Abstract
Introduction
Familiarity has been associated with integrity of the rhinal cortex. Thus, impairment in familiarity is expected in very early stages of Alzheimer's disease (AD). The apolipoprotein E (APOE) ε4 allele is a major risk factor for AD. Here, we investigated the effect of the APOE ε4 status on familiarity in cognitively normal aging individuals.
Methods
Eighty-one individuals aged between 55 and 80 years, 21 carriers and 60 noncarriers, were used in these analyses. A cognitive evaluation was performed on all participants to document the absence of objective cognitive deficits. The effect of APOE ε4 status on familiarity was tested using independent sample t test and an analysis of covariance controlling for age, gender, and education.
Results
The groups did not differ in term of age, education, and male/female ratio. APOE ε4 carriers showed a significant reduction in familiarity. No other cognitive deficit was observed in the group of ε4 carriers, relative to noncarriers.
Discussion
APOE ε4 is associated with a reduction in familiarity in the absence of other cognitive deficits. These results suggest that performance in familiarity could represent an early cognitive marker for individuals at risk of AD.
Keywords: Aging, Apolipoprotein E, Familiarity, Recollection, Cognitive marker, Alzheimer's disease
1. Introduction
Owing to the aging of the population, a worldwide phenomenon, the burden of Alzheimer's disease (AD) is expected to increase dramatically over the next decades [1]. Because of its insidious progression, AD is often only detected at a relatively advanced stage of neurodegeneration, when clinical manifestations and functional impairments are observable. Detecting AD at an earlier stage could contribute to the development of new interventions (pharmaceutical or not) to slow or halt the progression of the disease. The development of new cognitive tests sensitive to early cognitive changes associated with impending AD could contribute to this objective.
AD is a progressive and neurodegenerative condition that develops over several decades [2]. In the first stages of AD, also known as transentorhinal stages, the entorhinal and perirhinal cortices (EC/PC) are targeted by neurofibrillary tangles [3]. Histopathologic studies have demonstrated important neuronal loss in the EC at the earliest stages of dementia. A study by Gómez-Isla et al. [4] revealed that individuals with a clinical dementia rating scale score of 0.5 presented 32% less neurons in the EC relative to aged-matched controls. Magnetic resonance imaging volumetric studies also reported significant EC volume reduction in individuals with mild AD, with one study revealing a volume loss as high as 40% in this region for patients with AD [5]. The functionality of the EC/PC region is also altered in the course of pathologic aging. A longitudinal positron emission tomography study showed that glucose metabolism in the EC accurately predicted cognitive decline over a period of 3 years [6]. In comparison with other regions in the temporal and frontal lobes, the EC was found to be to the most accurate predictor of cognitive changes over time. These findings demonstrate that the EC/PC region is very sensitive to early pathologic alterations associated with AD. Thus, detecting an impaired functionality of these regions could allow early and specific identification of subjects with preclinical AD. However, this is hindered by the fact that transentorhinal stages of AD, also referred to as “silent stages,” are believed to occur before observable clinical manifestations [3].
The functional role of the EC/PC has been extensively studied over the past decades, but the vast majority of studies investigating its function have been performed in animals. Hence, its role in human cognition continues to be a matter of debate. Recent evidence suggests that the EC/PC is associated with familiarity [7], [8]. According to dual-process models, familiarity and recollection are two distinct and independent processes involved in the recognition of previously seen material. Recollection can be defined as a recognition episode accompanied by retrieval of contextual details associated with the encoding sequence. In contrast, familiarity is perceived as a recognition based on a feeling of “knowledge” that a stimulus has previously been encountered, despite a lack of retrieval of contextual details associated with the encoding episode. The existence of two distinct processes involved in recognition has received a substantial amount of empirical support (see Yonelinas 2002 for a review [9]).
Human lesion studies reveal impairment in familiarity following EC/PC lesions [7], [10]. In contrast, hippocampal lesions lead to impairment in recollection with preservation of familiarity [7]. These studies highlight a functional double dissociation between the hippocampus and EC/PC region, the latter being associated with familiarity. This was further corroborated by functional magnetic resonance imaging (fMRI) studies showing that activation in the EC/PC was associated with familiarity-based recognition but not with recollection [8], [11]. Taken together, these results support the involvement of the EC/PC region in familiarity-based recognition.
Recent articles have reviewed the literature looking at familiarity and recollection in aging individuals with normal cognition, mild cognitive impairment (MCI), and AD [12], [13]. Overall, these reviews suggest that, although recollection is impaired in the course of normal aging, familiarity is preserved [12]. The presence of familiarity deficits in MCI and AD patients is not corroborated by all studies. However, the results of these reviews indicate that familiarity deficits tend to be present in MCI individuals at increased risk of AD. Looking at familiarity performance in cognitively normal individuals at increased risk of AD could help defining whether the presence of familiarity deficits represents an early cognitive marker for the development of AD.
The apolipoprotein E (APOE) gene is involved in regulating metabolism and the transportation of lipids, such as cholesterol [14]. It is also involved in the growth and repair of neuronal and axonal membranes during development, and following lesions [14]. The APOE locus contains two alleles that are polymorphic and exist in three variants: ε2, ε3, and ε4. The ε3 allele is the most common and found in 78% of individuals [15]. The ε4 and ε2 alleles are far less common and are represented in 15% and 7% of individuals, respectively [15]. The ε4 allele of the APOE gene, the APOE ε4, is a well-documented risk factor for both familial and sporadic AD [16]. The ε4 allele has widely been associated with a higher risk and a younger age of onset of AD [17]. Furthermore, the risk related to the ε4 allele is “dose dependant”: individuals with only one ε4 allele show a 2.8–4.4 times increased risk for AD, whereas individuals with two ε4 alleles show a 7.0–19.3 times increased risk [16], [18]. Consequently, cognitively intact aging individuals with APOE ε4 are more likely to harbor neurofibrillary tangles at the transentorhinal level than noncarriers. Accordingly, a study showed a volume reduction in the medial temporal lobe of nondemented aging individuals, APOE ε4 carriers [19].
The objective of this study was to investigate the performance in familiarity of aging individuals carrying an APOE ε4 allele and compare it with aging individuals who are noncarriers. Owing to an increased likelihood of harboring AD neuropathology at a subclinical stage, we hypothesized that nondemented individuals with APOE ε4 will show decreased performance in familiarity-based recognition.
2. Methods
2.1. Participants
Older Caucasians adults aged between 55 and 80 years were recruited via advertisements in community newspapers. To be eligible for the study, participants had to meet the following criteria: French or English as a first language; absence of current or past neurologic, psychiatric, or severe medical conditions; no current or past history of substance abuse; no current medication known to cross the blood-brain barrier or alter cognitive functioning; >10 years of formal education; and geriatric depression scale [20] and Beck anxiety inventory [21] scores within the normal range. All data were collected as part of a larger fMRI study. The Douglas Mental Health University Institute's research ethics board approved the protocol of the study, and all participants provided informed written consent. Recruited participants underwent a cognitive test battery to allow the exclusion of individuals with suspected cognitive impairment, as defined by a performance of >1.5 standard deviations lower than age-adjusted mean. Participants were also asked to provide a saliva sample to determine their APOE genotype. They were informed that results from this genetic test would not be disclosed to them. A total of 88 older individuals completed all testing procedures. Four participants were removed from analyses because of suspected MCI. Three other participants were removed from the analyses because of a performance suggesting a lack of comprehension of the instructions associated with the recollection and familiarity task (as indicated by an absence of variance in responses). Thus, the final sample used in the analyses included 81 older adults (male/female: 38/43).
2.2. Cognitive evaluation
The tests administered in the cognitive evaluation included (1) mini-mental status examination [22]; (2) Montreal cognitive assessment [23]; (3) Rey auditory verbal learning test [24]; (4) the copy and immediate recall (3-minutes delay) of the Rey-Osterrieth complex figure test [25]; (5) letter (FAS) and category fluency (animals) [26]; (6) trail making tests A and B [27]; (7) digit symbol substitution—90-seconds version [28]; (8) Boston naming test—60 items [29]; (9) color naming, reading, and interference conditions from the Stroop color-word test [30]; (7) digit span subtest of the Wechsler adult intelligence scale III [31]; and the matrix reasoning subtest of the Wechsler abbreviated scale of intelligence II [32].
2.3. Quantification of familiarity and recollection
2.3.1. Stimuli
A total of 135 black and white neutral Caucasian faces were selected from the FACES database [33] and were divided into three lists of 45 faces. Each list was equated for age and gender of the faces. The first and second lists of faces were used as targets during the first and second encoding condition. Faces from the third list were used as distractors during the recognition.
2.3.2. Experimental paradigm
The paradigm of this experiment shows similarity with the process dissociation procedure (PDP) developed by Jacoby in 1991 [34] as it is also based on the assumption that, if the recognition of an item is based on recollection, participants should be able to provide contextual details associated with the encoding of this item. Consistently with the PDP, the encoding phase of our task consisted of two contextually distinct encoding conditions. However, in opposition to the PDP, it estimates recollection and familiarity from a unique recognition phase. This procedure has the advantages of being simpler to understand, faster to complete, and to avoid methodological biases associated with different response biases that can come with different conditions.
2.3.3. Encoding
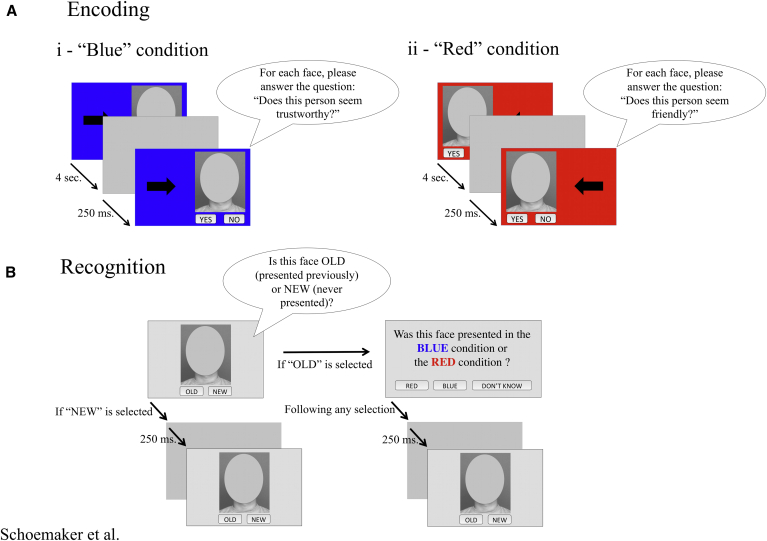
The encoding phase was divided into two distinguishable conditions: the “blue” and “red” encoding conditions. In each encoding condition, participants were presented with 45 faces in a randomized order, for a total of 90 faces. Faces were presented for 4 seconds with a 250-milliseconds interval. In the “blue” condition, faces were displayed on the right side of the screen, on a blue background, and, for each face, participants were asked to answer the question “Does this person looks trustworthy?” In the “red” condition, faces were presented on the left side, on a red background, and, for each face, participants were asked to answer the question “Does this person looks friendly?” These encoding questions were meant to favor a deeper encoding of the faces and provide additional anchors for recollection at the time of the recognition. Participants received the instructions pertaining the upcoming encoding conditions before the beginning of each condition. The order of presentation of the encoding conditions was counterbalanced across subjects. The encoding procedure is illustrated in Fig. 1A.
Fig. 1.
Experimental paradigm. Schematic representation of the procedure and events in the experimental paradigm. The encoding task consisted in the “blue” (A-i) and the “red” (A-ii) encoding condition. Forty-five Caucasian faces were presented in random order in each condition. Faces were presented on the screen for 4 seconds (s) with a 250-milliseconds (ms) interval. The recognition (B) was self-paced and consisted of a two-step procedure. Participants were presented with the 90 targets from the encoding phase as well as 45 new faces (distractors). First, participants were asked to determine whether the face presented was “old” or “new.” If the face was judged to be “old,” participants were then asked to determine in which encoding condition it was presented.
2.3.4. Recognition
After the encoding phase, participants received instructions pertaining the upcoming recognition procedure. The recognition procedure was self-paced. Participants were presented with all faces presented during the encoding phase as well as new unseen faces (distractors). Faces were presented one at a time in the center of the screen on a gray background. For each presented face, participants were first asked to define whether this face was “old” (presented during the encoding phase, in either condition) or “new” (not seen before). Second, if participants recognized the face as being “old,” they were then asked whether this face was presented in the “red” or the “blue” encoding condition. To avoid biases associated with guesses, participants were given the option to answer “can't remember” when they couldn't remember in which condition the face was presented. Participants were given three practice trials (two target faces and ne distractor, same for all participants) before starting the recognition. After completing the practice trials, participants saw the remaining 88 target and 44 distractor faces in random order. The recognition procedure is illustrated in Fig. 1B.
2.3.5. Quantification of responses
The hit rate was computed using the number of target faces correctly recognized as “old” divided by the total number of targets. The recollection rate was computed using the number of target faces correctly recognized as “old” and classified in the correct encoding condition, divided by the total number of targets. The familiarity rate was computed using the number of target faces correctly recognized as “old” but with a failure to provide a correct classification of the encoding condition, divided by the total number of targets. False alarm rate was computed using the number of “new” faces falsely identified as being “old” divided by the total number of distractors.
2.4. APOE genotyping
Saliva samples were collected using Norgen Biotek Corp. Saliva DNA Collection, Preservation and Isolation kits (Norgen Biotek Corp, Thorold, Canada). The extraction of the DNA was performed according to manufacturer specifications. The genotyping technique used for APOE polymorphism has been described previously [35]. Participants with one or more ε4 allele were classified as APOE ε4 positive and participants not presenting an ε4 allele as APOE ε4 negative. The distribution of APOE genotypes in our sample is consistent with previously published reports [36] and is presented in Table 1.
Table 1.
APOE genotypes distribution in the studied sample
| APOE genotype | n | % of sample |
|---|---|---|
| ε2-ε3 | 4 | 4.94 |
| ε3-ε3 | 55 | 69.14 |
| ε2-ε4 | 1 | 1.23 |
| ε3-ε4 | 20 | 24.69 |
| Total | 80 | 100 |
Abbreviation: APOE, apolipoprotein.
2.5. Statistical analysis
Analyses were performed using the Statistical Package for the Social Sciences (SPSS; IBM, Chicago, USA) version 20. Differences between APOE ε4 negative and APOE ε4 positive groups on demographic variables and cognitive measures were analyzed using independent samples t tests. The assumption of equal variance was verified using Levene's test. A χ2 test was used to assess the difference in the male/(female + male) ratio between groups.
The performance of the two groups on hit, recollection, familiarity, and false alarm rates obtained in the recollection and familiarity task was contrasted using independent samples t tests and Levene's test for equality of variances. To correct for multiple comparisons, the alpha for statistical significance was adjusted to P < .01 to account for the four performed comparisons, as per the Bonferroni procedure. A one-way analysis of covariance was also computed to compare performances of both groups on the task, while controlling for age, gender, and education.
3. Results
3.1. Demographic and cognitive characteristics of study subjects
Demographic characteristics and results on cognitive tests are summarized for each group separately in Table 2. APOE ε4 negative and positive groups did not significantly differ in terms of age and years of education. The χ2 test did not show any significant association between the APOE ε4 status and gender χ2 (1, n = 81) = 1.19, P > .05, suggesting that both groups did not significantly differ in terms of gender representation. When contrasting scores of the two groups on standard cognitive tests, only one comparison reached statistical significance, suggesting a higher performance on the immediate recall of the Rey-Osterrieth complex figure test for the APOE ε4 positive group. However, this result did not survive statistical correction for multiple comparisons.
Table 2.
Summary of demographic variables and cognitive performance on standard cognitive tests
| Demographics and cognitive performance |
APOE ε4 negative Mean (SD), n = 60 |
APOE ε4 positive Mean (SD), n = 21 |
Between-group comparison |
|---|---|---|---|
| Age | 65.15 (6.46) | 63.48 (6.22) | t = 1.03 (NS) |
| Education | 14.88 (2.54) | 15.29 (2.08) | t = −0.65 (NS) |
| Male/(male + female) | 0.43 | 0.57 | χ2 = 1.19 (NS) |
| MMSE | 29.31 (0.88) | 29.33 (0.73) | t = −0.13 (NS) |
| MoCA | 27.47 (2.43) | 27.14 (2.15) | t = 0.53 (NS) |
| RAVLT | |||
| Total learning | 53.19 (8.80) | 50.33 (12.00) | t = 1.15 (NS) |
| Recall after interference | 11.19 (3.06) | 10.24 (3.99) | t = 1.12 (NS) |
| Delayed recall | 11.17 (3.36) | 10.43 (3.83) | t = 0.84 (NS) |
| Recognition | 14.69 (0.70) | 14.42 (1.36) | t = 0.86 (NS) |
| Rey-Osterrieth complex figure | |||
| Copy | 30.90 (3.30) | 31.52 (3.40) | t = −0.74 (NS) |
| Immediate recall | 17.23 (5.53) | 20.21 (4.86) | t = −2.13 (P = .04*) |
| Letter fluency (FAS) | 42.62 (12.35) | 40.62 (10.89) | t = 0.66 (NS) |
| Category fluency (animals) | 20.84 (5.28) | 19.57 (3.43) | t = 1.03 (NS) |
| Digit symbol substitution test | 44.23 (10.21) | 45.67 (11.06) | t = −0.54 (NS) |
| Trail making test | |||
| A (letter) | 32.15 (11.13) | 31.76 (10.50) | t = 0.14 (NS) |
| B (letter-number) | 73.07 (29.00) | 75.43 (31.48) | t = −0.31 (NS) |
| Stroop color-word interference test | |||
| Color naming | 30.36 (4.76) | 32.29 (5.83) | t = −1.50 (NS) |
| Word reading | 23.37 (5.90) | 23.76 (5.72) | t = −0.26 (NS) |
| Inhibition | 60.51 (14.29) | 63.43 (12.96) | t = −0.82 (NS) |
| Digit span | |||
| Total forward | 6.66 (1.38) | 6.71 (1.19) | t = −0.16 (NS) |
| Total backward | 5.36 (1.26) | 5.24 (1.37) | t = 0.36 (NS) |
| Boston naming test | 54.17 (4.80) | 53.90 (4.00) | t = 0.23 (NS) |
| Matrix reasoning | 19.28 (4.53) | 19.05 (4.35) | t = 0.16 (NS) |
Abbreviations: APOE, apolipoprotein; SD, standard deviation; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; RAVLT, Rey auditory verbal learning test.
NOTE. All comparisons for continuous variables have been computed using independent sample t tests. A χ2 test was used to compare gender ratio between groups. The asterisk shows a significant between-group difference with P < .05. NS indicates absence of between-group significance.
3.2. Familiarity and recollection as a function of APOE ε4 status
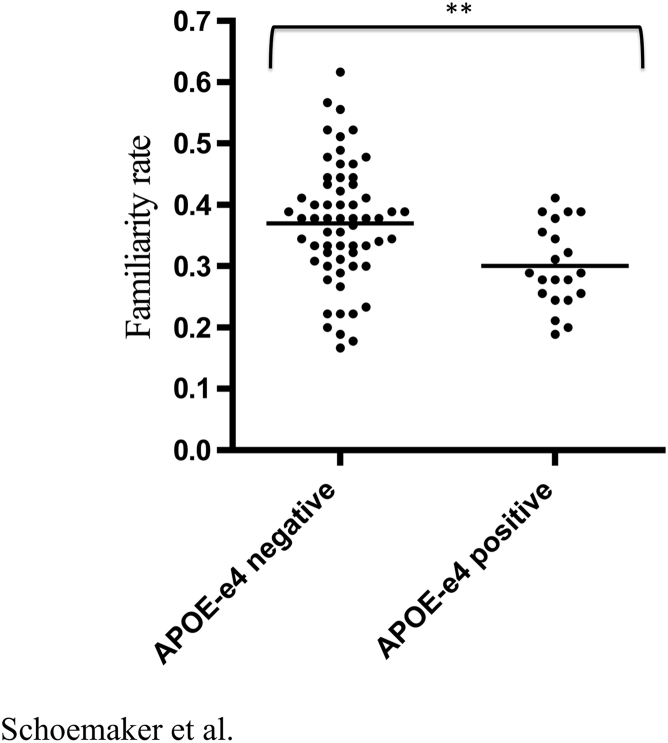
When contrasting the performance of APOE ε4 positive and negative groups on our recollection and familiarity task, the only significant difference was found in the familiarity rate. More precisely, the familiarity rate was significantly reduced in the APOE ε4 positive group t(1,79) = 2.79, P = .007. The hit rate t(1,79) = 1.31, NS, recollection rate t(1,79) = −0.79, NS, and false alarm rate t(1,79) = 1.46, NS did not differ significantly between the groups. These results are summarized in Table 3 and the difference in familiarity between groups is illustrated in Fig. 2.
Table 3.
Performance on the recollection and familiarity task as a function of APOE ε4 status
| Recollection and familiarity task |
APOE ε4 negative Mean (SD) |
APOE ε4 positive Mean (SD) |
|---|---|---|
| Hit rate | 0.61 (0.14) | 0.56 (0.14) |
| Recollection rate | 0.24 (0.10) | 0.26 (0.11) |
| Familiarity rate | 0.37 (0.10) | 0.30 (0.07)** |
| False alarm rate | 0.30 (0.11) | 0.25 (0.12) |
Abbreviations: APOE, apolipoprotein; SD, standard deviation; ANOVA, analysis of variance.
All between-group comparisons have been computed using a one-way ANOVA. Double asterisks show a significant group difference with a P < .01.
Fig. 2.
Familiarity performance as a function of APOE ε4 status. Individual performances in familiarity plotted separately for APOE ε4 negative and APOE ε4 positive groups. **Significant with P < .01.
The effect of APOE ε4 status on familiarity was still significant after controlling for age, gender, and education of participants F (1, 76) = 8.74, P = .004, whereas it remained nonsignificant for hit rate F (1, 76) = 2.60, NS, recollection rate F (1, 76) = 0.37, NS, and false alarm rate F (1, 76) = 1.54, NS.
4. Discussion
In this article, we investigated the effects of APOE ε4 status on familiarity in a sample of cognitively normal aging individuals. Our results highlight a significant reduction in familiarity in aging individuals who are carriers of the APOE ε4 allele, relative to noncarriers. Familiarity was the only cognitive domain that was significantly reduced in this group. Furthermore, our analysis demonstrated that this effect was not due to differences in age, education, or gender between groups.
Consistently with previous studies, we failed to find impairment in performance on standard cognitive tests in the APOE ε4 positive group [37], [38]. The APOE ε4 positive group even outperformed the APOE ε4 negative group on the immediate recall of the Rey-Osterrieth complex figure [25]. Because no other cognitive impairment was found in the APOE ε4 positive group, this suggests that familiarity is more sensitive to the effects of APOE ε4 than other cognitive measures administered. This also suggests that the effects of APOE ε4 on cognition might occur much before the onset of overt dementia. Individuals carrying the APOE ε4 are at an increased risk of AD compared with noncarriers [39], [40]. As a consequence, a greater frequency of individuals with neuropathologic alteration associated with AD is expected in this population. Familiarity has previously been linked to the integrity of EC/PC regions [7], [10]. Therefore, the specific impairment in familiarity observed in the group of ε4 carriers might reflect very early cognitive changes occurring as a consequence of neurofibrillary accumulation in these brain regions. Future studies should, thus, aim to investigate neuroanatomic and/or functional correlates of this impairment.
An overlap in the performance in familiarity between both groups is observed in the plot illustrating the results. Although it is rare that APOE ε4 carriers reach a high level of performance in familiarity, multiple noncarriers show a performance that is in the lower range. This overlap is expected and consistent with our hypothesis. We hypothesize that impairment familiarity is a marker for early neuropathologic alterations associated with subclinical AD. APOE ε4 is a well-documented and important risk factor for the development of AD, but it remains a risk factor only. Thus, many individuals with APOE ε4 will never develop AD over time. On the other hand, nondemented aging individuals without APOE ε4 can also have subclinical AD and harbor AD neuropathology at the transentorhinal stage. Consequently, as represented in our results, it is expected that some ε4 carriers show a normal performance in familiarity and that some noncarriers perform in a deficient manner. Here, longitudinal studies are necessary to define whether a low performance in familiarity is predictive of future cognitive decline and dementia.
Growing evidence indicates that the ε4 allele put women at increased risk of cognitive decline compared with men [14], [41]. Although causes behind this gender dimorphism are still a matter of debate, gender remains an important factor to take into consideration when investigating the effects of APOE ε4. In this study, both groups had an equivalent gender distribution. Furthermore, including gender as a covariate in a statistical model did not alter the significance of our findings. This suggests that our results were not explained by gender difference between groups.
To our knowledge, only one article has previously looked at the effect of APOE ε4 on familiarity [42]. As part of their study, Tse et al. used a memory exclusion paradigm that allows evaluating the ability to correctly reject familiar information that is contextually irrelevant to the demand of the task. As it directly opposes automatic and controlled recognition processes, this procedure is believed to also rely on executive control. Overall, results from the Tse et al. article highlight an increased reliance on familiarity and a reduced ability to control familiarity-based information in nondemented aging individuals with APOE ε4. It is important to note that in their article, although APOE ε4 carriers were judged cognitively normal, they showed poorer performance in a free recall and computational span test than the noncarriers, suggesting some a-priori differences in cognitive functioning between groups. The authors hypothesized that changes in mechanisms of attentional control might be responsible for the observed difference between groups. Although this article demonstrates some changes in the interplay between familiarity and recollection in APOE ε4 carriers, the used paradigm is different than the one used in the present article and does not provide a separate estimate of familiarity and recollection. It is, therefore, difficult to compare the results of Tse et al. to the findings obtained in the present study.
Although we did not find any evidence for other forms of cognitive impairment in the group of ε4 carriers, the presence of deficits in executive function and spatial attention has previously been shown in this population [43], [44]. It is possible that a more detailed cognitive evaluation would have exposed differences between the cognitive profile of APOE ε4 positive and negative groups. Accordingly, by using a specific visual cueing paradigm, Greenwood et al. [44] found evidence for reduced visual discrimination accuracy and visuospatial attention in nondemented individuals with one or two APOE ε4 alleles. Because the present recollection and familiarity task involves a spatial component (left vs. right side presentation), the experimental paradigm perhaps contributed to the observed familiarity deficits. Furthermore, past studies have demonstrated that performance in recollection and familiarity might be influenced by the stimulus modality [45], [46], [47]. For example, Embree et al. [46] showed that, although the stimulus type did not influence recollection, familiarity for words, but not for pictures, was impaired in MCI individuals. Previous articles have highlighted a possible hemispheric asymmetry in volume loss occurring over the course of AD [48], [49]. Because of the known hemispheric specialization for verbal functions, the replication of these findings using verbal instead of nonverbal material could provide interesting additional information related to familiarity and recollection performance in populations at risk of AD. Thus, future studies should aim to replicate the current findings using different experimental paradigms and different type of stimulus.
Memory evaluation is a central component of clinical investigation associated with the differential diagnosis of cognitive complains occurring in aging individuals. Here, we highlighted familiarity impairment in aging individuals at increased risk of AD, in the absence of any other observable cognitive deficits. Although the scope of this study is limited by the small sample of APOE ε4 positive individuals, the results of this article suggest that familiarity deficits might arise before other cognitive manifestations associated with beginning AD. Thus, the inclusion of a task evaluating familiarity in clinical evaluation might allow an earlier identification of individuals with subclinical AD. However, more research is needed to confirm the relevance of familiarity assessment in clinical settings.
Research in context.
-
1.
Systematic review: Relevant articles were identified via a search through online databases PubMed and PsycINFO with the use of the keywords “familiarity,” “recollection,” “recognition,” and “apolipoprotein E.” The abstract of every article retrieved from this search was reviewed. Pertinent articles are cited and discussed in the article.
-
2.
Interpretation: Our results suggest that performance in familiarity could represent an early cognitive marker for individuals at risk of Alzheimer's disease.
-
3.
Future directions: The article proposes multiple directions to further assess the predictive value of familiarity in the early detection of dementia. One of the most important aspects addressed in the article is the need for longitudinal studies to define whether baseline measures in familiarity can accurately predict cognitive deterioration over time. Furthermore, future research should aim to achieve a better understanding of the neuroanatomic basis of this differential impairment.
Acknowledgments
This work was supported by a research grant “Maladie d'Alzheimer et les Maladies Apparentées” jointly sponsored by the Agence Nationale de la Recherche (ANR), Fonds de Recherche du Québec - Santé (FRQS), and the Canadian Institutes of Health Research (CIHR) (grant 23638). DS doctoral training is funded by an FRQS doctoral award (grant 29793). JCP is supported by an FRQS Chercheur National salary award (grant 22507).
References
- 1.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Isla T., Price J.L., McKeel D.W., Jr., Morris J.C., Growdon J.H., Hyman B.T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juottonen K., Laakso M., Insausti R., Lehtovirta M., Pitkänen A., Partanen K. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiol Aging. 1998;19:15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 6.de Leon M.J., Convit A., Wolf O.T., Tarshish C.Y., DeSanti S., Rusinek H. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowles B., Crupi C., Pigott S., Parrent A., Wiebe S., Janzen L. Double dissociation of selective recollection and familiarity impairments following two different surgical treatments for temporal-lobe epilepsy. Neuropsychologia. 2010;48:2640–2647. doi: 10.1016/j.neuropsychologia.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Ranganath C., Yonelinas A.P., Cohen M.X., Dy C.J., Tom S.M., D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Yonelinas A.P. The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 10.Martin C.B., Bowles B., Mirsattari S.M., Köhler S. Selective familiarity deficits after left anterior temporal-lobe removal with hippocampal sparing are material specific. Neuropsychologia. 2011;49:1870–1878. doi: 10.1016/j.neuropsychologia.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Daselaar S.M., Fleck M.S., Dobbins I.G., Madden D.J., Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koen J.D., Yonelinas A.P. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer's disease on recollection and familiarity: A meta-analytic review. Neuropsychol Rev. 2014;24:332–354. doi: 10.1007/s11065-014-9266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoemaker D., Gauthier S., Pruessner J.C. Recollection and familiarity in aging individuals with mild cognitive impairment and Alzheimer's disease: A literature review. Neuropsychol Rev. 2014;24:313–331. doi: 10.1007/s11065-014-9265-6. [DOI] [PubMed] [Google Scholar]
- 14.Poirier J., Bertrand P., Kogan S., Gauthier S., Davignon J., Bouthillier D. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 15.Strittmatter W.J., Roses A.D. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy-Lahad E., Bird T.D. Genetic factors in Alzheimer's disease: A review of recent advances. Ann Neurol. 1996;40:829–840. doi: 10.1002/ana.410400604. [DOI] [PubMed] [Google Scholar]
- 17.Corder E., Saunders A., Strittmatter W., Schmechel D., Gaskell P., Small G. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 18.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honea R.A., Vidoni E., Harsha A., Burns J.M. Impact of APOE on the healthy aging brain: A voxel-based MRI and DTI study. J Alzheimers Dis. 2009;18:553. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yesavage J.A., Brink T., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Beck A.T., Steer R.A. Psychological Corporation; San Antonio, TX: 1990. BAI, Beck anxiety inventory. [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey auditory verbal learning test: A handbook. [Google Scholar]
- 25.Osterrieth P.A. Le test de copie d'une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 26.Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 27.Reitan R.M. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. Trail Making Test: Manual for administration and scoring. [Google Scholar]
- 28.Smith A. Testzentrale; Los Angeles, CA: 2000. Symbol digit modalities test: SDMT. [Google Scholar]
- 29.Kaplan E., Goodglass H., Weintraub S., Segal O., van Loon-Vervoorn A. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. Boston naming test: Pro-ed; 2nd edition. [Google Scholar]
- 30.Golden C., Freshwater S. Stoelting; Chicago, IL: 2002. A manual for the adult Stroop Color and Word Test; pp. 1–11. [Google Scholar]
- 31.Wechsler D. Psychological Corporation; San Antonio, TX: 1997. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. [Google Scholar]
- 32.Wechsler D., Hsiao-pin C. Pearson; San Antonio, TX: 2011. WASI-II: Wechsler abbreviated scale of intelligence. [Google Scholar]
- 33.Ebner N.C., Riediger M., Lindenberger U. FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behav Res Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- 34.Jacoby L.L. A process dissociation framework: Separating automatic from intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 35.Wiebusch H., Poirier J., Sévigny P., Schappert K. Further evidence for a synergistic association between APOEɛ4 and BCHE-K in confirmed Alzheimer's disease. Hum Genet. 1999;104:158–163. doi: 10.1007/s004390050929. [DOI] [PubMed] [Google Scholar]
- 36.Henderson A., Jorm A., Korten A., Christensen H., Jacomb P., Easteal S. Apolipoprotein E allele 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- 37.Smith G.E., Bohac D., Waring S., Kokmen E., Tangalos E., Ivnik R. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer's disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- 38.Small B.J., Graves A., McEvoy C., Crawford F., Mullan M., Mortimer J. Is APOE-ε4 a risk factor for cognitive impairment in normal aging? Neurology. 2000;54:2082–2088. doi: 10.1212/wnl.54.11.2082. [DOI] [PubMed] [Google Scholar]
- 39.Nalbantoglu J., Gilfix B.M., Bertrand P., Robitaille Y., Gauthier S., Rosenblatt D.S. Predictive value of apolipoprotein E genotyping in Alzheimer's disease: Results of an autopsy series and an analysis of several combined studies. Ann Neurol. 1994;36:889–895. doi: 10.1002/ana.410360614. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri S., Drachman D.A., Lippa C.F. Apolipoprotein E ε4 allele and the lifetime risk of Alzheimer's disease: What physicians know, and what they should know. Arch Neurol. 1995;52:1074–1079. doi: 10.1001/archneur.1995.00540350068018. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen E.L., Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57:89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- 42.Tse C.-S., Balota D.A., Moynan S.C., Duchek J.M., Jacoby L.L. The utility of placing recollection in opposition to familiarity in early discrimination of healthy aging and very mild dementia of the Alzheimer's type. Neuropsychology. 2010;24:49. doi: 10.1037/a0014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenwood P., Lambert C., Sunderland T., Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19:199. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood P.M., Sunderland T., Friz J.L., Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the 4 allele of the apolipoprotein E gene. Proc Natl Acad Sci U S A. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belleville S., MÈnard M.C., Lepage E. Impact of novelty and type of material on recognition in healthy older adults and persons with mild cognitive impairment. Neuropsychologia. 2011;49:2856–2865. doi: 10.1016/j.neuropsychologia.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Embree L.M., Budson A.E., Ally B.A. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50:2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barba G.D. Recognition memory and recollective experience in Alzheimer's disease. Memory. 1997;5:657–672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- 48.Shi F., Liu B., Zhou Y., Yu C., Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 49.Thompson P., Moussai J., Zohoori S., Goldkorn A., Khan A., Mega M. Cortical variability and asymmetry in normal aging and Alzheimer's disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]