Abstract
Purpose
Although dose-intensive strategies or high-dose therapy induction followed by autologous stem-cell transplantation have improved the outcome for patients with mantle-cell lymphoma (MCL), most eventually relapse and subsequently respond poorly to additional therapy. Bortezomib (in the United States) and temsirolimus (in Europe) are currently the only two treatments approved for relapsed disease. Lenalidomide is an immunomodulatory agent with proven tumoricidal and antiproliferative activity in MCL. The MCL-001 (EMERGE) trial is a global, multicenter phase II study examining the safety and efficacy of lenalidomide in patients who had relapsed or were refractory to bortezomib.
Patients and Methods
Lenalidomide 25 mg orally was administered on days 1 through 21 every 28 days until disease progression or intolerance. Primary end points were overall response rate (ORR) and duration of response (DOR); secondary end points included complete response (CR) rate, progression-free survival (PFS), overall survival (OS), and safety.
Results
In all, 134 patients were enrolled with a median age of 67 years and a median of four prior therapies (range, two to 10 prior therapies). The ORR was 28% (7.5% CR/CR unconfirmed) with rapid time to response (median, 2.2 months) and a median DOR of 16.6 months (95% CI, 7.7 to 26.7 months). Median PFS was 4.0 months (95% CI, 3.6 to 5.6 months), and median OS was 19.0 months (95% CI, 12.5 to 23.9 months). The most common grade 3 to 4 adverse events were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (8%), and fatigue (7%).
Conclusion
The MCL-001 study demonstrated durable efficacy of lenalidomide with a predictable safety profile in heavily pretreated patients with MCL who had all relapsed or progressed after or were refractory to bortezomib.
INTRODUCTION
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL),1 accounting for 3% to 6% of NHL.2–4 Median age at diagnosis is mid to late 60s and patients typically present with advanced-stage disease.2,5–7 Although overall survival (OS) has improved over the last two decades, MCL remains challenging, especially in the relapsed/refractory setting in which median OS is approximately 1 to 2 years with current therapies.8–10
Combination chemotherapy and immunotherapy is the foundation of first-line MCL treatment and, when feasible, dose-intensive/induction strategies followed by high-dose therapy and autologous stem-cell transplantation (HDT-ASCT) consolidation have improved outcomes.8,11–15 Alternative options in older patients or those with comorbidities include less intensive strategies (eg, bendamustine plus rituximab), some of which may incorporate maintenance strategies to improve duration of disease control.9,16–18 Following relapse, there are limited options, with minimal benefit from standard chemotherapy or HDT-ASCT, because patients often become chemotherapy resistant.13,19,20 Two therapeutic agents are currently approved in the relapsed/refractory setting: bortezomib (a proteasome inhibitor; United States) and temsirolimus (a mammalian target of rapamycin complex 1 inhibitor; Europe).21,22 Both are limited by intravenous administration and short duration of response (DOR),23–27 substantiating the need for novel alternatives for these patients.
Lenalidomide (Revlimid; Celgene, Summit, NJ) is an immunomodulatory agent initially studied in multiple myeloma and myelodysplastic syndromes.28–31 Preclinical studies showed antitumor and antiproliferative activities in leukemia and lymphoma, including MCL.32–34 Two phase II studies (NHL-002 and NHL-003) reported clinical activity of lenalidomide in heavily pretreated patients with relapsed/refractory aggressive NHL,35,36 including MCL.37,38 With a similar dosing schema (25 mg per day orally for 21 of 28 days), responses were consistent between studies, including 35% overall response rate (ORR) for both (12% to 13% complete response [CR]), median DOR of 6.2 months (NHL-002) and 10.6 months (NHL-003), and median progression-free survival (PFS) of 4.0 months (NHL-002) and 3.7 months (NHL-003) across all histologies.35,36 Interestingly, higher and more durable responses were seen in MCL versus other NHL subtypes. Patients with MCL in NHL-002 showed 53% ORR (20% CR), median DOR of 13.7 months, and median PFS of 5.6 months.36 Central review in the NHL-003 study showed 35% ORR (12% CR/ CR unconfirmed [CRu]), median DOR of 16.3 months, and median PFS of 8.8 months.38 Responses were independent of baseline characteristics or prior therapies; most common grade 3 to 4 adverse events (AEs) for patients with MCL in NHL-002 and NHL-003 were neutropenia (40% and 46%) and thrombocytopenia (33% and 30%), respectively.37,38 On the basis of these encouraging results and limited treatment options in relapsed/refractory MCL, the MCL-001 (EMERGE) phase II study was designed to examine the safety and efficacy of single-agent lenalidomide in heavily pretreated patients who had relapsed, progressed, or were refractory to bortezomib.
PATIENTS AND METHODS
Patients
The institutional review board or independent ethics committee at each participating institution reviewed and approved the study protocol, amendments, and patient's written informed consent before study initiation. Study design and conduct were in accordance with ethical principles of Good Clinical Practice according to International Conference on Harmonization Harmonized Tripartite Guidelines and the Declaration of Helsinki.
Key inclusion criteria were confirmed MCL diagnosis with cyclin-D1 overexpression by immunohistochemistry or t(11;14)(q13;q32) translocation by fluorescent in situ hybridization, age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance score 0 to 2, absolute neutrophil count ≥ 1,500/μL, platelets ≥ 60,000/μL, and adequate organ function. Diagnosis criteria included measurable lesion (≥ 2 cm by computed tomography [CT]). Patients were required to have prior anthracycline or mitoxantrone, cyclophosphamide, or rituximab therapy and documented relapsed, refractory, or progressive disease (PD) following bortezomib (alone or in combination). The definition of relapse was within 1 year of the last dose of bortezomib and following an initial CR to a bortezomib-containing regimen. Refractory to bortezomib was defined as PD without achieving at least a partial response (PR) during treatment after at least two cycles of a bortezomib-containing regimen. PD was within 1 year of the last dose of bortezomib after achieving a PR to a bortezomib-containing regimen. Patients who relapsed after HDT-ASCT were eligible, and there was no limitation for the number of prior therapies.
Key exclusion criteria included the presence of CNS disease, creatinine clearance (CrCl) < 30 mL/min, eligibility for HDT-ASCT or allogeneic stem-cell transplantation per investigator decision, corticosteroids ≤ 1 week (> 10 mg per day prednisone or equivalent), unwillingness to receive contraception or prophylaxis for deep vein thrombosis, desquamating rash with prior thalidomide, prior exposure to lenalidomide, chemotherapy ≤ 2 weeks, nitrosourea ≤ 6 weeks, monoclonal antibody ≤ 8 weeks, radioimmunoconjugate ≤ 12 weeks, or external radiotherapy ≤ 3 weeks.
Study Design
MCL-001 (EMERGE; NCT00737529) was a global, multicenter, single-arm, open-label phase II study of safety and efficacy of single-agent lenalidomide in patients who had relapsed, progressed, or were refractory to bortezomib. Primary end points were ORR and DOR; secondary end points included safety, CR/CRu, time to response (TTR), time to progression (TTP), time to treatment failure (TTF), PFS, and OS.
Lenalidomide 25 mg (10 mg for CrCl ≥ 30 to < 60 mL/min) was self-administered orally on days 1 through 21 of each 28-day cycle until PD, intolerance, or voluntary withdrawal. Dosing was based on prior NHL studies (including MCL)35–37 and approved dosing in multiple myeloma.39
Dose modification/interruption was planned in the event of grade ≥ 2 allergic reaction or hypersensitivity; > 3× upper limit of normal AST, ALT, or bilirubin; grade I or higher tumor lysis syndrome (TLS; by Cairo-Bishop grading system40); sustained grade ≥ 3 neutropenia for ≥ 7 days or associated with fever (≥ 38.5°C); thrombocytopenia (platelets < 50,000/μL); constipation; desquamating (blistering) rash (or grade 4 nondesquamating rash); venous thrombosis/embolism; new peripheral neuropathy; tumor flare reaction (TFR); or lenalidomide-related nonhematologic AE. Allopurinol 300 mg per day or equivalent was recommended for TLS prophylaxis with oral hydration during the first 7 days of treatment (or as indicated). Patients at high-risk for developing a thromboembolic event (TEE; defined as a history of TEE and/or concomitant medication with increased risk and/or known hypercoagulable state regardless of thromboembolic history) received prophylaxis (eg, aspirin 70 to 100 mg per day, low-molecular-weight heparin [LMWH], or warfarin, per investigator). Growth factors were not administered as prophylaxis but were allowed to treat severe hematologic events. Concomitant anticancer therapy was prohibited, although physiologic doses of steroids (≤ 10 mg per day) not prescribed for MCL were permitted.
Response and Safety Assessments
Safety assessments included AEs, pregnancy tests for females of childbearing age, second primary malignancies (SPMs), TLS, and TFR, hematology, serum chemistry, and other laboratory tests. CT scans were performed every two cycles (± 7 days) throughout treatment and every 90 days (± 14 days) after stopping lenalidomide until progression or initiation of subsequent antilymphoma therapy. Confirmatory bone marrow aspirate and unilateral biopsy was required within 28 days for patients achieving CR (by CT).
Efficacy analyses were performed in the intent-to-treat patient population as defined in the protocol. Response data were evaluated by investigators and an independent review committee (ie, central review) per modified International Workshop Lymphoma Response Criteria.24,41,42 Central reviewers prospectively reviewed efficacy data to provide an objective, unbiased independent review of clinical outcomes blinded to institution information, demographic information, and investigator assessments. Central reviewers consisted of four experts in radiology and hematology/oncology. Two radiologists first evaluated medical imaging data in a blinded independent radiology review, with adjudication by a third radiologist as needed, followed by an independent overall hematologist/oncologist review of radiology results in conjunction with pertinent clinical data to determine response. Central reviewers provided the primary efficacy results for this study.
Statistical Analyses
Primary efficacy end points were evaluated following six cycles (± 1 month) of lenalidomide or on treatment discontinuation. Patients discontinuing before achieving a response or who switched to another therapy were considered nonresponders. Response rates were calculated with two-sided exact 95% CIs, with a requirement of > 15% responders to validate efficacy. Waterfall plots were evaluated for patients with baseline and postbaseline lesion assessments for a maximum percentage change from baseline in tumor burden for target lesions. DOR was calculated from the day of first response (of PR or better) to PD or last tumor assessment. The Kaplan-Meier product limit method estimated the survivorship function for all time-to-event end points (eg, DOR, PFS, OS) with median estimates and two-sided 95% CIs. Censoring rules followed regulatory guidance and were prespecified before database lock. AEs were assessed according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Exploratory subgroup analyses included ORR and DOR assessments per baseline demographics and prior therapies. Multivariate logistic regression models evaluated possible baseline and prognostic factors predictive of response. Results were reported with a cutoff date of July 2, 2012, with continued follow-up until 70% patients had died or to a maximum of 4 years from last patient enrollment. All P values reported were two-sided.
RESULTS
Patient Characteristics
From January 2009 to July 2012, 134 patients at 45 study sites worldwide received one or more doses of lenalidomide. Median age was 67 years and 63% of patients were age 65 years or older (Table 1). Almost all patients (93%) had stage III to IV MCL, 57% had high tumor burden, 33% had bulky disease, and one-third had prior HDT-ASCT. In addition to study-required prior therapies, other prior treatments included vincristine (96%), glucocorticoids (92%), cytarabine (44%), etoposide (40%), bendamustine (25%), and platinum compounds (25%).
Table 1.
Patient Demographics, Baseline Disease Characteristics at Time of Study Entry, and Prior Antilymphoma Treatment (N = 134)
| Characteristic | No. of Patients | % | Median | Range |
|---|---|---|---|---|
| Age, years | 67 | 43-83 | ||
| ≥ 65 | 85 | 63 | ||
| Male | 108 | 81 | ||
| Stage III to IV | 124 | 93 | ||
| ECOG PS | ||||
| 0-1 | 116 | 87 | ||
| 2 | 18 | 13 | ||
| Moderate-severe renal insufficiency* | 29 | 22 | ||
| Time from original MCL diagnosis to enrollment, years | ||||
| < 3 | 52 | 39 | ||
| ≥ 3 | 82 | 61 | ||
| MIPI score group at enrollment | ||||
| Intermediate | 51 | 38 | ||
| High | 39 | 29 | ||
| Positive bone marrow involvement† | 55 | 41 | ||
| High tumor burden‡ | 77 | 57 | ||
| Bulky disease§ | 44 | 33 | ||
| No. of prior treatment regimens | 4 | 2-10 | ||
| No. of prior systemic antilymphoma therapies | ||||
| 2 | 29 | 22 | ||
| 3 | 34 | 25 | ||
| ≥ 4 | 71 | 53 | ||
| Received prior bortezomib | 134 | 100 | ||
| Refractory to prior bortezomib | 81 | 60 | ||
| Refractory to last therapy | 74 | 55 | ||
| Received prior high-dose or dose-intensive therapy‖ | 44 | 33 | ||
| Received prior bone marrow or autologous stem cell transplantation | 39 | 29 | ||
| Time from last prior systemic antilymphoma therapy, months | 3.1 | 0.3-37.7 | ||
| < 6 | 96 | 72 | ||
| ≥ 6 | 38 | 28 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MCL, mantle cell lymphoma; MIPI, MCL International Prognostic Index.
Moderate renal insufficiency defined as creatinine clearance (CrCl) ≥ 30 and < 60 mL/min; severe renal insufficiency defined as CrCl < 30 mL/min.
Bone marrow involvement was not required per protocol; prior data for bone marrow biopsy and aspirate were collected in 115 evaluable patients.
Defined as at least one lesion ≥ 5 cm in diameter or three or more lesions that were ≥ 3 cm in diameter by central radiology review.
Defined as at least one lesion ≥ 7 cm in diameter by central radiology review.
Includes stem cell transplantation, hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicine, dexamethasone), or R-hyper-CVAD (rituximab plus hyper-CVAD).
Efficacy
ORR by central review was 28% (95% CI, 20% to 36%; Table 2). The CR/CRu rate was 7.5% (95% CI, 4% to 13%). Responders showed a median DOR of 16.6 months (95% CI, 7.7 to 26.7 months; Fig 1), and median duration of CR/CRu of 16.6 months (95% CI, 16.6 months to not reached). At data cutoff, 18 patients had a DOR ≥ 6 months and 10 patients had a DOR ≥ 12 months (maximum DOR, 29.2+ months). Eleven of 39 patients maintained stable disease for ≥ 6 months, including four patients with stable disease for ≥ 12 months. Of note, efficacy results were similar for investigator assessments.
Table 2.
Efficacy Outcomes With Lenalidomide in Patients With Relapsed/Refractory MCL (N = 134)
| Efficacy Parameter | Central Review |
Investigator Review |
||||
|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | |
| ORR | 37 | 28 | 43 | 32 | ||
| CR/CRu | 10 | 7.5 | 22 | 16 | ||
| PR | 27 | 20 | 21 | 16 | ||
| SD | 39 | 29 | 36 | 27 | ||
| PD | 35 | 26 | 43 | 32 | ||
| Missing response assessment* | 23 | 17 | 12 | 9 | ||
| Median DOR, months | 16.6 | 7.7 to 26.7 | 18.5 | 12.8 to 26.7 | ||
| Median duration of CR/CRu, months | 16.6 | 16.6 to N/R | 26.7 | 26.7 to N/R | ||
| Median duration of PR, months | 9.2 | 5.7 to 20.5 | 7.7 | 3.7 to 21.4 | ||
| TTR, months | ||||||
| Median | 2.2 | 2.0 | ||||
| Range | 1.7-13.1 | 1.7-15.9 | ||||
| Time to CR/CRu, months | ||||||
| Median | 3.7 | 5.6 | ||||
| Range | 1.9-29.5 | 1.8-24.2 | ||||
| Median PFS, months | 4.0 | 3.6 to 5.6 | 3.8 | 3.5 to 6.8 | ||
| Median TTP, months | 5.4 | 3.7 to 7.5 | 4.0 | 3.6 to 7.5 | ||
| Median TTF months | 3.8 | 2.3 to 4.5 | 3.8 | 2.3 to 4.5 | ||
| Median OS, months | 19.0 | 12.5 to 23.9 | 19.0 | 12.5 to 23.9 | ||
Abbreviations: CR, complete response; CRu, unconfirmed complete response; DOR, duration of response; MCL, mantle cell lymphoma; ORR, overall response rate; OS, overall survival; N/R, not reached; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; TTF, time to treatment failure; TTP, time to progression; TTR, time to response.
Includes patients without or with incomplete postbaseline response assessment. For these 23 patients, the investigator's assessment for best ORR included 12 with progressive disease, 10 not assessable, and one CR (no identifiable target lesions by the central radiology reviewer who reported this patient as not evaluable, although a single GI [colon] lesion was reported by investigator readings). All 23 patients were included in the centrally reviewed response assessments as nonresponders.
Fig 1.
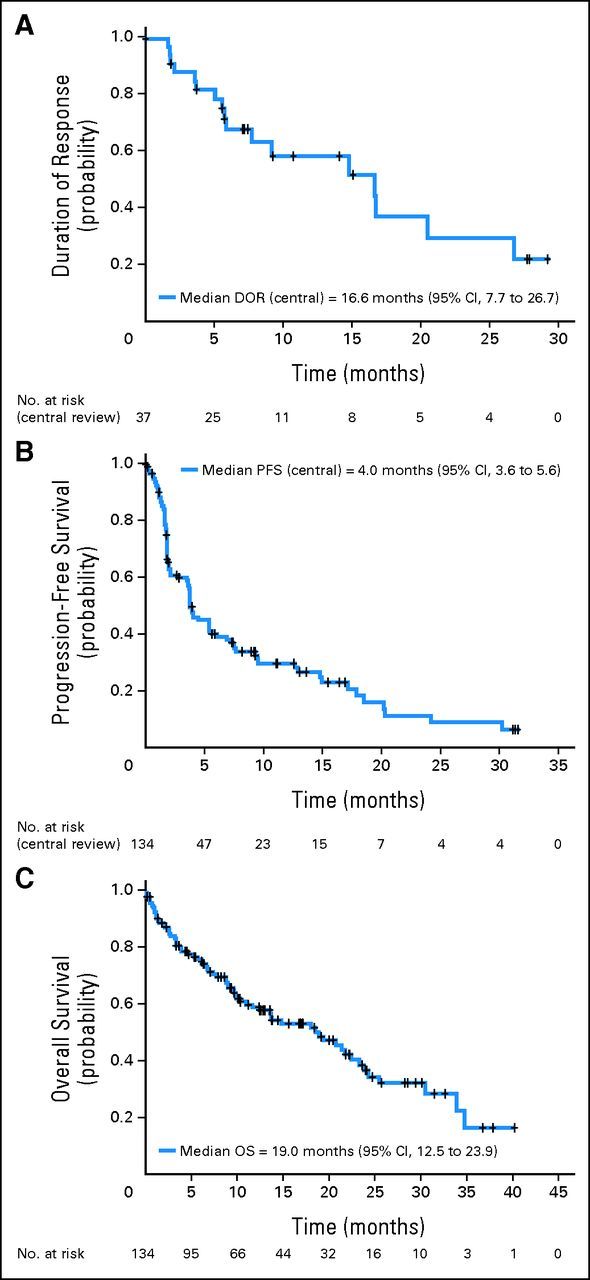
Duration of (A) response (DOR), (B) progression-free survival (PFS), and (C) overall survival (OS) after lenalidomide in relapsed/refractory mantle-cell lymphoma (by central review).
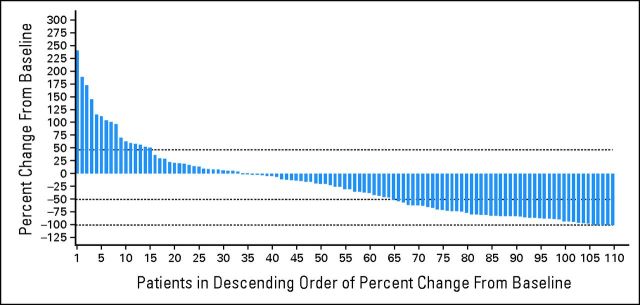
The median TTR was 2.2 months (3.7 months for CR/CRu), with 16 (43%) of 37 responders achieving at least PR by the first assessment (56 ± 7 days). Most responses were reported after two to four cycles of lenalidomide, although in some patients, up to 13 months of treatment was required to achieve best response. Reduction in tumor burden was based on maximum percentage change from baseline for target lesions by central review (Appendix Fig A1, online only). For 111 patients with baseline and postbaseline data available, 77 (69%) experienced a reduction, including 46 (41%) with a ≥ 50% reduction in tumor burden. Efficacy assessments using the waterfall plot calculated reductions in tumor burden that may not have met the stringent criteria for response even though there was a ≥ 50% reduction of all target lesions.
Median PFS was 4.0 months (95% CI, 3.6 to 5.6 months; Table 2 and Fig 1); median TTP and TTF were 5.4 months (95% CI, 3.7 to 7.5 months) and 3.8 months (95% CI, 2.3 to 4.5 months), respectively. With a median follow-up of 9.9 months, median OS was 19.0 months (95% CI, 12.5 to 23.9 months; Fig 1).
At data cutoff, 112 patients (84%) were off treatment and 22 (16%) continued treatment. Sixty-two patients (46%) received subsequent antilymphoma therapy following lenalidomide, with 13% ORR (eight of 62 patients) reported to date. The most common antilymphoma treatment following lenalidomide included rituximab alone (n = 5), rituximab/bendamustine (rituximab/bendamustine ± prednisone; n = 11), rituximab/bendamustine plus other chemotherapy/steroids (n = 12), and radiotherapy (n = 8). Four patients received lenalidomide following study completion (including one patient who discontinued therapy because of lack of PD postbortezomib, one with prolonged treatment delay in lenalidomide due to cytopenia, and two patients who were given lenalidomide as subsequent therapy following PD).
Response by Subgroup Analysis
Lenalidomide showed consistent ORR and DOR across subgroups (Table 3). Multivariate logistic regression analysis (central review) evaluated factors including demographic characteristics, baseline disease characteristics, number of and response to prior therapies, and the starting dose of lenalidomide. The only factor that was significant in both the univariate and multivariate models was high lactate dehydrogenase at baseline.
Table 3.
Summary of Subgroup Analyses of ORR and DOR by Baseline Demographics and Patient Characteristics With Lenalidomide in Evaluable Patients With Relapsed/Refractory MCL (central review)
| Characteristic | Total No. of Patients | ORR |
DOR |
||||
|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | Median | 95% CI | ||
| Median age, years | |||||||
| < 65 | 49 | 15 | 31 | 18 to 45 | 15 | 20.5 | 5.6 to N/A |
| ≥ 65 | 85 | 22 | 26 | 17 to 37 | 22 | 9.2 | 5.8 to 16.7 |
| Sex | |||||||
| Male | 108 | 28 | 26 | 18 to 35 | 28 | 16.7 | 9.2 to N/A |
| Female | 26 | 9 | 35 | 17 to 56 | 9 | 7.7 | 2.1 to 20.5 |
| ECOG PS | |||||||
| 0-1 | 116 | 31 | 27 | 19 to 36 | 31 | 16.7 | 14.8 to N/A |
| 2-4 | 18 | 6 | 33 | 13 to 59 | 6 | 7.7 | 1.7 to 9.2 |
| Renal function | |||||||
| Normal | 99 | 28 | 28 | 20 to 38 | 28 | 20.5 | 5.7 to N/A |
| Moderate insufficiency | 28 | 7 | 25 | 11 to 45 | 7 | 9.2 | 7.7 to 16.6 |
| Time from MCL diagnosis to first dose, years | |||||||
| < 3 | 52 | 12 | 23 | 13 to 37 | 12 | 16.6 | 5.1 to N/A |
| ≥ 3 | 82 | 25 | 31 | 21 to 42 | 25 | 14.8 | 5.8 to 20.5 |
| MCL (Ann Arbor) stage | |||||||
| I or II | 10 | 1 | 10 | 0.3 to 45 | 1 | 7.7 | N/A |
| III or IV | 124 | 36 | 29 | 21 to 38 | 36 | 16.6 | 9.2 to 26.7 |
| MIPI score at enrollment | |||||||
| Low | 39 | 14 | 36 | 21 to 53 | 14 | 20.5 | 5.6 to N/A |
| Intermediate | 51 | 12 | 23 | 13 to 38 | 12 | 16.7 | 5.7 to 26.7 |
| High | 39 | 10 | 26 | 13 to 42 | 10 | 7.7 | 3.6 to N/A |
| LDH | |||||||
| Normal | 84 | 32 | 38 | 28 to 49 | 32 | 16.7 | 14.8 to N/A |
| High | 47 | 5 | 11 | 4 to 23 | 5 | 5.8 | 1.7 to 7.7 |
| WBC count (× 109/L) | |||||||
| < 6.7 | 67 | 22 | 33 | 22 to 45 | 22 | 14.8 | 5.6 to 20.5 |
| 6.7 to < 10 | 41 | 7 | 17 | 7 to 32 | 7 | 26.7 | 7.7 to N/A |
| 10 to < 15 | 9 | 6 | 67 | 30 to 93 | 6 | N/A | 3.6 to N/A |
| ≥ 15 | 12 | 1 | 8 | 0.2 to 39 | 1 | N/A | N/A to N/A |
| Tumor burden | |||||||
| High* | 77 | 22 | 29 | 19 to 40 | 22 | 14.8 | 5.8 to 26.7 |
| Low | 54 | 15 | 28 | 17 to 42 | 15 | 16.6 | 5.6 to 16.6 |
| Bulky disease | |||||||
| Yes† | 44 | 13 | 30 | 17 to 45 | 13 | 14.8 | 5.7 to N/A |
| No | 87 | 24 | 28 | 19 to 38 | 24 | 16.6 | 5.8 to N/A |
| Prior bone marrow involvement‡ | |||||||
| Positive | 55 | 13 | 24 | 13 to 37 | 13 | 9.2 | 3.6 to N/A |
| Negative | 52 | 13 | 25 | 14 to 39 | 13 | 16.7 | 5.1 to N/A |
| Indeterminate | 8 | 4 | 50 | 16 to 84 | 4 | 14.8 | N/A to N/A |
| No. of prior systemic antilymphoma therapies | |||||||
| < 3 | 29 | 9 | 31 | 15 to 51 | 9 | 16.6 | 7.7 to N/A |
| ≥ 3 | 105 | 28 | 27 | 19 to 36 | 28 | 16.7 | 5.7 to 26.7 |
| Received prior stem cell transplantation | |||||||
| Yes | 39 | 12 | 31 | 17 to 48 | 12 | 16.7 | 3.6 to 16.7 |
| No | 95 | 25 | 26 | 18 to 36 | 25 | 14.8 | 5.8 to 26.7 |
| Received prior high-intensity therapy | |||||||
| Yes | 44 | 12 | 27 | 15 to 43 | 12 | 16.7 | 3.6 to 16.7 |
| No | 90 | 25 | 28 | 19 to 38 | 25 | 14.8 | 5.8 to 26.7 |
| Time from last prior systemic antilymphoma therapy, months | |||||||
| < 6 | 96 | 23 | 24 | 16 to 34 | 23 | 7.7 | 3.6 to 26.7 |
| ≥ 6 | 38 | 14 | 37 | 22 to 54 | 14 | 16.7 | 14.8 to N/A |
| Relapsed/refractory to prior bortezomib | |||||||
| Refractory | 81 | 22 | 27 | 18 to 38 | 22 | 20.5 | 7.7 to N/A |
| Relapsed/progressed | 51 | 15 | 29 | 18 to 44 | 15 | 16.6 | 5.1 to 16.7 |
| Relapsed/refractory to last prior therapy | |||||||
| Refractory | 74 | 20 | 27 | 17 to 39 | 20 | 26.7 | 5.6 to N/A |
| Relapsed/progressed | 53 | 16 | 30 | 18 to 44 | 16 | 14.8 | 5.7 to 20.5 |
| Starting dose of lenalidomide, mg | |||||||
| 10 | 29 | 6 | 21 | 8 to 40 | 6 | 9.2 | 7.7 to 14.8 |
| 25 | 104 | 31 | 30 | 21 to 40 | 31 | 16.7 | 5.7 to N/A |
Abbreviations: DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; MCL, mantle-cell lymphoma; MIPI, MCL International Prognostic Index; N/A, not applicable; ORR, overall response rate.
Defined as at least one lesion ≥ 5 cm in diameter or three or more lesions that were ≥ 3 cm in diameter by central radiology review.
Defined as at least one lesion ≥ 7 cm in diameter by central radiology review.
Bone marrow involvement was assessable in 115 evaluable patients.
Safety
The average daily dose of lenalidomide was 20 mg per day (± 6.5 mg per day [standard deviation]) received for a median duration of 95 days (range, 1 to 1,002 days). Fifty-eight percent of patients received three or more cycles of lenalidomide, 40% received six or more cycles, and 19% received 12 or more cycles. Dose interruptions were present in 57% of patients; median time to first dose interruption was 29 days (ie, after one cycle) with a median time to resume lenalidomide of 7 days (range, 1 to 59 days). Dose reductions due to AEs were reported in 51 patients (38%), with a median time to first dose reduction of 57 days (ie, after two cycles). Twenty-six patients (19%) discontinued lenalidomide due to AEs. The most common AEs leading to dose reductions, interruptions, or discontinuations were neutropenia and thrombocytopenia.
Ninety-nine percent of patients experienced at least one AE, including 66% grade ≥ 3 (Table 4). The most common grade ≥ 3 AEs were neutropenia (43%), thrombocytopenia (27%), and anemia (11%). Growth factors were administered to 35 patients (26%) to treat neutropenia. Platelet transfusions were reported in 15 patients (11%) for thrombocytopenia. Six patients were identified with bleeding events (including GI hemorrhage), none related to lenalidomide and one concurrently with thrombocytopenia.
Table 4.
All Treatment-Emergent AEs After Lenalidomide (regardless of attribution) in ≥ 10% of Patients With Relapsed/Refractory MCL (N = 134)
| AE | Any Grade |
Grade 3 |
Grade 4 |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Patients with one or more AEs | 132 | 99 | 47 | 35 | 41 | 31 |
| Hematologic | ||||||
| Neutropenia | 65 | 49 | 26 | 19 | 32 | 24 |
| Thrombocytopenia | 48 | 36 | 23 | 17 | 14 | 10 |
| Anemia | 41 | 31 | 11 | 8 | 4 | 3 |
| Leukopenia | 20 | 15 | 7 | 5 | 2 | 1 |
| Nonhematologic | ||||||
| Fatigue | 45 | 34 | 9 | 7 | 0 | 0 |
| Diarrhea | 42 | 31 | 8 | 6 | 0 | 0 |
| Nausea | 40 | 30 | 0 | 0 | 1 | < 1 |
| Cough | 38 | 28 | 1 | < 1 | 0 | 0 |
| Pyrexia* | 31 | 23 | 1 | < 1 | 1 | < 1 |
| Rash | 30 | 22 | 2 | 1 | 0 | 0 |
| Dyspnea* | 24 | 18 | 6 | 5 | 1 | < 1 |
| Pruritus | 23 | 17 | 1 | < 1 | 0 | 0 |
| Constipation | 21 | 16 | 1 | < 1 | 0 | 0 |
| Peripheral edema | 21 | 16 | 0 | 0 | 0 | 0 |
| Pneumonia† | 19 | 14 | 10 | 8 | 0 | 0 |
| Asthenia* | 19 | 14 | 2 | 1 | 1 | < 1 |
| Decreased appetite | 19 | 14 | 1 | < 1 | 0 | 0 |
| Back pain | 18 | 13 | 2 | 1 | 0 | 0 |
| Hypokalemia | 17 | 13 | 2 | 1 | 1 | < 1 |
| Muscle spasms | 17 | 13 | 1 | < 1 | 0 | 0 |
| Upper respiratory tract infection | 17 | 13 | 0 | 0 | 0 | 0 |
| Decreased weight | 17 | 13 | 0 | 0 | 0 | 0 |
| Vomiting | 16 | 12 | 0 | 0 | 1 | < 1 |
Abbreviations: AE, adverse event; MCL, mantle-cell lymphoma.
Denotes one grade 5 event per AE.
Two grade 5 pneumonia events.
Rash was observed in 30 patients (22%), mainly grade 1 to 2 (grade 3 in two patients), and was primarily managed with antihistamines or low-dose steroids. Thirteen patients (10%) experienced grade 1 to 2 TFRs, all occurring in cycle 1 with one additional event in cycle 11. TFRs were managed with steroids, analgesics, or nonsteroidal anti-inflammatory agents without interruption or modification of lenalidomide dosing. There were no reports of TLS. Ten patients (7%) reported venous TEEs (seven grade 3 to 4), including five grade 3 to 4 deep vein thrombosis, three pulmonary embolisms (one grade 2; two grade ≥ 3), and one each with grade 2 thrombophlebitis and grade 2 venous thrombosis. Further analysis indicated that 35 patients (26%) in this study received no prophylaxis; two (6%) of whom developed TEEs. Of the 99 patients who received prophylaxis, eight (8%) of 99 developed TEEs. Of these 99 patients, 81% received aspirin, eight received LMWH, and nine received warfarin. Treatment-related serious AEs were reported in 26 patients (19%); pneumonia was the most common in nine patients (7%), and all others were reported in less than 5% of patients.
At a median 13.4-month follow-up, six SPMs (4.5%) were reported, including three invasive SPMs consisting of one MDS in a patient with prior ASCT and two solid tumors (one metastatic colon cancer; one squamous cell carcinoma of skin metastasized to cervical lymph node) for an overall incidence rate of 2.21 per 100 person-years. Median time to SPM diagnosis from initiation of lenalidomide was 7.3 months (range, 3.1 to 9.7 months). Time to onset was 3.1 months for MDS, 7.3 months for metastatic squamous cell carcinoma, and 9.7 months for colon cancer. Four patients showed noninvasive nonmelanoma skin cancer, including one patient with noninvasive skin carcinoma that progressed to metastatic squamous cell carcinoma. There were no reports of acute lymphocytic leukemia, Hodgkin lymphoma, or diffuse large B-cell lymphoma. Four of six patients with SPMs were alive at data cutoff; two died as a result of PD from MCL.
Eighteen patients (13%) died within 30 days of the last dose of lenalidomide, 14 of 18 as a result of MCL progression. Other causes included one possibly treatment-related toxicity (Pseudomonas aeruginosa and neutropenic sepsis) and one unknown cause of death; deaths unrelated to lenalidomide were one PD/pneumonia and one non-neutropenic sepsis. Of 18 patients who died, nine were ≥ 70 years of age and eight had received one or fewer cycles of lenalidomide (including two of four patients with other causes of death).
DISCUSSION
The MCL-001 trial results demonstrate the efficacy of lenalidomide in a large series of 134 patients with heavily pretreated relapsed/refractory MCL (median of four prior therapies; range, two to 10 prior therapies). Prior bortezomib, cyclophosphamide, anthracyclines, and rituximab had failed all patients with relapsed/refractory MCL.
Consistent with established MCL characteristics, patients were older, most were males, and more than 93% had stage 3/4 disease.5 In addition, more than half had high tumor burden, one third had bulky disease, and three fourths had extranodal disease. All patients received prior bortezomib, including 60% of patients who were refractory to bortezomib. One third of patients had undergone prior HDT-ASCT, and 55% were refractory to last therapy.
The safety profile was consistent with other lenalidomide studies in relapsed/refractory NHL.35–38 The most common AEs were hematologic (primarily neutropenia and thrombocytopenia) and easily manageable with dose modifications and/or supportive treatment. There were no cases of TLS; grade 1 to 2 TFR was reported in 10%, mainly occurring in cycle 1 (with no correlation with efficacy). TEEs were reported in 10 patients (7%), eight of whom received anticoagulant prophylaxis and two of whom did not. Definite conclusions regarding TEE prophylaxis could not be drawn on the basis of the relatively small number of events in this single-arm study. SPMs were reported in six patients, with an overall incidence rate of 2.21/100 person-years; these patients were heavily pretreated and received multiple lines of prior cytotoxic therapy. Age-adjusted incidence rates of newly diagnosed invasive cancer were approximately 2.1/100 person-years for individuals age ≥ 65 years from the US Surveillance, Epidemiology, and End Results (SEER) program.43 Thus, the observed SPM rate here was consistent with the expected background occurrence of new invasive malignancies in this age group.
ORR with lenalidomide was 28% (7.5% CR/CRu), exceeding the prespecified 15% target, with prolonged responses showing median DOR of 16.6 months, including 18 patients who responded for ≥ 6 months and 10 who responded for ≥ 12 months (maximum, 29.2+ months). Response to lenalidomide was rapid (median TTR, 2.2 months); most patients responded by cycle 4, although longer treatment was needed for some patients to achieve best response. Secondary end points showed median PFS of 4.0 months, median TTP of 5.4 months, and median TTF of 3.8 months. In previous studies of the two therapeutic agents approved for relapsed/refractory MCL, temsirolimus was associated with ORR of 22% and DOR of 7.1 months (median, three prior therapies), and bortezomib was associated with ORR of 32% and DOR of 9.2 months (median, one prior therapy).25,27
Lenalidomide demonstrated efficacy in all MCL subgroups, including patients with bulky disease or high tumor burden, those who were refractory to their last therapy, or those for whom prior intensive therapies or HDT had failed, and regardless of the number and type of prior therapies (including seven of 33 responders [two CR/CRu] in patients who had also received prior bendamustine). A lower response rate occurred in patients with increased lactate dehydrogenase, a known adverse prognostic factor for patients with MCL44; consistent response rates and DOR were seen in spite of all other adverse prognostic factors.
Although the MCL-001 study lacked randomization with a comparator arm, key strengths were the large number of patients, multicenter design, heavily pretreated patients with a defined prior therapies requirement, and central review assessment. Ongoing studies evaluating T-cell subsets and natural killer cell changes in patients with MCL and future studies will hopefully help predict which patients will benefit the most from lenalidomide.
In conclusion, results from the MCL-001 trial demonstrated rapid and prolonged efficacy of lenalidomide with a predictable safety profile in heavily pretreated responding patients with MCL who had relapsed or progressed after or were refractory to prior therapies that included bortezomib. These findings support the clinical benefit of oral lenalidomide in heavily pretreated patients with MCL, including those with advanced-stage disease, regardless of tumor burden, and those with multiple prior therapies, including prior ASCT, thus providing evidence of an active treatment in this patient population after bortezomib.
Acknowledgment
Editorial support was provided by Julie Kern, PhD, of Bio Connections, funded by Celgene.
Appendix
Fig A1.
Reduction in tumor burden after lenalidomide in relapsed/refractory mantle-cell lymphoma.
Footnotes
Listen to the podcast by Dr Till at www.jco.org/podcasts
Supported by Celgene.
Presented in part at the 54th American Society of Hematology Meeting and Exposition, Atlanta, GA, December 8-11, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00737529.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Lei Zhang, Celgene (C); Sherri Cicero, Celgene (C); Tommy Fu, Celgene (C) Consultant or Advisory Role: Andre Goy, Celgene (U); Michael E. Williams, Celgene (C); Johannes Drach, Celgene (C); Thomas E. Witzig, Celgene (U) Stock Ownership: Lei Zhang, Celgene; Sherri Cicero, Celgene; Tommy Fu, Celgene Honoraria: Michael E. Williams, Celgene, Data Safety Monitoring Committee member; Johannes Drach, Celgene Research Funding: Andre Goy, Celgene; Michael E. Williams, Celgene; Thomas E. Witzig, Celgene Expert Testimony: Thomas E. Witzig, Celgene (U) Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Andre Goy, Rajni Sinha, Lei Zhang, Sherri Cicero, Tommy Fu, Thomas E. Witzig
Provision of study materials or patients: Andre Goy, Sevgi Kalayoglu Besisik, Thomas E. Witzig
Collection and assembly of data: Andre Goy, Michael E. Williams, Sevgi Kalayoglu Besisik, Johannes Drach, Radhakrishnan Ramchandren, Lei Zhang, Sherri Cicero, Thomas E. Witzig
Data analysis and interpretation: Andre Goy, Michael E. Williams, Radhakrishnan Ramchandren, Lei Zhang, Sherri Cicero, Tommy Fu, Thomas E. Witzig
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Armitage JO. Treatment of non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1023–1030. doi: 10.1056/NEJM199304083281409. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113:791–798. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 3.Turner JJ, Hughes AM, Kricker A, et al. WHO non-Hodgkin's lymphoma classification by criterion-based report review followed by targeted pathology review: An effective strategy for epidemiology studies. Cancer Epidemiol Biomarkers Prev. 2005;14:2213–2219. doi: 10.1158/1055-9965.EPI-05-0358. [DOI] [PubMed] [Google Scholar]
- 4.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma: The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [No authors listed] [PubMed] [Google Scholar]
- 5.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 6.Bosch F, López-Guillermo A, Campo E, et al. Mantle cell lymphoma: Presenting features, response to therapy, and prognostic factors. Cancer. 1998;82:567–575. doi: 10.1002/(sici)1097-0142(19980201)82:3<567::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Tiemann M, Schrader C, Klapper W, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): A clinicopathological study from the European MCL Network. Br J Haematol. 2005;131:29–38. doi: 10.1111/j.1365-2141.2005.05716.x. [DOI] [PubMed] [Google Scholar]
- 8.McKay P, Leach M, Jackson R, et al. Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol. 2012;159:405–426. doi: 10.1111/bjh.12046. [DOI] [PubMed] [Google Scholar]
- 9.Goy A, Kahl B. Mantle cell lymphoma: The promise of new treatment options. Crit Rev Oncol Hematol. 2011;80:69–86. doi: 10.1016/j.critrevonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Vose JM. Mantle cell lymphoma: 2012 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2012;87:604–609. doi: 10.1002/ajh.23176. [DOI] [PubMed] [Google Scholar]
- 11.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 13.Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: An active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–3809. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie DS, Seymour JF, Grigg AP, et al. The hyper-CVAD-rituximab chemotherapy programme followed by high-dose busulfan, melphalan and autologous stem cell transplantation produces excellent event-free survival in patients with previously untreated mantle cell lymphoma. Ann Hematol. 2007;86:101–105. doi: 10.1007/s00277-006-0193-2. [DOI] [PubMed] [Google Scholar]
- 15.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 16.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 17.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 18.Rummel MJ, Niederle N, Maschmerye G, et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent and mantle cell lymphomas (MCL): Updated results from the StiL NHL1 study. J Clin Oncol. 2012;30(suppl):6s. abstr 3. [Google Scholar]
- 19.Evens AM, Winter JN, Hou N, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: Long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. doi: 10.1111/j.1365-2141.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 20.Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millenium Pharmaceuticals. Cambridge, MA: Millennium Pharmaceuticals; 2012. Velcade (bortezomib) for injection prescribing information. [Google Scholar]
- 22.Torisel (temsirolimus) prescribing information. Catania, Italy: Wyeth Lederle; 2012. [Google Scholar]
- 23.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: A phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 25.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: Updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 27.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 28.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 29.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 31.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 32.Qian Z, Zhang L, Cai Z, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res. 2011;35:380–386. doi: 10.1016/j.leukres.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–559. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 35.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 36.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011;22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 37.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 38.Zinzani PL, Vose JM, Czuczman MS, et al. Phase II multicenter study of the safety and efficacy of single-agent lenalidomide in subjects with relapsed/refractory mantle cell lymphoma: Long-term follow-up analysis of the NHL-003 study. 54th American Society of Hematology Annual Meeting and Exposition; December 8-11, 2012; Atlanta, GA. (abstr 2738) [Google Scholar]
- 39.Celgene. Lenalidomide prescribing information. Summit, NJ: Celgene; 2011. [Google Scholar]
- 40.Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 41.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 42.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 43.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 44.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]