored subject, particularly in what concerns the contribution of mononuclear phagocytes to amyloid beta (Aβ) clearance. We reviewed PubMed for studies addressing the role of blood phagocytes in health and disease and found reports suggesting benefic effects in animal models of AD. However, little information is available concerning these cells in the context of human disease.
2.Interpretation: Our study provides new evidence that the CCR2/CCL2 axis, crucial for chemotaxis, is deregulated in AD patients. Differences were also detected in the phagocytosis of the Aβ peptide. We correlated these results with significant changes in the expression of immune-related microRNAs (miRNAs) and new AD-associated proteins, such as TREM2.
3.Future directions: Our findings point to important systemic functional impairments in AD patients and suggest caution when translating the knowledge obtained from AD mouse models to human patients. Future work includes exploring the link between the identified miRNAs and the new AD risk proteins from mechanistic and therapeutic perspectives.
Acknowledgments
This work was supported by grants from the Portuguese Foundation for Science and Technology (J.R.G.: SFRH/BD/51677/2011, A.M.C.: SFRH/BPD/99613/2014 and PTDC/BIM-MEC/0651/2012) and FEDER/COMPETE (PEst-C/SAU/LA0001/2013-2014). From CNC, the authors thank Prof. João Nuno Moreira for providing the LysoTracker® and Prof. Ana Cristina Rego, Dr. John Jones, and Filipa Simões for their help regarding the production of Aβ fibrils.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.11.004.
Supplementary data
References
- 1.Lebson L., Nash K., Kamath S., Herber D., Carty N., Lee D.C. Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J Neurosci. 2010;30:9651–9658. doi: 10.1523/JNEUROSCI.0329-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaud J.P., Bellavance M.A., Prefontaine P., Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013;5:646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Koronyo Y., Salumbides B.C., Sheyn J., Pelissier L., Li S., Ljubimov V. Therapeutic effects of glatiramer acetate and grafted CD115+ monocytes in a mouse model of Alzheimer's disease. Brain. 2015;138:2399–2422. doi: 10.1093/brain/awv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 5.Naert G., Rivest S. Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. Mol Med. 2012;18:297–313. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiala M., Lin J., Ringman J., Kermani-Arab V., Tsao G., Patel A. Ineffective phagocytosis of amyloid-ß by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- 8.Naert G., Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickman S.E., Kingery N.D., Ohsumi T.K., Borowsky M.L., Wang L.C., Means T.K. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso A.L., Guedes J.R., Pereira de Almeida L., Pedroso de Lima M.C. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. doi: 10.1111/j.1365-2567.2011.03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guedes J.R., Custodia C.M., Silva R.J., de Almeida L.P., Pedroso de Lima M.C., Cardoso A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer's disease triple transgenic mouse model. Hum Mol Genet. 2014;23:6286–6301. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- 14.Butovsky O., Siddiqui S., Gabriely G., Lanser A.J., Dake B., Murugaiyan G. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinberger G., Yamanishi Y., Suarez-Calvet M., Czirr E., Lohmann E., Cuyvers E. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 16.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohs R.C., Rosen W.G., Davis K.L. The Alzheimer's disease assessment scale: An instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19:448–450. [PubMed] [Google Scholar]
- 19.Guerreiro M. [Contribution of Neuropsychology to the study of dementia]. University of Lisbon; Lisbon: 1998. Contributo da Neuropsicologia para o estudo das demências. (Unpublished doctoral dissertation) [Google Scholar]
- 20.Morris J.C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 26.Frevert C.W., Wong V.A., Goodman R.B., Goodwin R., Martin T.R. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods. 1998;213:41–52. doi: 10.1016/s0022-1759(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 27.Resende R., Ferreiro E., Pereira C., Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Volpe S., Cameroni E., Moepps B., Thelen S., Apuzzo T., Thelen M. CCR2 acts as scavenger for CCL2 during monocyte chemotaxis. PLoS One. 2012;7:e37208. doi: 10.1371/journal.pone.0037208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baik S.H., Cha M.Y., Hyun Y.M., Cho H., Hamza B., Kim D.K. Migration of neutrophils targeting amyloid plaques in Alzheimer's disease mouse model. Neurobiol Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 33.Wunderlich P., Glebov K., Kemmerling N., Tien N.T., Neumann H., Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and gamma-secretase-dependent intramembranous cleavage. J Biol Chem. 2013;288:33027–33036. doi: 10.1074/jbc.M113.517540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dweep H., Sticht C., Pandey P., Gretz N. miRWalk–database: Prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Lu M.H., Li C.Z., Hu C.J., Fan Y.H., Wang S.M., Wu Y.Y. microRNA-27b suppresses mouse MSC migration to the liver by targeting SDF-1alphain vitro. Biochem Biophys Res Commun. 2012;421:389–395. doi: 10.1016/j.bbrc.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Freilich R.W., Woodbury M.E., Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naert G., Rivest S. A deficiency in CCR2+ monocytes: The hidden side of Alzheimer's disease. J Mol Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- 38.Mahad D., Callahan M.K., Williams K.A., Ubogu E.E., Kivisakk P., Tucky B. Modulating CCR2 and CCL2 at the blood-brain barrier: Relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- 39.Galimberti D., Fenoglio C., Lovati C., Venturelli E., Guidi I., Corra B. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Reale M., Iarlori C., Feliciani C., Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. J Alzheimers Dis. 2008;14:147–159. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- 41.Pellicano M., Bulati M., Buffa S., Barbagallo M., Di Prima A., Misiano G. Systemic immune responses in Alzheimer's disease: In vitro mononuclear cell activation and cytokine production. J Alzheimers Dis. 2010;21:181–192. doi: 10.3233/JAD-2010-091714. [DOI] [PubMed] [Google Scholar]
- 42.Parachikova A., Cotman C.W. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–153. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krabbe G., Halle A., Matyash V., Rinnenthal J.L., Eom G.D., Bernhardt U. Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu N., Tan M.S., Yu J.T., Sun L., Tan L., Wang Y.L. Increased expression of TREM2 in peripheral blood of Alzheimer's disease patients. J Alzheimers Dis. 2014;38:497–501. doi: 10.3233/JAD-130854. [DOI] [PubMed] [Google Scholar]
- 46.Tiribuzi R., Crispoltoni L., Porcellati S., Di Lullo M., Florenzano F., Pirro M. miR128 up-regulation correlates with impaired amyloid beta(1-42) degradation in monocytes from patients with sporadic Alzheimer's disease. Neurobiol Aging. 2014;35:345–356. doi: 10.1016/j.neurobiolaging.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Fiala M., Liu P.T., Espinosa-Jeffrey A., Rosenthal M.J., Bernard G., Ringman J.M. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.