Abstract
An asymmetric approach to the synthesis of neurotrophic seco-prezizaane sesquiterpenes is described that is based on strategic application of a hydroxyl-directed metallacycle-mediated [2+2+2] annulation and an intramolecular radical cyclization cascade. Targets prepared are among the most potent members of the natural product class and include (1R,10S)-2-oxo-3,4-dehydroneomajucin, (2S)-hydroxy-3,4-dehydroneomajucin and (−)-jiadifenin. In addition to representing the first application of the alkoxide-directed metallacycle-mediated hydrindane-forming annulation reaction in natural product synthesis and the first total synthesis of (2S)-hydroxy-3,4-dehydroneomajucin, these pursuits have resulted in the elucidation of a complex radical cascade process for installation of the C5 quaternary center common to the natural product class.
TOC Figure
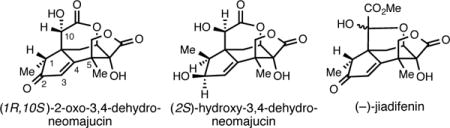
The search for agents that promote regeneration and growth of neurons is of great current interest, as axon degeneration and neuronal atrophy accompany chronic neurodegenerative disease and acute spinal cord injury.1 While proteins that serve in this regard (neurotrophins) have been investigated as potential therapeutic agents, they suffer from a variety of suboptimal characteristics that negatively impact their potential utility in the clinic (i.e. low serum stability, poor oral bioavailability, and inefficient penetration into the central nervous system).1a, 2 As such, there has been growing interest in identifying small molecule neurotrophic agents that have a more favorable pharmacokinetic profile.3 While early investigations of the seeds of the Japanese star anise (Illicium anisatum, L.) delivered anisatin,4 a neurotoxic noncompetitive GABA antagonist,5 more recent studies of Illicium terrestrial plants (evergreen shrubs/trees) have delivered a collection of complex carbocyclic natural products that have been shown to possess potent neurotrophic properties (Figure 1).3 Perhaps not surprisingly, molecules in this class have emerged as attractive targets for chemical synthesis due to the combination of their potential therapeutically relevant biological activity and their interesting carbocyclic structures.3,6 In 1990 Kouno and coworkers described the isolation of (1R, 10S)-2-oxo-3,4-dehydroneomajucin (1) and (2S)-hydroxy-3,4-dehydroneomajucin (2) in 0.0006 and 0.007% yield, respectively, from the dried fruit of the Chinese Illicium majus.7,8 These natural products, while structurally related to anisatin, did not exhibit convulsive toxicity in mice. Fourteen years later, in efforts targeting the synthesis of (+/−)-jiadifenin, Danishefsky reported that 1 is a highly active neurotrophic agent in vitro: 184% vs. control at 300 nM6c, 6e – an activity that was later supported by structure–activity relationships reported by Theodorakis.6n Here, we report the first of our studies aimed at establishing an asymmetric synthesis of neurotrophic seco-prezizaane natural products and demonstrate the first application of an alkoxide-directed metallacycle-mediated annulative coupling reaction in natural product synthesis.9 In addition, a powerful intramolecular radical cascade reaction has been found to be useful for installing the sterically congested C5 quaternary center common to the natural product class.
Figure 1.
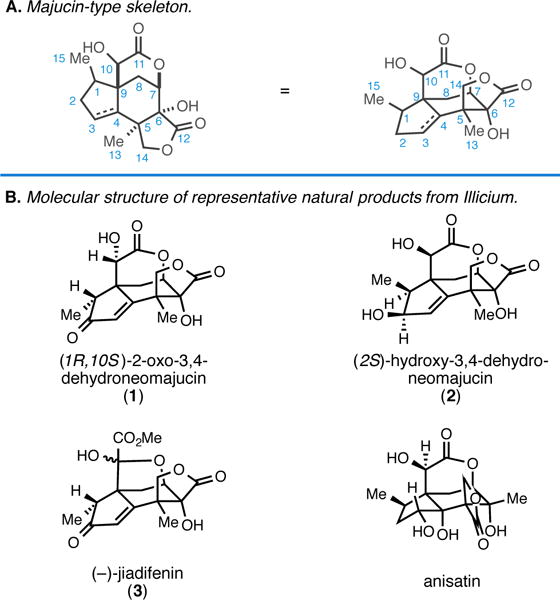
Introduction to majucin-type sesquiterpenes from Illicium.
Our pursuits began by targeting the synthesis of 1 by Bu3SnH-mediated intramolecular 5-exo trig cyclization of the phenylseleno-substituted radical precursor 4 (Figure 2). Synthesis of this substrate was planned from hydrindane 5 and would require differential functionalization of the TMS- and Bu3Sn-substituted alkene. Finally, hydrindane 5 was expected to be accessible from union of enyne 6 with alkyne 7 by application of a recently developed regio- and stereoselective hydroxyl-directed titanium-mediated annulation reaction.
Figure 2.
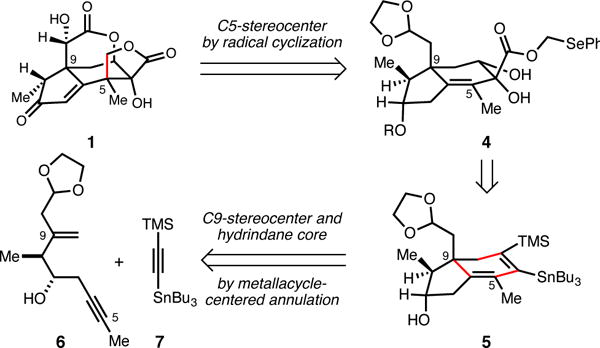
Retrosynthetic strategy for 1.
Synthesis was initiated by regioselective addition of the organometallic reagent derived from 9 to chiral epoxide 810 (Figure 3A).11 Conversion of the resulting homoallylic alcohol to enyne 6 was then accomplished by a sequence of desilylation (TBAF, THF), epoxide formation (TsCl, Et3N, DMAP, then NaH, THF), and nucleophilic addition of propynyl lithium. In accord with our earlier observations regarding the regio- and stereosleective coupling of 4-hydroxy-1,6-enynes with TMS-alkynes,9, 12 exposure of stannyl-substituted TMS-acetylene 7 to the combination of Ti(Oi-Pr)4 and n-BuLi (−78 to 50 °C), followed by addition of the Lialkoxide of enyne 6 (−78 °C to rt) generated hydrindane 5 in 73% yield (rs ≥ 20:1), where the C9 quaternary center was established with high levels of stereoselectivity (ds ≥ 20:1).
Figure 3.
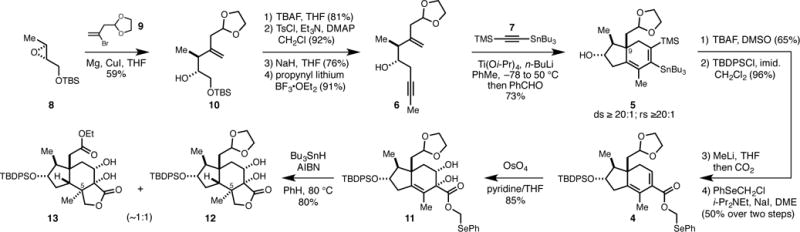
Establishment of the carbocyclic skeleton of the seco-prezizaane sesquiterpenes.
Treatment of hydrindane 5 with TBAF resulted in removal of the TMS-group and was followed by silylation of the secondary alcohol (TBDPSCl, imid., CH2Cl2). Subsequently, tin-lithium exchange was followed by carboxylation,13 and esterification with PhSeCH2Cl – a sequence that ultimeatley delivered the selenophenyl methyl ester 4.14 Site- and stereoselective dihydroxylation15 then generated 11 in 85% yield as a single observable stereoisomer.
We next turned our attention to the radical cyclization process that was anticipated to establish the sterically congested C5 quaternary center. Heating selenophenyl methyl ester 11 in the presence of Bu3SnH and AIBN resulted in the formation of two cyclized products 12 and 13 in 80% combined yield (notably, each of these products possessed the desired C5-quaternary center). While the formation of 12 was expected, observation of the ethyl ester-containing product 13 was unanticipated and, on further consideration, warmly welcomed. We speculate that this latter compound is produced from the sequence summarized in Figure 4, where formation of radical I is followed by stereoselective 5-exo trig cyclization16 en route to II. While intermolecular quenching of this tertiary radical with Bu3SnH results in the expected product 12, intramolecular hydrogen atom transfer from the acetal carbon (II → III), fragmentation (III → IV), and reduction delivers the ethyl ester-containing carbocycle 13.
Figure 4.
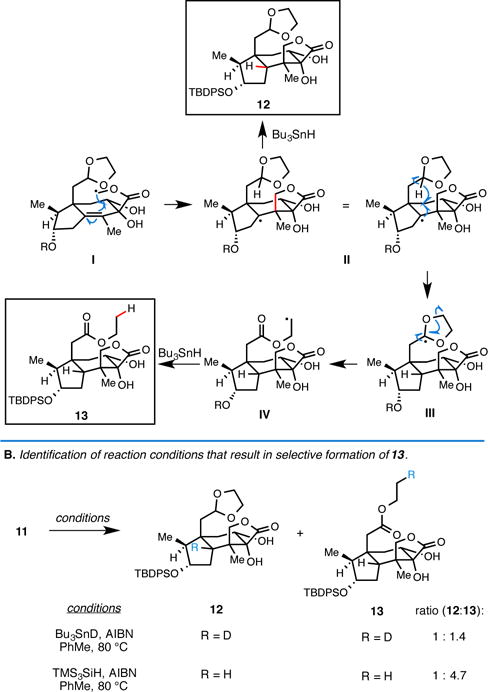
Proposed mechanism for the radical cascade reaction.
With the goal of influencing the course of this radical cascade reaction, we anticipated that slowing the rate of intermolecular reduction of II would result in the production of a greater quantity of ester 13. While simply decreasing the concentration of Bu3SnH would be anticipated to have such an effect, slow addition by syringe pump (over 3 h) did little to effect selectivity. Use of Bu3SnD was hypothesized as an alternative means to slow the rate of reduction of II through exploiting the primary deuterium isotope effect,17 yet this modification resulted in only a minor shift in selectivity favoring the formation of 13(D) (1.4: 1; Figure 4B). Satisfyingly, use of TMS3SiH instead of Bu3SnH in this radical cascade reaction resulted in a more substantial change in ratio to favor the ethyl ester-containing product 13 (4.7: 1).
As illustrated in Figure 5, both products of the radical cyclization were converted to the tetracyclic bis-lactone 14 by a sequence of straightforward functional group manipulations. Desilylation of 14 with TBAF and sequential oxidation (IBX, then Saegusa) then delivered enone 15. Finally, removal of the tertiary TMS-ether that was generated during the Saegusa oxidation, and α-hydroxylation18 delivered (1S,10R)-2-oxo-3,4-dehydroneomajucin (1) through a process that was accompanied by epimerization at C1. As reported by Danishefsky, oxidation and methanolysis of 1 was successful for producing synthetic (−)-jiadifenin (3).6c, 6i, 6k
Figure 5.
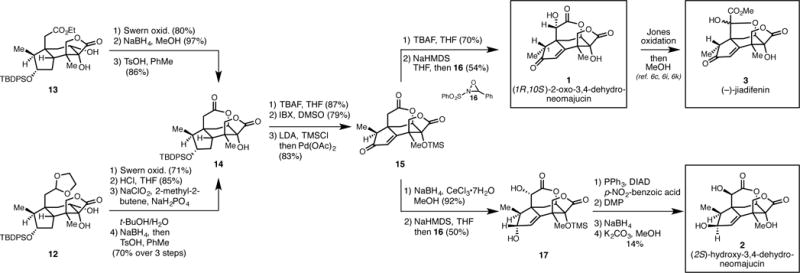
Total syntheses of (1R,10S)-2-oxo-3,4-dehydroneomajucin, (−)-jiadifenin, and (2S)-3,4-dehydroneomajucin.
The complex tetracyclic intermediate 15 also served as a precursor to one of the most active neurotrophic agents in this natural product family, (2S)-hydroxy-3,4-dehydroneomajucin (2).7b First, stereoselective reduction of the enone and α-hydroxylation of the lactone delivered 17. Subsequent Mitsunobu reaction with p-nitrobenzoic acid, followed by a sequence of oxidation to the α-keto-lactone (Dess Martin periodinane), stereoselective reduction (NaBH4), hydrolysis of the p-nitrobenzoate and deprotection of the TMS ether (K2CO3, MeOH) furnished the first synthetic sample of (2S)-3,4-dehydroneomajucin 2.
Overall, we report an enantioselective pathway for the synthesis of several neurotrophic seco-prezizaanes by way of an alkoxide-directed metallacycle-mediated [2+2+2] annulation and intramolecular radical cyclization. This marks the first demonstration of such a metallacycle-centered annulation reaction in natural product synthesis and demonstrates the utility of a selenophenyl-methyl ester-based radical cyclization cascade to generate the congested quaternary center at C5 of this natural product family. We look forward to further developing chemistry capable of producing neurotrophic agents inspired by the seco-prezizaanes and to exploring the broad utility of alkoxide-directed metallacycle-centered annulative cross-coupling reactions in natural product synthesis.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the National Institutes of Health–NIGMS (GM080266). The authors also thank Dr. Haruki Mizoguchi and Dr. Christopher amEnde for helpful discussions, and Professor Yoshiyasu Fukuyama for NMR spectra of 2.
Footnotes
SUPPORTING INFORMATION PARAGRAPH:
Experimental procedures and tabulated spectroscopic data for new compounds (PDF) are available free of charge via the Internet at http://pubs.acs.org/page/jacsat/submission/authors.html.
References
- 1.(a) Price RD, Milne SA, Sharkey J, Matsuoka N. Pharmacol Therapeut. 2007;115:292–306. doi: 10.1016/j.pharmthera.2007.03.005. [DOI] [PubMed] [Google Scholar]; (b) Dakas P-Y, Parga JA, Höing S, Schöler HR, Sterneckert J, Kumar K, Waldmann H. Angew Chem Int Ed. 2013;52:9576–9581. doi: 10.1002/anie.201302045. [DOI] [PubMed] [Google Scholar]; (c) Burch P, Binaghi M, Scherer M, Wentzel C, Bossert D, Eberhardt L, Neuburger M, Scheiffele P, Gademann K. Chem Eur J. 2013;19:2589–2591. doi: 10.1002/chem.201203746. [DOI] [PubMed] [Google Scholar]
- 2.Skaper DS, Walsh FS. Mol Cell Neurosci. 1998;12:179–193. doi: 10.1006/mcne.1998.0714. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Lacoske MH, Theodorakis EA. Angew Chem Int Ed. 2014;53:956–987. doi: 10.1002/anie.201302268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Lane JF, Kock WT, Leeds NS, Gorin G. J Am Chem Soc. 1952;74:3211–3215. [Google Scholar]; (b) Yamada K, Takada S, Nakamura S, Hirata Y. Tetrahedron. 1968;24:199–229. [Google Scholar]
- 5.Kudo Y, Oka J-I, Yamada K. Neurosci Lett. 1981;25:83–88. doi: 10.1016/0304-3940(81)90105-1. [DOI] [PubMed] [Google Scholar]
- 6.(a) Birman VB, Danishefsky SJ. J Am Chem Soc. 2002;124:2080–2081. doi: 10.1021/ja012495d. [DOI] [PubMed] [Google Scholar]; (b) Inoue M, Sato T, Hirama M. J Am Chem Soc. 2003;125:10772–10773. doi: 10.1021/ja036587+. [DOI] [PubMed] [Google Scholar]; (c) Cho YS, Carcache DA, Tian Y, Li Y-M, Danishefsky SJ. J Am Chem Soc. 2004;126:14358–14359. doi: 10.1021/ja045939p. [DOI] [PubMed] [Google Scholar]; (d) Meng Z, Danishefsky SJ. Angew Chem Int Ed. 2005;44:1511–1513. doi: 10.1002/anie.200462509. [DOI] [PubMed] [Google Scholar]; (e) Carcache DA, Cho YS, Hua Z, Tian Y, Li Y-M, Danishefsky SJ. J Am Chem Soc. 2006;128:1016–1022. doi: 10.1021/ja056980a. [DOI] [PubMed] [Google Scholar]; (f) Inoue M, Sato T, Hirama M. Angew Chem Int Ed. 2006;45:4843–4848. doi: 10.1002/anie.200601358. [DOI] [PubMed] [Google Scholar]; (g) Mehta G, Singh SR. Angew Chem Int Ed. 2006;45:953–955. doi: 10.1002/anie.200503618. [DOI] [PubMed] [Google Scholar]; (h) Mehta G, Shinde HM. Tetrahedron Lett. 2007;48:8297–8300. [Google Scholar]; (i) Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Org Lett. 2011;13:4554–4557. doi: 10.1021/ol201742j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Xu J, Trzoss L, Chang WK, Theodorakis EA. Angew Chem Int Ed. 2011;50:3672–3676. doi: 10.1002/anie.201100313. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Yang Y, Fu X, Chen J, Zhai H. Angew Chem Int Ed. 2012;51:9825–9828. doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]; (l) Mehta G, Shinde HM. J Org Chem. 2012;77:8056–8070. doi: 10.1021/jo301258g. [DOI] [PubMed] [Google Scholar]; (m) Chen J, Gao P, Yu F, Yang Y, Zhu S, Zhai H. Angew Chem Int Ed. 2012;51:5897–5899. doi: 10.1002/anie.201200378. [DOI] [PubMed] [Google Scholar]; (n) Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Chem Eur J. 2013;19:6398–6408. doi: 10.1002/chem.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Paterson I, Xuan M, Dalby SM. Angew Chem Int Ed. 2014;53:7286–7289. doi: 10.1002/anie.201404224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Siler DA, Mighion JD, Sorensen EJ. Angew Chem Int Ed. 2014;53:5332–5335. doi: 10.1002/anie.201402335. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Lu H-H, Martinez MD, Shenvi RA. Nat Chem. 2015;7:604–607. doi: 10.1038/nchem.2283. [DOI] [PubMed] [Google Scholar]; For recent reviews, see:; (r) Wilson RM, Danishefsky SJ. Acc Chem Res. 2006;39:539–549. doi: 10.1021/ar068018n. [DOI] [PubMed] [Google Scholar]; (s) Urabe D, Inoue M. Tetrahedron. 2009;65:6271–6289. [Google Scholar]; and ref. 3.
- 7.Kouno I, Baba N, Hashimoto M, Kawano N, Takahashi M, Kaneto H, Yang C-S. Chem Pharm Bull. 1990;38:422–425. doi: 10.1248/cpb.38.291. [DOI] [PubMed] [Google Scholar]
- 8.(2S)-hydroxy-3,4-dehydroneomajucin has also recently been isolated from Illicium jiadifengpi, along with a collection of related seco-prezizaane-type sesquiterpenes, see:; (a) Yokoyama R, Huang J-M, Yang C-S, Fukuyama Y. J Nat Prod. 2002;65:527–531. doi: 10.1021/np010571k. [DOI] [PubMed] [Google Scholar]; (b) Liu J-F, Li Y-C, Wang L, Wang Y-F, Jia L, Bi Y-F, Zhang Y-B. Tetrahedron Lett. 2014;55:2942–2944. [Google Scholar]
- 9.(a) Greszler SN, Reichard HA, Micalizio GC. J Am Chem Soc. 2012;134:2766–2774. doi: 10.1021/ja2105043. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jeso V, Aquino C, Cheng X, Mizoguchi H, Nakashige M, Micalizio GC. J Am Chem Soc. 2014;136:8209–8212. doi: 10.1021/ja504374j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheng X, Micalizio GC. Org Lett. 2014;16:5144–5147. doi: 10.1021/ol502496d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J Am Chem Soc. 1987;109:5765–5780. [Google Scholar]
- 11.For a similar regioselective addition reaction, see:; Baker R, Castro JL. J Chem Soc, Chem Commun. 1989:378–381. [Google Scholar]
- 12.Mizoguchi H, Micalizio GC. J Am Chem Soc. 2015;137:6624–6628. doi: 10.1021/jacs.5b02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Boeckman RK, Wrobleski ST. J Org Chem. 1996;61:7238–7239. doi: 10.1021/jo961585b. [DOI] [PubMed] [Google Scholar]; (b) Lander PA, Hegedus LS. J Am Chem Soc. 1994;116:8126–8132. [Google Scholar]; (c) Fleming I, Taddei M. Synthesis. 1985;898 [Google Scholar]
- 14.(a) Beckwith ALJ, Pigou PE. Aust J Chem. 1986;39:77–87. [Google Scholar]; (b) Singh R, Singh GC, Ghosh SK. Eur J Org Chem. 2007:5376–5385. [Google Scholar]; (c) Kumamoto H, Takahashi N, Shimamura T, Tanaka H, Nakamura KT, Hamasaki T, Baba M, Abe H, Yano M, Kato N. Tetrahedron. 2008;64:1494–1505. [Google Scholar]
- 15.(a) Yaji K, Shindo M. Tetrahedron. 2010;66:9808–9813. [Google Scholar]; (b) Ohyoshi T, Funakubo S, Miyazawa Y, Niida K, Hayakawa I, Kigoshi H. Angew Chem Int Ed. 2012;51:4972–4975. doi: 10.1002/anie.201201383. [DOI] [PubMed] [Google Scholar]; (c) Caldwell JJ, Colman R, Kerr WJ, Magennis EJ. Synlett. 2001:1428–1430. [Google Scholar]
- 16.Baldwin JE. J Chem Soc, Chem Commun. 1976:734–736. [Google Scholar]
- 17.For examples of the use of isotope effects in synthesis, see:; (a) Clive DLJ, Cantin C, Khodabocus A, Kong X, Tao Y. Tetrahedron. 1993;49:7917–1930. [Google Scholar]; (b) Vedejs E, Little J. J Am Chem Soc. 2002;124:748–749. doi: 10.1021/ja0120835. [DOI] [PubMed] [Google Scholar]; (c) Dudley GB, Danishefsky SJ, Sukenick G. Tetrahedron Lett. 2002;43:5605–5606. [Google Scholar]; (d) Miyashita M, Sasaki M, Hattori I, Sakai M, Tanino K. Science. 2004;305:495–499. doi: 10.1126/science.1098851. [DOI] [PubMed] [Google Scholar]
- 18.(a) Davis FA, Nadir Upender K, Kluger EW. J Chem Soc, Chem Commun. 1977:25–26. [Google Scholar]; (b) Davis FA, Lamendola J, Nadir U, Kluger EW, Sedergran TC, Panunto TW, Billmers R, Jenkins R, Turchi IJ, Watson WH, Chen JS, Kimura M. J Am Chem Soc. 1980;102:2000–2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


