Abstract
Introduction
For early detection of Alzheimer's disease (AD), the field needs biomarkers that can be used to detect disease status with high sensitivity and specificity. Apolipoprotein J (ApoJ, also known as clusterin) has long been associated with AD pathogenesis through various pathways. The aim of this study was to investigate the potential of plasma apoJ as a blood biomarker for AD.
Methods
Using the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging, the present study assayed plasma apoJ levels over baseline and 18 months in 833 individuals. Plasma ApoJ levels were analyzed with respect to clinical classification, age, gender, apolipoprotein E (APOE) ε4 allele status, mini-mental state examination score, plasma amyloid beta (Aβ), neocortical Aβ burden (as measured by Pittsburgh compound B-positron emission tomography), and total adjusted hippocampus volume.
Results
ApoJ was significantly higher in both mild cognitive impairment (MCI) and AD groups as compared with healthy controls (HC; P < .0001). ApoJ significantly correlated with both “standardized uptake value ratio” (SUVR) and hippocampus volume and weakly correlated with the plasma Aβ1–42/Aβ1–40 ratio. Plasma apoJ predicted both MCI and AD from HC with greater than 80% accuracy for AD and greater than 75% accuracy for MCI at both baseline and 18-month time points.
Discussion
Mean apoJ levels were significantly higher in both MCI and AD groups. ApoJ was able to differentiate between HC with high SUVR and HC with low SUVR via APOE ε4 allele status, indicating that it may be included in a biomarker panel to identify AD before the onset of clinical symptoms.
Keywords: Biomarkers, Apolipoprotein J, Plasma, Brain amyloid beta, Hippocampus volume
1. Introduction
Apolipoprotein J (ApoJ), also popularly known as clusterin, is an extracellular chaperone protein that is part of the defense machinery acting against extracellular protein misfolding [1], [2]. ApoJ has been previously associated with Alzheimer's disease (AD) pathogenesis [3]. Studies have shown that apoJ inhibits formation of amyloid beta (Aβ) deposits and thereby its toxicity by interacting with prefibrillar species and inhibiting fibril formation [4], [5]. Various genome-wide association studies have also linked apoJ with AD by identifying the clusterin gene (CLU) as one of the strong genetic loci for AD [6], [7], [8]. This has led many groups to investigate apoJ as a potential peripheral diagnostic marker for AD [7], [9], [10]. Plasma apoJ levels have previously associated with entorhinal cortex atrophy and disease severity; however, this study found no differences in actual plasma levels between healthy and AD participants [10]. In another study, plasma apoJ levels were found to be higher in AD participants compared with healthy controls (HC) and correlated with disease severity [11]. IJsselstijn et al. [12] showed that serum apoJ levels were not increased in presymptomatic AD compared with controls in a set of 43 participants who were diagnosed with AD during a 10-year follow-up study. Another study concluded apoJ to be not good enough as a diagnostic biomarker for AD where they showed similar plasma apoJ levels in controls, AD, and patients with other dementias [13]. This body of literature clearly indicates that there is a general lack of consensus as to the efficacy of apoJ as a blood biomarker for AD. The aim of this present study was to address this lack of agreement by assaying the levels of apoJ in plasma samples obtained at baseline and follow-up time points from the highly characterized Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging cohort. AIBL consists of volunteers from different clinical categories namely HCs, mild cognitively impaired (MCI), and patients with AD.
2. Methods
2.1. Population sample
2.1.1. The AIBL cohort
The AIBL study is a prospective, longitudinal study, following participants at 18-month intervals. The cohort recruitment process including the neuropsychological, lifestyle, and mood assessments have been previously described in detail [14]. In brief, the AIBL study recruited a total of 1166 participants aged >60 years at baseline, of whom 54 were excluded because of comorbid disorders or consent withdrawal. Using the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) international criteria for AD diagnosis [15], a clinical review panel determined disease classifications at each assessment time point to ensure accurate and consistent diagnoses among the participants. According to these diagnostic criteria, participants were classified into one of three groups; AD, MCI, or HC. At baseline, there were a total of 768 HC, 133 MCI, and 211 AD subjects.
This study reports on 833 individuals at baseline and 824 individuals at 18 months who completed the full study assessment and corresponding blood sample collection at both baseline and 18-month follow-up. A subgroup of these participants also underwent brain imaging with carbon-11-labeled Pittsburgh compound B-positron emission tomography (11C-PiB-PET) and magnetic resonance imaging (MRI) as listed in Table 1.
Table 1.
Demographics of the cohort analyzed in this study, means and standard deviation for age, and PiB-PET SUVR and interquartile range for MMSE
| Categories | HC |
MCI |
AD |
P value |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | |
| Count (n) | 590 | 576 | 93 | 70 | 150 | 178 | ||
| Age, mean (±SD) | 70.72 (6.9) | 72.07 (6.73) | 75.84 (7.31) | 76.67 (7.44) | 77.89 (7.66) | 79.31 (7.65) | <.0001 | <.0001 |
| Gender (n, %F) | 335 (57) | 331 (58) | 52 (56) | 38 (54) | 88 (59) | 105 (59) | .890 | .800 |
| APOE ε4 positive, % | 189 (32) | 182 (32) | 49 (53) | 30 (43) | 99 (66) | 121 (68) | <.0001 | <.0001 |
| MMSE (IQR) | 29 (2) | 29 (2) | 27 (3) | 27 (3) | 20 (5) | 18 (9) | <.0001 | <.0001 |
| PiB-PET subgroup (n) | 144 | 140 | 42 | 27 | 21 | 22 | ||
| PiB SUVR | 1.41 (0.4) | 1.39 (0.38) | 1.91 (0.58) | 1.84 (0.64) | 2.34 (0.46) | 2.35 (0.44) | <.0001 | <.0001 |
| MRI subgroup (n) | 137 | 132 | 36 | 23 | 16 | 18 | ||
| Hippocampus volume (mean) | 0.0041 | 0.0041 | 0.0038 | 0.0038 | 0.0036 | 0.0034 | <.0001 | <.0001 |
| Hippocampus volume (SD) | 0.0003 | 0.0003 | 0.0005 | 0.0005 | 0.0004 | 0.0004 | <.0001 | <.0001 |
Abbreviations: SD, standard deviation; PiB-PET, Pittsburgh compound B-positron emission tomography; SUVR, standardized uptake value ratio; MMSE, mini-mental state examination; HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer's disease; APOE ε4, apolipoprotein ε4; IQR, interquartile range; MRI, magnetic resonance imaging.
NOTE. P values shown are unadjusted.
The institutional ethics committees of Austin Health, St. Vincent's Health, Hollywood Private Hospital, and Edith Cowan University granted ethics approval for the AIBL study. All volunteers gave written informed consent before participating in the study.
2.2. Sample collection and apolipoprotein E (APOE) genotyping
Plasma was isolated from whole blood and collected in standard EDTA tubes with prostaglandin E1 (33.3 ng/mL, Sapphire Biosciences, NSW, Australia) added. On completion of blood fractionation, samples were aliquoted and immediately stored in liquid nitrogen until required for analysis. DNA was isolated from whole blood using a QIAamp DNA Blood Midi Kit (Qiagen, VIC, Australia) according to the manufacturer's protocol, and APOE genotype was determined through TaqMan genotyping assays (Life Technologies, Mulgrave, VIC, Australia) for rs7412 (Assay ID: C____904973_10) and rs429358 (Assay ID: C___3084793_20) [16]. For TaqMan assays, polymerase chain reactions and real-time fluorescence measurements were carried out on a QuantStudio real-time PCR system (Applied Biosystems, VIC, Australia) using the TaqMan® GTXpress Master Mix (Life Technologies) methodology as per manufacturer's instructions.
2.3. Plasma apoJ assay
Plasma apoJ levels were assayed using a commercial quantitative sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, USA). The plasma samples were thawed on ice, centrifuged for 10 minutes at 12,000× g, and diluted 4000-fold using the supplied Calibrator Diluent RD5T. All the reagents were brought to room temperature before use. The kit uses a monoclonal antibody specific for apoJ that has been precoated onto a microplate. Briefly, 100 μL of Assay Diluent RD1-19 was added to each well, followed by 50 μL of standard or sample per well. The plate is then kept at room temperature on a horizontal orbital microplate shaker for incubation for 2 hours. The plate is then washed to remove any unbound substances; an enzyme-linked monoclonal antibody specific for apoJ is added to the wells. After a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of apoJ bound in the initial step. The color development is stopped, and the intensity of the color was then measured using a BMG microplate reader at 450 nm.
2.4. Evaluation of neocortical Aβ and hippocampus volume via PiB-PET and MRI
A subset of the AIBL cohort (n = 287) underwent 11C-PiB-PET imaging at baseline to measure cerebral amyloid load, as previously described [17]. Imaging was carried out using a Phillips Allegro PET camera. The sorted sinograms were reconstructed using a three-dimensional row-action maximization likelihood algorithm (RAMLA) algorithm. Aβ burden was calculated as the average of the mean of frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate region of interest (ROI) activity per voxel divided by the cerebellar gray matter voxel activity and termed the standardized uptake value ratio (SUVR). The cerebellar cortex was used as a reference region because of no PiB binding shown in either controls or AD. Of the total 833 participants reported here, 207 individuals underwent 11C-PiB-PET imaging at baseline and 189 underwent at 18-month follow-up. In addition, 3D T1 MPRAGE (magnetization-prepared rapid acquisition gradient echo) and a T2 TurboSpin echo and FLAIR (fluid-attenuated inversion recovery) sequence MRI were acquired for screening and co-registration with the PET images. MRI measurements were performed on Siemens Avanto 1.5 T scanner. Co-registration of the PET images with each individual's MRI was performed with SPM2 (statistical parametric mapping). For registration purposes, the initial frames of the dynamic PET studies were summed. Mean radioactivity values were obtained from ROIs for cortical, subcortical, and cerebellar regions. Hippocampal volume was calculated from T1 MPRAGE images and was normalized by dividing with the total intracranial volume consisting of the sum of the cerebrospinal fluid, gray matter, and white matter volumes. Of the total 833 participants reported here, 189 individuals underwent MRI at baseline, whereas 173 individuals were scanned at the 18-month follow-up.
2.5. Statistical methodology
Descriptive statistics including means, standard deviations (SDs), and frequencies were calculated across clinical classifications. Comparisons in frequencies of gender and APOE ε4 allele comparisons were calculated using χ2 test and Fisher's exact where necessary. P values, calculated from the analysis of mean apoJ levels between clinical classifications adjusted for age, gender, and APOE ε4 allele status, were derived using polynomial ordered logistic regression and generalized linear modeling (GLM) for three-group and two-group comparisons, respectively. Linear mixed models (LMM) were used to define P values for the comparison of mean apoJ levels between HC and MCI, and HC and AD participants over time, adjusted for age, gender, and APOE ε4 allele status. Spearman's correlation coefficients were calculated to describe the relationship between apoJ and plasma Aβ, SUVR, and hippocampal volume. Testing across the lower range of SUVR values, four different threshold values (1.3, 1.4, 1.5, and 1.8) for SUVR were tested to find the most appropriate criterion for biomarker evaluation before the 1.8 cutoff being chosen. GLM combined with receiver operating characteristic (ROC) analyses were combined to perform 100-fold cross-validated disease predictions. The R statistical software environment, version 2.15, was used for all statistical analyses (Team, R Development Core. 2009. R: A Language and Environment for Statistical Computing Manual).
3. Results
3.1. Population demographics
Baseline and 18-month follow-up demographic data, APOE genotype, and mini-mental state examination (MMSE) for the AIBL cohort are presented in Table 1. SUV ratios for PiB-PET and hippocampal volume from MRI for the AIBL imaging subcohort are also presented in Table 1. ApoJ data were available for 590 HCs, 93 participants with MCI, and 150 participants with AD at baseline, and 576 HC, 93 participants with MCI, and 178 participants with AD at 18 months. Nine participants were withdrawn from the AIBL study in the 18-month interim period. Age, APOE ε4 allele status, and MMSE were significantly different between clinical classifications at both baseline and 18 months (P < .0001; Table 1). There was no difference in the proportion of females to males at either time point (P > .05). Total number of participants from the AIBL imaging subcohort was lower compared with that in the total group; however, we found no significant difference in mean apoJ levels between those participants included in the complete cohort as compared with those in the imaging subcohort (P > .05). SUVR was significantly higher and total adjusted hippocampus volume was significantly lower in the MCI and AD groups as compared with those in the HC group (P < .0001; Table 1).
3.2. Mean apoJ levels are higher in MCI/AD participants compared with those in HCs
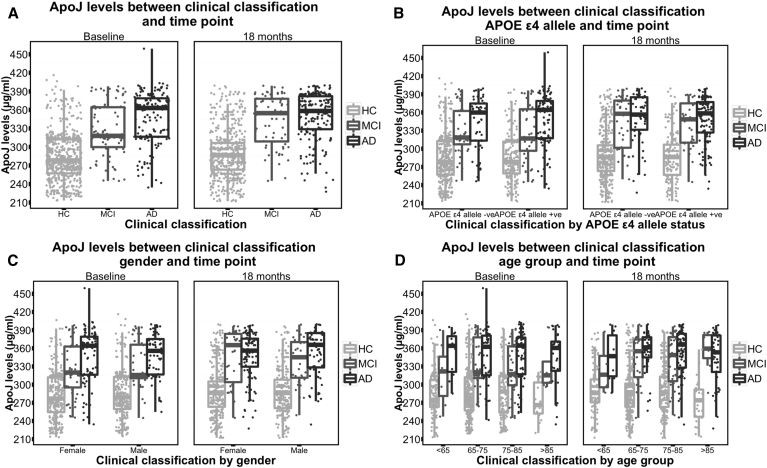
ApoJ levels were consistent between time points (Pearson's correlation coefficient R = 0.7, P < .0001). Mean apoJ levels were significantly higher in both MCI and AD groups as compared with those in the HC group at both baseline and 18 months (P values for all tests where there were sufficient numbers to analyze the data were <.0001, Table 2, Table 3, Fig. 1A); however, the levels were not different between MCI and AD groups (P > .05, data not shown). We conducted stratified analyses for APOE ε4 allele status (Fig. 1B), gender (Fig. 1C), and age group (Fig. 1D), with significant differences in apoJ identified between HC and both MCI and AD for most stratification categories (P values for all tests where there were sufficient numbers to analyze the data were <.05). ApoJ levels were not significantly different between males and females, APOE ε4 allele carriers and noncarriers, and older and younger participants across both time points (P > .05, data not shown). Adjustment for age, gender, and APOE ε4 allele status did not affect the outcome compared with unadjusted analyses. Using the longitudinal nature of the AIBL study, we compared mean apoJ levels between clinical classifications over time (HC vs. MCI and HC vs. AD, LMM). We found that the difference in apoJ levels between clinical classifications remained statistically significant (P < .0001 for both comparisons) and that there was no significant change in apoJ levels between baseline and 18 months (P > .05).
Table 2.
ApoJ means and standard deviations for stratified subgroup analyses
| Categories | Mean (SD) |
|||||
|---|---|---|---|---|---|---|
| HC mean (SD) |
MCI mean (SD) |
AD mean (SD) |
||||
| Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | |
| Total | 283.76 (38.98) | 289.8 (39.63) | 327.33 (41.41) | 342.58 (40.87) | 347.17 (42.09) | 349.38 (38.91) |
| APOE ε4 −ve | 283.69 (39.58) | 290.9 (39.94) | 328.33 (37.61) | 344.27 (42.74) | 343.03 (43.22) | 350.03 (41.36) |
| APOE ε4 +ve | 283.92 (37.78) | 287.4 (38.94) | 326.44 (44.92) | 340.31 (38.83) | 349.31 (41.55) | 349.07 (37.88) |
| Female | 282.13 (37.72) | 290.82 (40.2) | 323.96 (43.78) | 346.32 (42.91) | 347.04 (45.07) | 347.16 (39.51) |
| Male | 285.91 (40.55) | 288.41 (38.87) | 331.6 (38.3) | 338.13 (38.49) | 347.36 (37.81) | 352.56 (38.08) |
| Age | ||||||
| <65 | 282.92 (36.65) | 290.75 (41.29) | 316.3 (51.12) | 326.75 (45.23) | 351.31 (36.9) | 346.7 (39.36) |
| 65–75 | 283.78 (39.89) | 289.08 (38.45) | 333.82 (41.66) | 343.69 (40.44) | 347.94 (45.56) | 354.66 (33.77) |
| 75–85 | 286.22 (40.44) | 293.49 (40.89) | 323.61 (40.42) | 341.09 (42.6) | 347.74 (39.83) | 352.68 (37.22) |
| >85 | 272.24 (31.3) | 272.65 (36.66) | 329.86 (40.72) | 354.86 (36.01) | 343.3 (45.61) | 343.62 (42.59) |
Abbreviations: APoJ, apolipoprotein J; SD, standard deviation; HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer's disease; APOE ε4, apolipoprotein ε4.
NOTE. NA means not enough participants in group to calculate mean and standard deviation.
Table 3.
P values for the mean difference between HC and MCI and HC and AD groups at both baseline and at 18 mo for each of the stratified categories
| Stratification | All groups |
HC versus MCI |
HC versus AD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||||
| Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | Baseline | 18 mo | |
| Total | 1.73E-64 | 5.80E-67 | 1.30E-35 | 1.30E-35 | 8.38E-22 | 7.28E-24 | 7.06E-21 | 8.97E-23 | 1.28E-57 | 2.31E-58 | 2.11E-48 | 9.83E-50 |
| APOE ε4 −ve | 5.53E-27 | 6.95E-30 | 6.74E-19 | 1.46E-19 | 3.96E-12 | 1.15E-14 | 4.70E-13 | 1.40E-14 | 2.56E-21 | 7.70E-23 | 1.66E-21 | 8.91E-23 |
| APOE ε4 +ve | 1.43E-32 | 8.97E-35 | 1.00E-17 | 1.65E-17 | 1.19E-10 | 6.07E-11 | 6.81E-08 | 7.84E-09 | 2.23E-32 | 2.41E-33 | 1.80E-24 | 2.72E-25 |
| Female | 3.39E-38 | 5.44E-36 | 1.39E-21 | 3.94E-20 | 1.94E-12 | 1.61E-14 | 5.40E-12 | 3.23E-14 | 6.94E-36 | 4.30E-31 | 3.10E-30 | 1.81E-26 |
| Male | 4.63E-27 | 5.26E-32 | 1.32E-15 | 1.18E-17 | 8.05E-11 | 6.03E-11 | 3.47E-10 | 7.49E-10 | 1.77E-23 | 3.65E-29 | 9.91E-20 | 6.02E-25 |
| Age | ||||||||||||
| <65 | 3.37E-08 | 0.0002 | 6.06E-06 | 0.0004 | 0.0222 | 1.0000 | 0.0441 | 1.0000 | 1.57E-08 | 8.50E-05 | 6.71E-09 | 0.0001 |
| 65–75 | 1.75E-23 | 1.75E-26 | 7.21E-16 | 1.05E-16 | 3.13E-11 | 4.97E-11 | 5.03E-12 | 1.33E-11 | 6.60E-19 | 9.09E-22 | 8.20E-18 | 8.45E-22 |
| 75–85 | 6.53E-21 | 3.48E-23 | 1.14E-13 | 1.52E-15 | 2.75E-07 | 6.26E-09 | 1.11E-05 | 7.98E-09 | 2.32E-20 | 5.44E-22 | 2.19E-16 | 7.81E-20 |
| >85 | 9.53E-07 | 2.87E-09 | 0.0001 | 4.05E-05 | 0.0005 | 1.54E-05 | 1.0000 | 0.0001 | 3.20E-07 | 3.16E-09 | 1.0000 | 5.73E-07 |
Abbreviations: HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer's disease; APOE ε4, apolipoprotein ε4.
NOTE. A value of 1.000 represents not enough participants in the subgroup for a valid statistical test
Fig. 1.
Box and whisker plot of apoJ levels between clinical classification and time point. (A) ApoJ by clinical classification, (B) ApoJ by classification and apolipoprotein ε4, (C) ApoJ by clinical classification and gender, and (D) ApoJ by clinical classification and age group. Abbreviations: APoJ, apolipoprotein J; HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer's disease; APOE ε4, apolipoprotein ε4.
3.3. Association between apoJ and hippocampus volume and SUVR
We conducted Spearman's correlation analyses between both adjusted total hippocampus volume and SUVR with apoJ levels at both baseline and 18 months. ApoJ was negatively correlated with adjusted total hippocampus volume in the whole group (baseline, R = −0.257, P = .0004; 18 months, R = −0.178, P = .019). Assessing the correlation within clinical classification showed the strongest association was within the AD group but only at the 18-month time point (R = −0.445, P = .066, Table 4). Using the three clinical classifications together identified a positive correlation between SUVR and apoJ at both baseline and 18 months (baseline R = 0.242, P = .0004; 18 months, R = 0.277, P = .0001; Table 4), whereas assessing the groups individually did not reveal any significant correlations between apoJ and SUVR (Table 4).
Table 4.
Spearman's correlations R and associate P values for both whole groups and groups stratified by clinical classification
| Clinical classification | Characteristic | Baseline |
18 mo |
||
|---|---|---|---|---|---|
| R | P value | R | P value | ||
| Whole group | Hippocampus | −0.257 | .0004 | −0.178 | .019 |
| HC | Hippocampus | −0.081 | .347 | 0.065 | .460 |
| MCI | Hippocampus | −0.141 | .410 | −0.121 | .582 |
| AD | Hippocampus | −0.032 | .908 | −0.445 | .066 |
| Whole group | SUVR | 0.242 | .0004 | 0.277 | .0001 |
| HC | SUVR | 0.027 | .749 | 0.078 | .359 |
| MCI | SUVR | −0.003 | .983 | 0.358 | .067 |
| AD | SUVR | −0.327 | .148 | −0.134 | .551 |
Abbreviations: HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer's disease; hippocampus, total hippocampus volume adjusted for intracranial volume; SUVR, standardized uptake value ratio.
3.4. APOE ε4 allele–specific apoJ comparisons
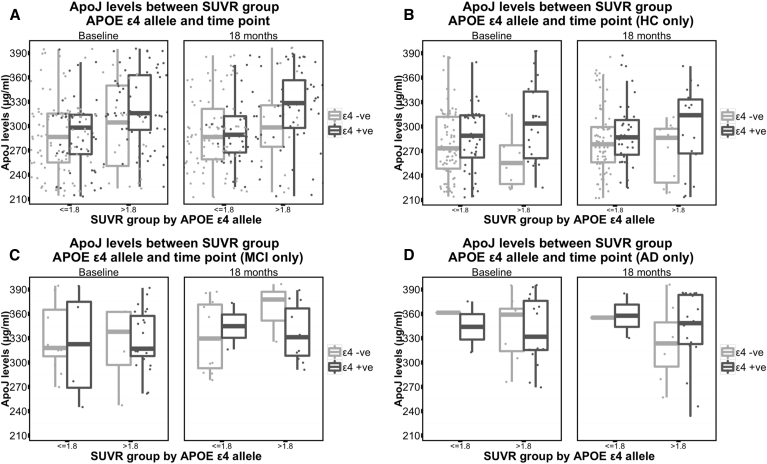
Investigation of the imaging subcohort identified a significant interaction for apoJ levels between APOE ε4 allele status and neocortical Aβ burden (as measured by 11C-PiB-PET ≤1.8/>1.8, HC group only, P = .03 [GLM]). When comparing HC participants without an APOE ε4 allele, those participants with an SUVR of >1.8 had lower mean apoJ levels (baseline mean apoJ 264.4 [SD ± 33.3], 18-month mean apoJ 273.9 [SD ± 34.0]) compared with those with an SUVR of ≤1.8 (baseline mean apoJ 279.9 [SD ± 41.2], 18-month mean apoJ 284.8 [SD ± 41.3]). Conversely, those HC participants with an APOE ε4 allele with an SUVR of >1.8 had much higher mean apoJ levels (baseline mean apoJ 310.4 [SD ± 45.8], 18-month mean apoJ 301.9 [SD ± 49.6]) compared with those of an SUVR of ≤1.8 (baseline mean apoJ 285.0 [SD ± 40.4], 18-month mean apoJ 292.1 [SD ± 37.4]; Fig. 2B). A similar relationship was shown for the MCI group (Fig. 2C). Using the standard SUVR threshold of 1.5 and lower (1.3 and 1.4) did not show the same interactions.
Fig. 2.
ApoJ levels between apolipoprotein ε4 allele status. (A) Complete PiB-PET imaging subgroup, (B) HC from the PiB-PET imaging subgroup only, (C) MCI from the PiB-PET imaging subgroup only, and (D) AD from the PiB-PET imaging subgroup only. Abbreviations: APoJ, apolipoprotein J; APOE ε4, apolipoprotein ε4; SUVR, standardized uptake value ratio; AD, Alzheimer's disease; MCI, mild cognitive impairment; HC, healthy control; PiB-PET, Pittsburgh compound B-positron emission tomography.
3.5. Association between apoJ and plasma Aβ
Investigating the relationship between plasma Aβ and apoJ identified weakly negative, but significant, correlation between the ratio of Aβ1–42/Aβ1–40 at both baseline and 18-month time points (baseline R = −0.08, P = .004; 18 months, R = −0.08, P = .004). ApoJ was also weakly correlated with Aβn-40 at the 18-month time point (R = −0.10, P = .0001). Subgroup correlations identified a significant but weak negative correlation between Aβ1–40 and apoJ for MCI participants at baseline (R = −0.232, P = .008), but this was not observed at the 18-month time point.
3.6. Disease predictions using clinical classification
Using the GLM and ROC analyses to predict AD using apoJ, age, gender, and APOE ε4 allele status, we identified an 8% increase in cross-validated accuracy over age, gender, and APOE ε4 allele status alone at baseline and a 6% increase in cross-validated accuracy at the 18-month time point (sensitivity and specificity at baseline, 84% and at the 18-month time point, 80%). Similar to the AD predictions, using apoJ to predict MCI at baseline was approximately 7% better than using age, gender, and APOE ε4 allele status alone (sensitivity and specificity, 75%). However, the same prediction using the 18-month time point identified a 13% increase in accuracy over age, gender, and APOE ε4 allele status alone (sensitivity and specificity, 80%). Predictions for AD did not include data from the MCI group, and predictions for the MCI group did not include data from the AD group.
4. Discussion
The AIBL study of aging is world leading in terms of the thorough clinical classification of participants, rigorous blood sample preparation, and storage. The longitudinal nature and presence of associated clinical data makes AIBL a very unique cohort to track the biochemical changes of a protein blood biomarker over a period of time. This cohort is particularly strengthened by the inclusion of state-of-the-art brain amyloid imaging through 11C-PiB-PET and presence of brain MRI data. Using such a well-characterized and large study, we were able to investigate (1) the associations of plasma apoJ levels with the presence and severity of AD and (B) if plasma apoJ might be a suitable candidate as an early diagnostic marker for AD.
The present study shows that apoJ levels are significantly higher in MCI and AD groups compared with those in the HC group at both baseline and 18-month follow-up. These findings suggest early involvement of apoJ in the disease process.
Our results also suggest a relationship between apoJ levels and brain Aβ as determined by SUVR. Plasma apoJ levels showed a positive correlation with SUVR derived from PiB-PET suggesting higher apoJ levels with increasing severity of the disease as indicated by increased deposition of Aβ in the brain. The carriage of APOE ε4 allele had a significant impact on the association between apoJ and brain Aβ levels. In APOE ε4 allele carriers, participants with SUVR above the cutoff of 1.8 had higher plasma apoJ levels compared with the ones who had SUVR below the 1.8 cutoff. However, this relationship was reversed in APOE ε4 allele noncarriers, as participants with SUVR above the cutoff of 1.8 had lower plasma apoJ levels compared with the ones who had SUVR below the 1.8 cutoff. This relationship between plasma apoJ levels and SUVR, however, was only seen in HC and MCI clinical categories. We have previously shown that levels of another potential biomarker namely apolipoprotein E (apoE) go down in plasma, APOE ε4 carriers, and during AD pathogenesis [18]. Our observation of positive correlation of apoJ levels with SUVR scores specifically in APOE ε4 carriers could be as a result of a compensatory mechanism exerted by the chaperonic activity of apoJ in plasma. Differences such as these provide us with an indication that plasma apoJ levels may be used in conjunction with APOE ε4 allele carriage information to identify candidates in need of PET imaging for assessment of neocortical amyloid burden. This combined information may also be used to distinguish those HC and MCI participants who are at an increased risk of progressing further into the disease. Hence, plasma apoJ levels may be used as the first step in a multistep neurodiagnostic process with all screen positives referred for neuroimaging for confirmatory diagnostic processes. This is exactly how cardiology, oncology, and infection disease screens and diagnostic and treatment approaches work in the existing medical infrastructure.
Our finding of a positive correlation of apoJ in HC and MCI with brain Aβ load demonstrates that apoJ is raised very early in AD pathogenesis and is not just an end-stage response to this etiopathologic event. Previous studies have shown that apoJ is a chaperonic protein and helps in the regulation of Aβ by a clearance mechanism where binding of Aβ to apoJ leads to its efflux from the brain [1], [19]. Our finding of inverse relationship between plasma Aβ and apoJ possibly demonstrates the chaperonic effect of apoJ in the periphery. Hence, it could be speculated that apoJ has a protective action and not only increases as a result of drastic events leading to AD.
A similar observation between plasma apoJ levels and disease severity was made with hippocampus volume derived from brain MRI where plasma apoJ levels correlated negatively with the hippocampal volume, thus indicating higher apoJ levels with increased hippocampal atrophy. ApoJ has earlier been shown to be associated with atrophy of the hippocampus, clinical progression, and disease severity [10]. Hippocampal atrophy is known to happen in the early stages of the disease, during the conversion from MCI to AD, and even considered as a marker in the late stages of AD [20], [21].
These findings were consistent with some of the earlier studies [10], [11], [22]. Thambisetty et al. [9], 2012, showed a correlation of plasma apoJ concentration with the longitudinal atrophy changes in several regions of the brain in the MCI group. Another study also showed apoJ levels increased in post mortem brain tissue in specific regions such as hippocampus and frontal cortex [23]. Thambisetty et al. [10], 2010, showed increased apoJ messenger RNA in the blood of AD patients, but there was no change in gene or protein expression of apoJ due to variation in the gene single nucleotide polymorphisms (SNPs). Mullan et al. [22] showed that plasma apoJ levels were not only higher in MCI and AD compared with controls but MCI stage subjects had even higher levels compared with AD indicating that increase in plasma apoJ levels may occur as a response to the disease process. Differences in assaying techniques used (mass spectrometry based vs. ELISA) and varying blood collection protocols (fasting vs. nonfasting) may have contributed to the contradictory results obtained in some of the earlier studies [12], [13].
Although the present study has found strong differences in plasma apoJ between HC and MCI/AD groups, the smaller imaging subgroup correlation analyses did not demonstrate the same strength and magnitude. Although some correlations were statistically significant, the magnitude was quite low, indicating potential relationships hidden behind considerable variance and small sample size. Another limitation of this study is the use of differential threshold levels for SUVR. The relationship identified with the APOE ε4 carrier status was only present in the higher threshold, as compared with the lower and more commonly used thresholds. This may suggest that the interaction with the APOE genotype only occurs at a late stage during brain Aβ deposition.
Overall, our findings reinforce the role and implications of amyloid chaperonic proteins in AD pathogenesis suggesting that further examination of this biochemical pathway may be useful to identify peripheral markers of AD.
Research in context.
-
1.
Systematic review: Apolipoprotein J (ApoJ; clusterin) has attracted a great deal of attention in recent times in regard to early diagnosis and monitoring of Alzheimer's disease (AD); however, its value as a blood biomarker for AD has not been established yet.
-
2.
Interpretation: We have used plasma samples from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging cohort to specifically answer the question if plasma apoJ can be used for diagnostic accuracy and whether it has any association with brain amyloid beta (Aβ) accumulation. Our results show that apoJ levels were higher in mild cognitive impairment (MCI) and AD compared with controls and it also correlated positively with neocortical Aβ.
-
3.
Future directions: We still need to answer how plasma apoJ levels change specifically in the “MCI progressors” by analyzing longitudinal samples from further time points of collection. This work is currently underway in our laboratory as AIBL is a longitudinal study.
Acknowledgments
The authors thank all the participants who took part in this study and the clinicians who referred participants. The AIBL study (www.AIBL.csiro.au) is a collaboration between CSIRO, Edith Cowan University (ECU), the Florey Institute of Neuroscience and Mental Health (FINMH), National Ageing Research Institute (NARI), and Austin Health. It also involves support from CogState Ltd., Hollywood Private Hospital, and Sir Charles Gairdner Hospital. The study received funding support from CSIRO, the Science and Industry Endowment Fund (www.SIEF.org.au), NHMRC via project grant APP1009292 and Dementia Collaborative Research Centres (DCRC), Alzheimer's Australia (AA), Alzheimer's Association, and the McCusker Alzheimer's Research Foundation, as well as industry sources. The authors acknowledge the financial support of the CRC for Mental Health; the Cooperative Research Centre (CRC) program is an Australian Government Initiative. Pfizer International has contributed financial support to assist with analysis of blood samples and to further the AIBL research program. “We would like to dedicate this paper to the memory of our friend and fellow co-author ‘Alan Rembach’ who recently passed away unexpectedly.”
References
- 1.Yerbury J.J., Poon S., Meehan S., Thompson B., Kumita J.R., Dobson C.M. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- 2.Yerbury J.J., Stewart E.M., Wyatt A.R., Wilson M.R. Quality control of protein folding in extracellular space. EMBO Rep. 2005;6:1131–1136. doi: 10.1038/sj.embor.7400586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May P.C., Lampert-Etchells M., Johnson S.A., Poirier J., Masters J.N., Finch C.E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990;5:831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- 4.Killick R., Ribe E.M., Al-Shawi R., Malik B., Hooper C., Fernandes C. Clusterin regulates beta-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry. 2014;19:88–98. doi: 10.1038/mp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oda T., Wals P., Osterburg H.H., Johnson S.A., Pasinetti G.M., Morgan T.E. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 6.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 7.Schurmann B., Wiese B., Bickel H., Weyerer S., Riedel-Heller S.G., Pentzek M. Association of the Alzheimer's disease clusterin risk allele with plasma clusterin concentration. J Alzheimers Dis. 2011;25:421–424. doi: 10.3233/JAD-2011-110251. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S., Fitzpatrick A.L., Ikram M.A., DeStefano A.L., Gudnason V., Boada M., CHARGE Consortium. GERAD1 Consortium. EADI1 Consortium Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thambisetty M., An Y., Kinsey A., Koka D., Saleem M., Guntert A. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. Neuroimage. 2012;59:212–217. doi: 10.1016/j.neuroimage.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thambisetty M., Simmons A., Velayudhan L., Hye A., Campbell J., Zhang Y. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrijvers E.M., Koudstaal P.J., Hofman A., Breteler M.M. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011;305:1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 12.IJsselstijn L., Dekker L.J., Koudstaal P.J., Hofman A., Sillevis Smitt P.A., Breteler M.M. Serum clusterin levels are not increased in presymptomatic Alzheimer's disease. J Proteome Res. 2011;10:2006–2010. doi: 10.1021/pr101221h. [DOI] [PubMed] [Google Scholar]
- 13.Silajdzic E., Minthon L., Bjorkqvist M., Hansson O. No diagnostic value of plasma clusterin in Alzheimer's disease. PLoS One. 2012;7:e50237. doi: 10.1371/journal.pone.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis K.A., Bush A.I., Darby D., De Fazio D., Foster J., Hudson P. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 15.Tierney M.C., Fisher R.H., Lewis A.J., Zorzitto M.L., Snow W.G., Reid D.W. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer's disease: A clinicopathologic study of 57 cases. Neurology. 1988;38:359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- 16.Hixson J.E., Vernier D.T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 17.Pike K.E., Savage G., Villemagne V.L., Ng S., Moss S.A., Maruff P. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 18.Gupta V.B., Laws S.M., Villemagne V.L., Ames D., Bush A.I., Ellis K.A. Plasma apolipoprotein E and Alzheimer disease risk: The AIBL study of aging. Neurology. 2011;76:1091–1098. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M.R., Yerbury J.J., Poon S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst. 2008;4:42–52. doi: 10.1039/b712728f. [DOI] [PubMed] [Google Scholar]
- 20.Nestor P.J., Scheltens P., Hodges J.R. Advances in the early detection of Alzheimer's disease. Nat Med. 2004;10(Suppl):S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- 21.Hampel H., Burger K., Pruessner J.C., Zinkowski R., DeBernardis J., Kerkman D. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62:770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- 22.Mullan G.M., McEneny J., Fuchs M., McMaster C., Todd S., McGuinness B. Plasma clusterin levels and the rs11136000 genotype in individuals with mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res. 2013;10:973–978. doi: 10.2174/15672050113106660162. [DOI] [PubMed] [Google Scholar]
- 23.Lidstrom A.M., Bogdanovic N., Hesse C., Volkman I., Davidsson P., Blennow K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer's disease. Exp Neurol. 1998;154:511–521. doi: 10.1006/exnr.1998.6892. [DOI] [PubMed] [Google Scholar]