Abstract
Introduction
We investigate whether longitudinal callosal atrophy could predict conversion from mild cognitive impairment (MCI) to Alzheimer's disease (AD).
Methods
Longitudinal (baseline + 1-year follow-up) MRI scans of 132 MCI subjects from the Alzheimer's Disease Neuroimaging Initiative were used. A total of 54 subjects did not convert to AD over an average (±SD) follow-up of 5.46 (±1.63) years, whereas 78 converted to AD with an average conversion time of 2.56 (±1.65) years. Annual change in the corpus callosum thickness profile was calculated from the baseline and 1-year follow-up MRI. A logistic regression model with fused lasso regularization for prediction was applied to the annual changes.
Results
We found a sex difference. The accuracy of prediction was 84% in females and 61% in males. The discriminating regions of corpus callosum differed between sexes. In females, the genu, rostrum, and posterior body had predictive power, whereas the genu and splenium were relevant in males.
Discussion
Annual callosal atrophy predicts MCI-to-AD conversion in females more accurately than in males.
Keywords: Alzheimer's disease, Mild cognitive impairment, Brain, Corpus callosum, Magnetic resonance imaging, Shape analysis, Longitudinal analysis
1. Introduction
Mild cognitive impairment (MCI) is generally considered as the prodromal phase of Alzheimer's disease (AD). It is of great importance to identify individuals with MCI who are likely to progress to AD in the near future, for it would allow for early intervention.
Significant efforts have been made to find biomarkers that predict conversion from MCI to AD. The potential biomarkers include genetic, CSF proteins, cognitive measurements, glucose metabolism (FDG-PET), and structural/functional brain abnormalities (magnetic resonance imaging [MRI], fMRI). In a comprehensive review of a number of studies, Landau et al. [1] compared these biomarkers in predicting conversion using data from the Alzheimer's Disease Neuroimaging Initiative (ADNI). They found 73%–88% specificity but a disappointing sensitivity of around 40% for all these biomarkers in classification. Another notable study of predicting conversion is that of Killiany et al. [2]. They used structural MRI to predict conversion and found the entorhinal cortex, the banks of the superior temporal sulcus, and the anterior cingulate most useful with 75% accuracy, but low specificity 48%. Davatzikos et al. [3] tried the combined information from MRI and CSF biomarkers in prediction and reported top accuracy of 61.7% in their analysis. Thus, more studies are required to find a way to improve the accuracy in predicting MCI-to-AD conversion.
Up to now, almost all efforts have been made based on baseline measures. Given the neurodegenerative nature of AD progression, we conjecture that observing temporal changes in brain structures may improve the accuracy in predicting conversion and provide a measure for the progression to AD. In addition, observing the temporal change within each subject could naturally diminish potential confounding factors in prediction because the progression in AD may depend on factors such as sex, age, education, and diet. The heterogeneity in cohorts may be a reason of poor accuracy in existing classification studies. The insidious manner of progression in AD requires reliable and accurate measures to detect subtle structural changes in the brain over a fairly short time period, say, at most 1 year. The hippocampus and medial temporal lobes are main targets as neuroimaging markers for AD, but measurements to detect subtle changes reliably and accurately are difficult. Therefore, we focus on the corpus callosum (CC) as the mid-sagittal plane cross-sectional area of the CC is well visualized in structural MRI scans and can be reliably measured with good accuracy [4].
The CC is the largest white matter tract interconnecting the cerebral hemispheres. Both cross-sectional and longitudinal studies have reported atrophy of the CC in MCI and AD [4], [5], [6], [7], [8]. CC atrophy has been proposed as a consequence of two possible mechanisms: direct myelin breakdown [9], [10], and Wallerian degeneration wherein callosal fibers are lost as a result of distal injury to the callosal projecting neurons [11]. However, the use of longitudinal CC atrophy to predict future MCI conversion to AD has not been studied extensively.
Therefore, this study aims to investigate whether patterns of longitudinal CC atrophy predict conversion from MCI to AD. To the best of our knowledge, this is the first attempt to predict future MCI conversion to AD using longitudinal structural callosal change from MRI scans. For this purpose, we used longitudinal scans from 132 MCI subjects in ADNI. Annual change in CC thickness profile was calculated for each subject from two MRI scans that were 1 year apart. A logistic regression model with fused lasso regularization [4] was trained on the callosal thickness profiles and used for predicting conversion.
2. Methods
2.1. Subjects
Data used in the preparation of this article were obtained from the ADNI database (http://adni.loni.usc.edu). The primary goal of the ADNI has been to test whether serial MRI, positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of the ADNI is Michael W. Weiner, MD, VA Medical Center and University of California-San Francisco. The first phase, ADNI1, was intended to enroll 800 subjects, including normal controls, MCI, and AD subjects. For up-to-date information, see www.adni-info.org.
In our longitudinal study of CC atrophy we used the ADNI1 “3-year complete standardized data set” [12], which includes 1.5-T longitudinal structural MRI scans from 148 individuals initially diagnosed as MCI. Summaries of demographic and diagnostic data were downloaded in October, 2013. We divided the MCI subjects into two groups, those whose diagnoses indicated a conversion to AD at any time within 3 years after their initial evaluation (mild cognitive impairment-converted [MCI-C]) and those who did not convert (mild cognitive impairment-nonconverted [MCI-NC]) during the follow-up period. Note that the later group includes subjects who may convert at a later unknown time or not convert at all. Therefore, our classification can be considered to be between incipient AD patients vs. “others”. ADNI1 followed nonconverters for up to 7.5 years (mean = 5.5, SD = 1.6). In ADNI1, the subjects were classified as MCI when their Mini-Mental State Examination (MMSE) score was between 24 and 30 (inclusive) and had a memory complaint, objective memory loss measured by education adjusted scores on Wechsler Memory Scale Logical Memory II, a clinical dementia rating (CDR) of 0.5, absence of significant levels of impairment in other cognitive domains, especially preserved activities of daily living, and an absence of dementia. The subjects were considered as mild AD if their MMSE score was in the 20–26 range (inclusive), had a CDR of 0.5 or 1.0, and met the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria for probable AD. Cognitive assessment and imaging were conducted at baseline, 6 months, and 12 months, and yearly thereafter [13]. Preliminary examination showed a difference in the sex ratio between the MCI-C and MCI-NC groups. A description of the demographic data is given in Table 1.
Table 1.
Demographic data (mean ± SD)
| Female |
Male |
|||||
|---|---|---|---|---|---|---|
| MCI-NC | MCI-C | P value | MCI-NC | MCI-C | P value | |
| N | 12 | 25 | 44 | 51 | ||
| Age (y) | 72.54 ± 8.44 | 71.75 ± 8.67 | NS | 74.63 ± 7.13 | 75.22 ± 5.64 | NS |
| Education (y) | 14.8 ± 2.39 | 15.52 ± 2.97 | NS | 15.93 ± 3.17 | 16.14 ± 3.00 | NS |
| MMSE | 27.5 ± 1.51 | 26.41 ± 1.95 | NS | 27.66 ± 1.77 | 26.8 ± 1.63 | .016 |
| Interval (y) | 1.00 ± 0.02 | 1.00 ± 0.03 | NS | 1.00 ± 0.07 | 1.00 ± 0.47 | NS |
Abbreviations: NS, not significant; Interval, time elapsed between baseline and follow-up scans.
2.2. MRI imaging
Subject scans were 1.5 T, T1-weighted magnetization prepared rapid gradient echo images, using matrix sizes of 192 × 192 × 160–170 or 256 × 256 × 166–184. The in-plane voxel dimensions were 0.94 to 1.25 mm, whereas the slice thickness was kept very close to 1.2 mm. Repetition time values were 2300–2400 ms for multicoil-phased array head coils and 3000 ms for birdcage or volume head coils. Inversion time was 1000 ms, and flip angle was 8°. Phase encodes were in the anterior-to-posterior direction. Detailed information about MRI acquisition and pre-processing procedures is available in Jack et al. [14].
2.3. CC segmentation
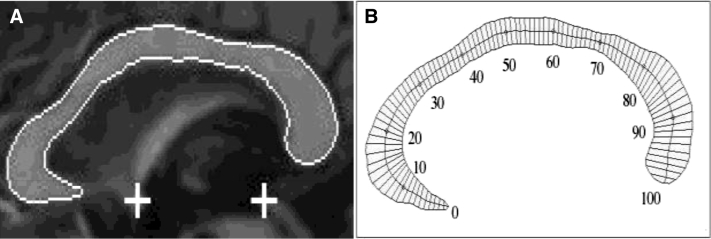
The CC was segmented using our Automatic Registration Toolbox (ART) software module Yuki, available online (http://www.nitrc.org/projects/art). Given a 3D T1-weighted structural MRI volume, Yuki segments the mid-sagittal cross-sectional area of the CC by performing the following steps. (1) A mid-sagittal plane (MSP) is determined so as to maximize bilateral symmetry [15]. (2) The coordinates of the anterior commissures (AC) and posterior commissures (PC) are automatically located on the MSP [16]. (3) From the original MRI volume, a single standardized MSP image of matrix size 512 × 512 and pixel size 0.5 × 0.5 mm2 is reconstructed by tri-linear interpolation. The location and orientation of this image is standardized so that the image left-to-right axis corresponds to subject's anterior-to-posterior axis parallel to the AC-PC line, the image top-to-bottom axis is the subject's superior-to-inferior axis, and field-of-view center coincides with the mid-point between AC and PC (Fig. 1A). (4) From a set of 628 atlases currently available and distributed with the software, a subset of 49 are selected automatically based on correlation between the atlas and the test image inside a rectangular sub-image containing the CC on the standardized MSP reconstructed in step 3. (5) The 49 selected atlases are nonlinearly registered to the test volume's MSP using ART's nonlinear registration approach [17]. The CC labels of the 49 atlases are projected onto the test MSP using the resulting nonlinear transformations. The projected labels are averaged to obtain a fuzzy segmentation of the CC on the MSP of the test volume. (6) Finally, the fuzzy label map obtained in step 5 is thresholded using an automatically determined threshold level to yield the final binary CC segmentation. The threshold level is selected such that the Fisher's linear discriminant ratio (FLDR) [18] between the intra-callosal and extra-callosal pixels within the support of the fuzzy label set in step 5 is maximized. The entire segmentation process takes about 7 seconds running in parallel on 7 cores of a 2.4 GHz Dual Quad-Core Linux workstation.
Fig. 1.
(A) The outline shows the detected corpus callosum. The automatically detected anterior commissure (AC) and posterior commissure (PC) are shown by the plus signs. (B) 99 thickness superimposed on a segmented corpus callosum cross-sectional area.
As baseline-to-follow-up changes in CC shape and size are small during the roughly 1-year interval from baseline to follow-up, it is important to have a consistent CC segmentation technique. To this end, it is very important to perform the above segmentation steps on the same MSP on both baseline and follow-up images. In addition, it is important to use the same selected 49 atlases and the same threshold level determined from FLDR analysis. To ensure this, we implemented the following protocol. (a) The baseline and follow-up volumes of each subject were registered using ART's rigid-body registration software Atra, which is an inverse-consistent symmetric rigid-body registration method, that is, the exact same linear transformation matrix is obtained regardless of whether the baseline or the follow-up volume is taken as the reference in the registration process and both images undergo interpolation to be registered at the exact half-way orientation between the original scans. (b) Using the registration matrix obtained in step (a), the baseline and follow-up images were averaged to obtain a single image per subject. (c) The CC segmentation software Yuki was applied to the average image in step (b) saving as auxiliary information the linear transformation that defines the standardized MSP, the list of 49 automatically selected atlases, and the value of the FLDR derived threshold. (d) Finally, the CC was segmented using Yuki on the baseline and follow-up images independently, whereas disabling automatic MSP detection, atlases selection, and FLDR analysis. Instead, Yuki was instructed to use the auxiliary information saved in step (c) for both baseline and follow-up segmentations. This way, both the baseline and follow-up scans were treated in exactly the same way in the CC segmentation process. We have shown using analysis of structural MRI volumes taken in two separate scanning sessions from the same set of subjects on the same day that this approach has significantly higher test-retest reliability as compared to the case where the two images are segmented independently (results not presented).
The results of CC segmentation were visually inspected in all 148 cases. Ten subjects were excluded because CC segmentations were deemed inaccurate on baseline and/or follow-up scans. Thus, there remained 138 subjects who had been diagnosed as MCI at the time of their initial ADNI scan with fully automatic segmentations of the CC at baseline and follow-up scans.
2.4. CC thickness profile
The CC thickness profile is specified in terms of 99 nonzero thickness values at equally spaced intervals along the length of the CC (Fig. 1B). Our method for finding these values is similar to those of Clarke et al. [19] and Denenberg et al. [20]. Thickness values are lengths of line segments that connect pairs of points on the upper and lower boundaries of the CC. The line segments are chosen perpendicular to the medial axis of the CC and intersect the medial axis at equal intervals.
An additional six subjects, from the 138 with successful automatic CC segmentations, were excluded from further analysis because their CC thickness profiles were not produced correctly by our automated algorithm at baseline and/or follow-up, leaving 132 subjects to be analyzed. These excluded cases in CC segmentation and profiling can be remedied by minor manual interventions. However, we decided to exclude them from the statistical analysis to keep the processing completely automatic and consistent. More importantly, we can avoid possible bias/noise introduced from manual interventions by doing so.
2.5. Logistic regression model with fused lasso regularization
The annual rate of corpus callosum thickness change was computed by subtracting the 99 nonzero CC thickness values of the baseline scan from those of the follow-up scan and dividing the results by the inter-scan interval in years. These 99 annual atrophy rates were used as predictors for MCI-to-AD conversion. We applied a logistic regression model to the annual atrophy rates of the CC thickness profiles. As CC thickness points are spatially ordered (from rostrum to splenium), and we aim to detect locally homogeneous and spatially contiguous regions rather than individual thickness points, we applied a fused lasso penalty on the coefficients of the model and their successive differences. As a result, only the coefficients of CC thickness points which significantly contribute to prediction will be nonzero and locally constant.
Suppose, we have n subjects , where , if the ith subject is in the MCI-C group, and 0 otherwise, and is the t-dimensional set of annualized CC thickness profile atrophy rates. The logistic regression model without fused lasso regularization can be written as:
where . This leads us to the log-likelihood function of :
After adding the fussed lasso regularization term , the problem becomes one minimizing
| (1) |
where
Detailed steps for minimizing (1) are given in Lee et al. [4]. Briefly, we approximate the objective function (1) by a 2nd order Taylor series expansion, take the derivatives with respect to βi's and set them to zero, and apply a modified coordinate descent algorithm iteratively until converge to a solution [4]. We used q-fold cross-validation to find the tuning parameters associated with the fused lasso penalty term by a grid-search for the minimum prediction error.
3. Results
A total of 132 MCI subjects (95 male and 37 female) had fully automatic CC thickness profile measurements at both baseline and follow-up. Of these subjects, 76 (51 male and 25 female) converted to AD with an average baseline-to-conversion time of 2.56 (SD = 1.65) years, whereas the diagnosis of 56 subjects remained MCI over the average observation period of 5.46 (SD = 1.63) years. Statistical analysis of the demographic data given in Table 1 showed that the MCI-NC and MCI-C groups were not significantly different in age or education in females or males. The male MCI-NC and MCI-C groups differed significantly in their mean MMSE scores (P = .016) but not in the female group. The mean inter-scan interval of roughly 1 year was not statistically different between the MCI-NC and MCI-C groups. There was no significant cognitive difference between males and females at baseline in terms of the Rey Auditory Verbal Learning Test (RAVLT) and Alzheimer's Disease Assessment Scale (ADAS) (P values = .90, .46, respectively), which indicates that the severity of the disease at baseline was not different between sexes.
From the total of 37 female MCI subjects, 25 (68%) converted to AD, whereas 51 of the 95 male subjects (54%) converted. The MCI-C to MCI-NC ratio was significantly different between male and female subjects (P = .025). Therefore, we applied the logistic model with fused lasso regularization to female and male subjects separately. We estimated the accuracy of MCI-to-AD prediction ability of the model by 6-fold cross-validation.
3.1. MCI-to-AD prediction in females
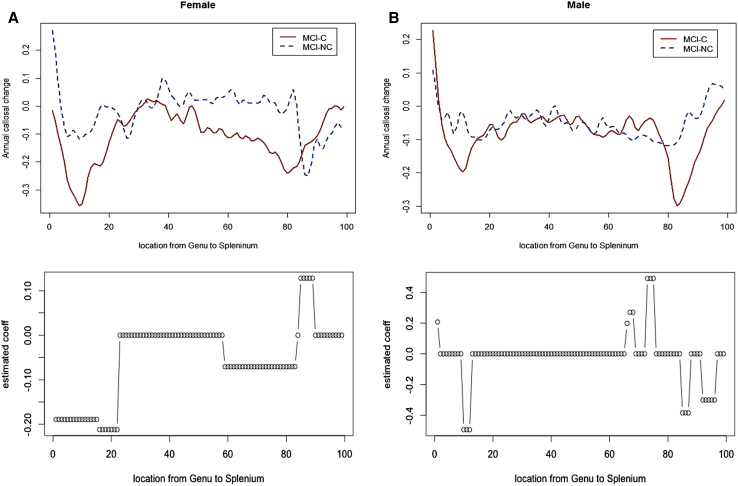
The accuracy of discrimination between the MCI-NC and MCI-C groups was 84% (sensitivity = 92%; specificity = 67%) females. The upper panel in Fig. 2A shows the mean annual callosal change at each location for female MCI-NC (blue) and female MCI-C (red) subjects. The lower panel in Fig. 2A shows the estimated coefficients of the logistic regression model. The atrophy in genu and/or rostrum and posterior body is much faster in female MCI-C subjects, whereas the atrophy in splenium seems a little slower in MCI-C as compared with MCI-NC.
Fig. 2.
The upper panel shows the mean curves of annual CC atrophy profiles for MCI-NC (blue-dotted line) and MCI-C (red line); and the lower panel plots the estimated coefficients of corpus callosum by FLLR (negative: faster in MCI-C; positive: slower in MCI-C) in females (A) and males (B).
3.2. MCI-to-AD prediction in males
The accuracy of the discrimination between the MCI-NC and MCI-C groups was 60% (sensitivity = 59%; specificity = 61%) in males. The upper panel in Fig. 2B shows the mean annual callosal change at each location for male MCI-NC (blue) and male MCI-C (red) subjects. The lower panel in Fig. 2B shows the estimated coefficients of the logistic regression model. The atrophy in genu and splenium is faster in male MCI-C relative to MCI-NC.
4. Discussion
Longitudinal studies on callosal change in MCI subjects are very rare. To our knowledge, only one study has been published. Elahi et al. [8] reported that changes in CC morphology were large enough to be detectable over a 1-year period on structural MRI scans. The CC became less circular with time and with faster decline in MCI-C. Callosal atrophy rate was higher in female MCI-C relative to female MCI-NC, whereas in contrast, the rate did not differ between groups in males. These findings suggested that annual callosal change could be a potential biomarker to predict future MCI-to-AD conversion particularly in females. Therefore, this article investigated whether the pattern of annual callosal change is able to predict future conversion from MCI to AD.
To do so, we applied a logistic model with fused lasso regularization to the annual change in the CC thickness profile for female and male groups separately. We also considered including cognitive measures such as MMSE, ADAS, or RAVLT at baseline in our logistic regression prediction model, which turned out not to improve the prediction power. We found that the annual callosal change has better predictive power of future MCI-to-AD conversion in females (accuracy = 84%) over males (accuracy = 60%). To ensure that the results are not affected by the unbalanced samples sizes over sex, we computed the 95% confidence intervals for sensitivity and specificity assuming a binomial distribution of the success probability of estimated sensitivity and specificity (Table 2). The sex difference in sensitivity is statistically significant.
Table 2.
Prediction results
| Female |
Male |
|||
|---|---|---|---|---|
| MCI-NC | MCI-C | MCI-NC | MCI-C | |
| MCI-NC | 8 | 4 | 27 | 17 |
| MCI-C | 2 | 23 | 21 | 30 |
| Female | Male | |
|---|---|---|
| Sensitivity | 92.00 (72.50–98.60) | 58.82 (44.21–72.11) |
| Specificity | 66.67 (35.43–88.73) | 61.36 (45.51–75.25) |
| Accuracy | 83.78 | 60.00 |
NOTE: MCI-NC and MCI-C indicate the predicted labels. The 95% confidence intervals are shown inside parenthesis.
The 84% accuracy in females is higher than that reported in the existing studies for prediction of MCI-to-AD conversion (top previously reported value is around 75%) albeit their accuracy are for male and female combined. Encouragingly, the sensitivity (92%) in females is much higher than reported in the existing studies. For a fair comparison, we estimated the accuracy using hippocampal volumes (provided in ADNI) along with age, APOE phenotype, and the intracranial volume by 6-fold cross-validation based on the logistic regression model. The accuracy was 70% for females and 63% for males.
Interestingly, we discovered that the locations along the CC length where atrophy has predictive power are different between female and male groups. In males, the discriminating parts of CC were narrow areas on the genu and splenium. In contrast, the discriminating parts of CC in females were broader areas of the genu and/or rostrum, posterior body, and splenium. In general, these findings agree with a previous study [8] that measured differences in broad areas where CC was segmented into five regions. This article looks at differences on a much finer scale in discrimination analysis. Sexual dimorphism is not new in healthy controls. A series of studies have reported that the female CC tends to be bigger than males on average after accounting for head size [21]. However, to our knowledge, this is the first study to report sex differences in progression to AD in predicting future conversion based on annual CC atrophy.
In conclusion, we have shown that the spatial and temporal (in this study, a year apart) patterns of CC morphologic change have predictive power for future conversion to AD, particularly in female MCI patients. We also discovered that the annual atrophy is faster in broad areas of the genu and/or rostrum, posterior body, and the splenium of the CC in female MCI converters than nonconverters, whereas the atrophy is faster in limited areas of the genu and splenium in male converters relative to nonconverters. Thus, our study provides two insights in neuroimaging-based prediction of MCI-to-AD conversion: (1) sex differences in brain atrophy should be taken into account; and (2) measuring temporal changes can improve the prediction power. This study also suggests that annual MRI brain scans can greatly improve the accuracy of measuring progression of AD.
As we targeted incipient conversion to AD in MCI subjects rather than “ultimate” conversion, it is possible that nonconverters would convert to AD at a later point than the current follow-up period (up to 7.5 years in this study). Therefore, being in the MCI-NC group does not necessarily mean absence of disease. Rather, they may differ from converters on the stage of progression of AD. A survival analysis on a longer follow-up data set would be required to resolve the issue. Despite this limitation, this study suggests that our approach of observing the rate of brain atrophy can be more objective for diagnosis and assessment of AD which can only be diagnosed conclusively postmortem.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources (PubMed, conference abstracts). Albeit various efforts have been made to predict progression from MCI to AD, it needs to be improved. Longitudinal change in brain has never been tried in prediction study.
-
2.
Interpretation: The spatial and temporal patterns of CC morphologic change have predictive power for conversion to AD in female MCI patients. The annual atrophy is faster in broad areas of the genu/rostrum, posterior body, and part of the splenium of the CC in female converters than nonconverters, whereas the atrophy is faster in limited areas of the genu and splenium in male converters than nonconverters.
-
3.
Future directions: The study suggests that sex differences should be taken into account in prediction study and temporal brain changes can improve the prediction power. Further studies such as a shorter period than a year and other areas (e.g., hippocampus) are required to elucidate the findings.
Acknowledgments
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- 1.Landau S.M., Harvey D., Madison C.M., Reiman E.M., Foster N.L., Aisen P.S., ADNI Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killiany R.J., Gomez-Isla T., Moss M., Kikinis R., Sandor T., Jolesz F. Use of structural magnetic resonance imaging to predict who will get Alzheimer's Disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 3.Davatzikos C., Bhatt P., Shaw L.M., Batmanghelich K.N., Trojanowski J.Q. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, pattern classification. Neurobiol Aging. 2011;32:2319–2322. doi: 10.1016/j.neurobiolaging.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S.H., Yu D., Bachman A.H., Lim J., Ardekani B.A. Application of fused lasso logistic regression to the study of corpus callosum thickness in early Alzheimer's disease. J Neurosci Methods. 2014;221:78–84. doi: 10.1016/j.jneumeth.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Paola M., Luders E., Di Iulio F., Cherubini A., Passafiume D., Thompson P.M. Callosal atrophy in mild cognitive impairment and Alzheimer's disease: Different effects in different stages. Neuroimage. 2010;49:141–149. doi: 10.1016/j.neuroimage.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardekani B.A., Bachman A.H., Figarsky K., Sidtis J.J. Corpus callosum shape changes in early Alzheimer's disease: An MRI study using the OASIS brain database. Brain Struct Funct. 2014;219:343–352. doi: 10.1007/s00429-013-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachman A.H., Lee S.H., Sidtis J.J., Ardekani B.A. Corpus callosum shape and size changes in early Alzheimer's disease: A longitudinal MRI study using the OASIS brain database. J Alzheimers Dis. 2014;39:71–78. doi: 10.3233/JAD-131526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elahi S., Bachman A.H., Lee S.H., Sidtis J.J., Ardekani B.A., ADNI Corpus callosum atrophy rate in mild cognitive impairment and prodromal Alzheimer's disease. J Alzheimers Dis. 2015;45:921–931. doi: 10.3233/JAD-142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Paola M., Caltagirone C., Spalletta G. What does the corpus callosum tell us about brain changes in the elderly? Expert Rev Neurother. 2011;11:1557–1560. doi: 10.1586/ern.11.130. [DOI] [PubMed] [Google Scholar]
- 10.Ryberg C., Rostrup E., Paulson O.B., Barkhof F., Scheltens P., van Straaten E.C., LADIS study group Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: A 3-year follow-up of the LADIS study cohort. J Neurol Sci. 2011;307:100–105. doi: 10.1016/j.jns.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Di Paola M., Di Iulio F., Cherubini A., Blundo C., Casini A.R., Sancesario G. When, where, and how the corpus callosum changes in MCI and AD: A multimodal MRI study. Neurology. 2010;74:1136–1142. doi: 10.1212/WNL.0b013e3181d7d8cb. [DOI] [PubMed] [Google Scholar]
- 12.Wyman B.T., Harvey D.J., Crawford K., Bernstein M.A., Carmichael O., Cole P.E., Alzheimer's Disease Neuroimaging Initiative Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement. 2013;9:332–337. doi: 10.1016/j.jalz.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen R.C., Asien P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack C.R., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardekani B.A., Kershaw J., Braun M., Kanno I. Automatic detection of the mid-sagittal plane in 3-D brain images. IEEE Trans Med Imaging. 1997;16:947–952. doi: 10.1109/42.650892. [DOI] [PubMed] [Google Scholar]
- 16.Ardekani B.A., Bachman A.H. Model-based automatic detection of the anterior and posterior commissures on MRI scans. Neuroimage. 2009;46:677–682. doi: 10.1016/j.neuroimage.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardekani B.A., Guckemus S., Bachman A., Hoptman M.J., Wojtaszek M., Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Fisher R.A. The use of multiple measurements in taxonomic problems. Ann Eugen. 1936;7:179–188. [Google Scholar]
- 19.Clarke M., Kraftsik R., van der Loos H., Innocenti G.M. Forms and measures of adult and developing human corpus callosum: Is there sexual dimorphism? J Comp Neurol. 1989;280:213–230. doi: 10.1002/cne.902800205. [DOI] [PubMed] [Google Scholar]
- 20.Denenberg V.H., Cowell P.E., Fitch R.H., Kertesz A., Kenner G.H. Corpus callosum: Multiple parameter measurements in rodents and humans. Physiol Behav. 1991;49:433–437. doi: 10.1016/0031-9384(91)90261-l. [DOI] [PubMed] [Google Scholar]
- 21.Ardekani B.A., Figarsky K., Sidtis J.J. Sexual dimorphism in the human corpus callosum: an MRI study using the OASIS brain database. Cereb Cortex. 2012;23:2514–2520. doi: 10.1093/cercor/bhs253. [DOI] [PMC free article] [PubMed] [Google Scholar]