Abstract
Purpose
To determine the maximum-tolerated dose (MTD) and assess the safety, pharmacokinetics, and preliminary evidence of antitumor activity of YM155, a small-molecule inhibitor of survivin.
Patients and Methods
Patients with advanced solid malignancies or lymphoma were treated with escalating doses of YM155 administered by 168-hour continuous intravenous infusion (CIVI). Plasma and urine samples were assayed to determine pharmacokinetic parameters and excretion.
Results
Forty-one patients received 127 cycles of YM155 at doses ranging from 1.8 to 6.0 mg/m2/d by 168-hour CIVI every 3 weeks. Overall, the most common grade 1 to 2 toxicities were stomatitis, pyrexia, and nausea, whereas grade 3 and 4 toxicities were rare. Reversible elevation in serum creatinine in two patients, with one developing acute tubular necrosis, was dose-limiting at 6.0 mg/m2. The MTD was 4.8 mg/m2. At the MTD, the mean steady-state concentration, clearance, volume of distribution at steady-state, and terminal elimination half-life were 7.7 ng/mL, 47.7 L/h, 1,763 L, and 26 hours, respectively. One complete and two partial responses lasting 8, 24+ and 48+ months occurred in three patients with non-Hodgkin's lymphoma, two patients with hormone- and docetaxel-refractory prostate cancer had prostate-specific antigen responses, and one patient with non–small-cell lung cancer had a minor response.
Conclusion
YM155 can be administered safely at 4.8 mg/m2/d 168 hours CIVI every 3 weeks. The absence of severe toxicities, attainment of plasma concentrations active in preclinical models, and compelling antitumor activity warrant further disease-directed studies of this agent alone and in combination with chemotherapy in a broad array of tumors.
INTRODUCTION
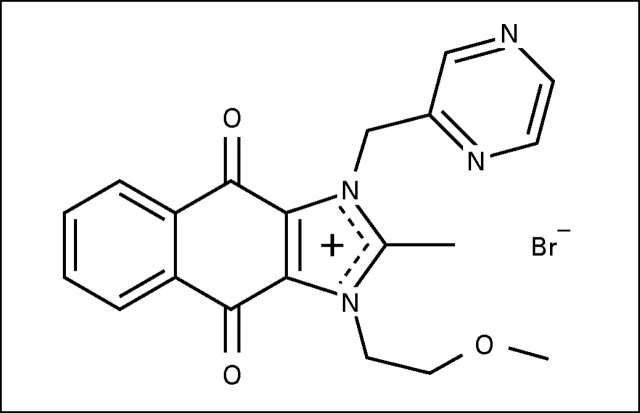
YM155 (1-(2-Methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide) is a small-molecule inhibitor of the antiapoptosis protein survivin (Appendix Fig A1, online only). YM155 was selected as a specific inhibitor of survivin expression from high through-put screening using a survivin promoter luciferase assay.
Apoptosis is a tightly regulated process, with both pre-, post-, and nonmitochondrial events resulting in caspase activation that degrades critical proteins required for viability. Survivin, a microtubule-associated protein and a member of the inhibitor of apoptosis family of proteins (IAP), abrogates cell death signaling by binding to the caspase-associated recruitment domain of the second mitochondrial activator of caspases (SMAC), sequestering it from interactions with other IAP family members.1-3 Following an apoptotic signal, the mitochondrial membrane undergoes depolarization that results in release of cytochrome C, adenosine triphosphate, and SMAC. Cytochrome C results in the formation of the apoptosome and downstream activation of caspases that methodically degrade proteins. As a final regulatory step, caspases are inhibited by the presence of IAP members (eg, XIAP, cIAP1, cIAP2, NAIP, and livin). SMAC functions by binding to the caspase-associated recruitment domains of IAPs, preventing them from interacting with and inhibiting caspases. Survivin, by binding to and sequestering SMAC, diminishes SMAC/IAP family member interaction, and with IAPs no longer restrained, caspase activation and apoptosis are inhibited.4-6
Survivin is expressed in the majority of human malignancies.7 In contrast, survivin is not expressed in most normal adult tissues, the exceptions being CD34+ cells, placenta, thymus, and some cells within the basal crypt layer of the GI tract. The presence of survivin expression is a negative prognostic feature in many malignancies, predicting early recurrence and death.8-13
In preclinical experiments, YM155 inhibited survivin mRNA and protein expression in a dose- and time-dependent manner, resulting in activation of caspases and apoptosis induction in a broad array of human tumor cell lines.14 YM155 induced tumor regressions in mice-bearing established human tumor xenografts (PC3 [prostate], RL [non-Hodgkin's lymphoma], and CALU6 and NCI-H358 [lung] tumors).14 Furthermore, YM155 enhanced the antitumor activity of cytotoxic agents in several cell lines (data on file, Astellas, Deerfield, IL).
YM155 demonstrated preclinical schedule-dependent antitumor activity, with 3- and 7-day continuous infusion schedules superior to intravenous daily bolus or intermittent schedules.14 Preclinical pharmacokinetic evaluation of YM155 demonstrated a brief elimination half-life in mice, rapid distribution to tissues, and a 20-fold increase in tumor tissue concentrations as compared with plasma.
Toxicologic evaluation in rats and dogs demonstrated hematologic and GI toxicities and reversible acute renal tubular necrosis at the highest doses examined and at plasma steady-state concentrations (Css) of 12 to 16 ng/mL in dogs.
The impetus for the clinical development of YM155 included the critical role and differential expression of the target, survivin, and the aforementioned safety and antitumor activity of YM155. The principal objectives of this phase I, first in human, and pharmacokinetic study of YM155 were to determine (1) the maximum-tolerated dose (MTD) administered over 168 hours by continuos intravenous infusion (CIVI), (2) the toxicities, (3) the pharmacokinetic behavior, and (4) preliminary evidence of anticancer activity in patients with advanced solid malignancies.
PATIENTS AND METHODS
Patient Selection
Eligible patients had pathologically confirmed solid malignancies refractory to standard therapy or for whom no standard therapy existed; age ≥ 18 years; life expectancy ≥ 12 weeks; an Eastern Cooperative Oncology Group performance status of 0 to 2; previous chemotherapy ≥ 4 weeks (6 weeks for prior mitomycin or a nitrosourea); hemoglobin ≥ 9 g/dL; absolute neutrophil count (ANC) ≥ 1,500/μL; platelets ≥ 100,000/μL; creatinine ≤ upper limit of normal (ULN) and calculated creatinine clearance more than 60 mL/min, bilirubin ≤ 1.5× ULN; AST and ALT ≤ 2.5× ULN; absence of pregnancy, HIV and hepatitis B/C sero-positivity, or brain metastases; and no coexisting severe medical conditions. Patients gave written informed consent according to federal and institutional guidelines before treatment.
Drug Administration
YM155 was administered by CIVI over 168 hours every 21 days, and treatment cycles were repeated in patients who met re-treatment criteria. From the starting dose of 1.8 mg/m2/d, subsequent dose levels were increased using standard Modified Fibonacci scheme. Initially, the cohorts included three patients. If one patient experienced dose-limiting toxicity (DLT), the cohort was expanded to six patients. The MTD was defined during cycle 1 as the highest dose at which fewer than 33% of patients experienced treatment-related DLT. DLT was defined as any grade 3 or 4 nonhematologic toxicity (including grade ≥ 3 nausea, vomiting, or diarrhea despite optimal prophylaxis), grade 4 thrombocytopenia, and grade 4 neutropenia lasting more than 4 days or accompanied by fever, or treatment delays more than 2 weeks owing to failure to meet re-treatment criteria. Toxicity was graded according to the National Cancer Institute's Common Toxicity Criteria, version 3. Patients experiencing DLT were treated at the next lowest dose level demonstrated to be safe or were discontinued from treatment.
YM155 was supplied in vials containing 30 mg of YM155 in 3-mL lactic acid-based buffer (pH3.6) by Astellas Pharma US Inc (Deerfield, IL). Each admixture was prepared in a controlled light and temperature environment with 5% dextrose as a diluent.
YM155 was administered via an indwelling intravenous catheter using polyethylene-lined tubes and polypropylene syringes, both light protected, for 168 hours via a programmable syringe pump. Syringes were exchanged every 24 hours.
Pretreatment and Follow-Up Studies
A complete history, physical examination, and routine laboratory studies, including CBC, WBC, chemistry including electrolytes, BUN, creatinine, uric acid, glucose, alkaline phosphatase, lactate dehydrogenase, ALT, AST, total bilirubin, calcium, total protein, albumin, cholesterol, triglycerides, creatine phosphokinase, HIV and hepatitis B/C serologic studies, ECG, relevant radiologic studies, and tumor markers and urinalysis were performed pretreatment. During the study, radiologic studies for disease status were repeated after every other cycle. Response was assessed by Response Evaluation Criteria in Solid Tumors.
In all cycles, patients were observed in the inpatient setting for 10 days after initiation of treatment. Vital signs were monitored 1, 10, and 30 minutes after beginning of infusion (BOI); 1, 4, 12, 24, 48, 72, 96, 120, 144, and 168 hours after BOI; and 1, 24, and 48 hours after the end of infusion (EOI). In the first two cycles, serial ECG and creatine phosphokinase assessments were performed on days 1, 3, and 5 and EOI. Urinalysis was performed on days 1 through 7 and EOI. Laboratory assessments (CBC and chemistry) were also performed on days 1 through 10 and on day 15 of all cycles.
Plasma and Urine Pharmacokinetic Sampling and Assay
On cycle 1 and 2, blood samples were collected into heparinized tubes immediately before infusion and at 15 and 30 minutes and at 1, 2, 3, 4, 6, 12, 24, 48, 72, 96, 120, 144, and 168 hours after BOI, and then at 15 and 30 minutes and 1, 2, 3, 4, 6, 12, 24, and 48 hours after EOI. Blood samples were centrifuged at 700× g for 10 minutes immediately after collection, and the plasma was stored frozen at −70°C until assayed. Urine samples were collected pretreatment and then relative to the start of infusion 0 to 6, 6 to 12, 12 to 24, 24 to 48, 48 to 72, 72 to 96, 96 to 120, 120 to 144, and 144 to 168 hours, then 168 to 174, 174 to 180, 180 to 192, and 192 to 216 hours.
For plasma, 500-μL study samples were pretreated by protein precipitation followed by solid phase extraction. The sample and the internal standard were eluted with 400 μL of methanol, and 100 μL of 100 mmol/L formate buffer (pH = 4.8) was added. For urine, 100-μL study samples were diluted followed by solid phase extraction eluted with 400 μL of acetonitrile, and 400 μL of 100 mmol/L formate buffer (pH = 4.8) was added.
The mixture (20 μL) was injected onto a reverse-phase high-performance liquid chromatography column (Thermo Aquasil C18, 3 micron, 2 × 100 mm; Thermo Fisher Scientific, Waltham, MA). Separation occurred using gradient elution, with mobile phases consisting of 100 mmol/L of ammonium formate buffer (pH = 4.8), water, and acetonitrile. The peaks were identified using single reaction monitoring on a TSQ 7000 mass spectrometer (Thermo Fisher Scientific) equipped with electrospray operating in positive ion mode. Peak area ratios (compound/internal standard) were fitted to a weighted (1/concentration2) least-squares linear regression analysis to calculate the line of best-fit from the data. The linear ranges for YM155 are 0.5 to 50 ng/mL and 1.0 to 500 ng/mL for plasma and urine, respectively. YM155 concentrations are expressed as the concentrations of the cationic moiety in nanograms per milliliter.
Pharmacokinetic and Pharmacodynamic Analyses
Individual plasma YM155 concentration-time profiles for each patient were analyzed individually, and noncompartmental analysis was used to determine pharmacokinetic parameters. Dose proportionality was assessed using a one-way analysis of variance at α = 0.05 significance level, with Bonferroni's multiple comparison for post tests.
The relationships between toxicities and YM155 dose and pharmacokinetic parameters area under the plasma concentration-time curve (AUC), peak plasma concentration observed, and Css were explored.
RESULTS
General
Forty-one patients, whose pertinent characteristics are listed in Table 1, received a total of 127 cycles of YM155 at doses ranging from 1.8 to 6.0 mg/m2/d for 168 hours. The total number of new patients treated and cycles at each dose level, as well as the overall dose-escalation scheme, are listed in Table 2. The median number of cycles administered per patient was three(range, one to 16+ cycles). Doses were reduced for DLT in three patients on four occasions.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients |
|---|---|
| No. of patients | 41 |
| No. of courses/patient | |
| Median | 3 |
| Range | 1-16+ |
| Age, years | |
| Median | 61 |
| Range | 29-78 |
| Performance status | |
| 0 | 9 |
| 1 | 30 |
| 2 | 2 |
| Sex | |
| Male | 31 |
| Female | 10 |
| Previous therapy | |
| Chemotherapy | 41 |
| Radiation | 26 |
| No. of prior chemotherapy regimens | |
| Median | 5 |
| Range | 2-17 |
| Tumor type | |
| Prostate | 9 |
| Colorectal | 5 |
| Non-Hodgkin's lymphoma | 5 |
| Head and neck | 1 |
| Sarcoma | 4 |
| Breast | 2 |
| Liver | 2 |
| NSCLC | 2 |
| Melanoma | 2 |
| Ovarian | 2 |
| Small-cell lung, endometrial, thyroid, esophageal, pancreatic, renal, thymus | 1 each |
Abbreviation: NSCLC, non–small-cell lung cancer.
Table 2.
Dose-Escalation Scheme
| YM155 Dose Level (mg/m2/d) | No. of Patients |
No. of Courses | Patients With DLT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| New | Modified To Level* | Total | First Course | All Courses | |||||
| 1.8 | 8 | 0 | 8 | 15 | 1/8 | 1/8 | |||
| 3.6 | 6 | 1 | 7 | 12 | 0/6 | 0/7 | |||
| 4.8 | 25 | 1 | 26 | 98 | 2/25 | 2/26 | |||
| 6.0 | 2 | 0 | 2 | 2 | 2/2 | 2/2 | |||
| Total | 41 | 127† | |||||||
Abbreviation: DLT, dose-limiting toxicity.
Patients whose doses were reduced to the next lowest dose for DLT.
Before extension study. One patient transferred to an extension study remains on treatment > 24 months with partial response.
At the first dose level of 1.8 mg/m2/d, one patient with hepatocellular carcinoma and baseline grade 1 AST and ALT elevation developed grade 3 transaminase elevation, prompting expansion of this dose level. Because two additional patients were not assessable because of YM155 infusion interruption (one patient experienced pulmonary embolism and one patient had central line sepsis), a total of eight patients were entered onto this dose level, and no further evidence of DLT was observed. At the 3.6 mg/m2/d dose level, three patients were treated initially without a DLT and one patient was entered at the next highest dose level of 6.0 mg/m2/d. At this dose on day 3, the patient's serum creatinine level increased, and despite saline loading, the patient experienced oliguric acute tubular necrosis, accompanied by grade 4 neutropenia and grade 3 stomatitis, requiring discontinuation of the infusion on day 5. These adverse events were reversible, although the patient required transient hemodialysis. Given the severity of this toxicity, three additional patients were entered at the previous dose level (3.6 mg/m2/d) without further DLT, and the protocol was amended to include an intermediate dose level of 4.8 mg/m2/d. Once six patients were treated without DLT at 4.8 mg/m2, one additional patient was entered at the 6.0 mg/m2/d dose level; however, increasing serum creatinine and severe stomatitis led to discontinuation on day 5 and precluded further dose escalation. Additional patients were entered at the 4.8 mg/m2/d, with two of 25 patients experiencing DLT (febrile grade 4 neutropenia and grade 3 stomatitis). The MTD for YM155 was therefore 4.8 mg/m2/d for 168 hours CIVI.
Adverse Events
Nonhematologic toxicities.
The principal toxicities of YM155 were transient reversible stomatitis (22%), pyrexia (19.5%), nausea (14.6%), and arthralgias (12.2%). Although reversible elevation in serum creatinine was the DLT, these cases occurred only at the highest dose level. The distributions of related toxicities as a function of dose are listed in Table 3. The onset of the related adverse events, including elevations in creatinine, were typically within 3 to 5 days of the start of infusion and recovered by day 15 in all patients.
Table 3.
Adverse Events (≥ 5% incidence overall) Related to YM155
| Adverse Event | No. of Patients Experiencing Toxicity During the First Course/All Courses by Grade |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.8 mg/m2/d (n = 8) |
3.6 mg/m2/d (n = 6) |
4.8 mg/m2/d (n = 25) |
6.0 mg/m2/d (n = 2) |
||||||||||||||||||||
| 1/2 | 3 | 4 | 1/2 | 3 | 4 | 1/2 | 3 | 4 | 1/2 | 3 | 4 | ||||||||||||
| Stomatitis* | 2/2 | 0 | 0 | 0 | 0 | 0 | 4/6 | 1/1 | 0 | 0/1 | 1/1 | 0 | |||||||||||
| Pyrexia | 1/2 | 0 | 0 | 0 | 0 | 0 | 3/5 | 0 | 0 | 1/1 | 0 | 0 | |||||||||||
| Nausea | 2/3 | 0 | 0 | 0/1 | 0 | 0 | 1/2 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Vomiting | 1/1 | 0 | 0 | 1/1 | 0 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 2/3 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Diarrhea | 0 | 0 | 0 | 1/1 | 0 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Arthralgia | 0 | 0 | 0 | 1/1 | 0 | 0 | 1/3 | 0 | 0 | 0/1 | 0 | 0 | |||||||||||
| Constipation | 0 | 0 | 0 | 1/1 | 0 | 0 | 1/2 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Increased transaminase | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | 1/1 | 0 | 0 | 0 | 0 | |||||||||||
| Increased creatinine† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2/2 | 0 | 0 | |||||||||||
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1/1 | 0 | 0 | 1/1 | |||||||||||
| Thrombocytopenia | 0 | 0 | 0 | 0/1 | 0 | 0 | 1/1 | 0 | 0 | 0 | 0 | 0 | |||||||||||
Stomatitis includes categories of mucosal inflammation, mucositis, and stomatitis.
One patient had oliguric renal failure requiring dialysis but met criteria for grade 2 increase in creatinine.
Hematologic toxicity.
Clinically significant effects on ANC or platelets were rare and only occurred at the two 3.6 and 6.0 mg/m2 dose levels. Only two patients had significant changes in ANC values during cycle 1, with the aforementioned patient at 6.0 mg/m2/d who had grade 4 neutropenia (and again on cycle 4 [grade 3] at the reduced dose of 3.6 mg/m2/d). One patient (3.6 mg/m2/d) with breast cancer bone metastases had transient grade 3 thrombocytopenia on cycle 3 and was discontinued for progressive disease.
Pharmacokinetic and Pharmacodynamic Analysis
All patients had measurable plasma YM155 concentrations, with the pharmacokinetic parameters listed in Table 4 and concentration-time profiles illustrated in Figure 1. There was marked interpatient variability across all dose levels. Both patients treated at the 6.0 mg/m2/d dose had YM155 infusions interrupted because of declining renal function, and because YM155 excretion is largely renal, the estimates of YM155 clearance, terminal elimination half-life, and Css at this dose level were potentially distorted. Values for Css and AUC0-t increased in a dose-proportional manner (Fig 2), whereas clearance was independent of dose over the range of 1.8 to 4.8 mg/m2/d.
Table 4.
Mean and Median Non-Compartmental Pharmacokinetic Parameters of YM155 Cycle 1
| Dose Level (mg/m2/d) | No. Assessable |
Cmax (ng/mL) |
AUC0-∞ (ng/mL×hr) |
T1/2 (hours) |
CL (L/h) |
Vdss (L) |
Css (ng/mL) |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Cycles | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | ||||||||||||||
| 1.8 | 8 | 13 | 10.1 | 9.8 | 5.5 | 968 | 521 | 843 | 21.9 | 10.4 | 20.4 | 27.6 | 12.5 | 24.6 | 1,020 | 859 | 691 | 4.7 | 1.7 | 5.1 | |||||||||||||
| 3.6 | 7 | 13 | 8.5 | 1.7 | 8.3 | 1,116 | 223 | 1,106 | 19.6 | 5.0 | 19.8 | 46.3 | 10.5 | 42.9 | 1,296 | 337 | 1,406 | 6.4 | 1.5 | 6.2 | |||||||||||||
| 4.8 | 25 | 46 | 10.7 | 4.8 | 9.4 | 1,439 | 427 | 1,408 | 26.3 | 14.1 | 24.0 | 47.7 | 17.8 | 42.5 | 1,763 | 1,057 | 1,591 | 7.7 | 2.6 | 7.8 | |||||||||||||
| 6.0* | 2 | 22.5 | 11.4 | 1,749 | 889 | 22.1 | 2.0 | 29.5 | 6.2 | 950 | 280 | 12.9 | 6.2 | ||||||||||||||||||||
Abbreviations: AUC0-∞, area under the concentration-time curve extrapolated to infinity; Cmax, peak plasma concentration observed; CL, clearance; T1/2, half-life of elimination; Vdss, volume of distribution at steady-state; and Css, steady-state concentration; SD, standard deviation.
Estimates based on two patients who terminated infusions early because of toxicity.
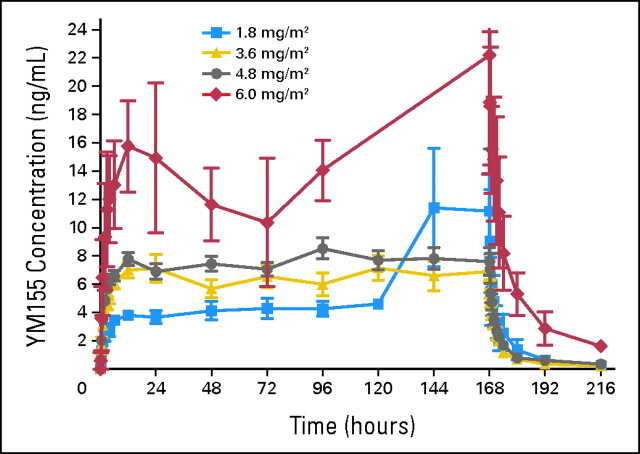
Fig 1.
Mean YM155 plasma concentration profile as a function of dose and time.
Fig 2.
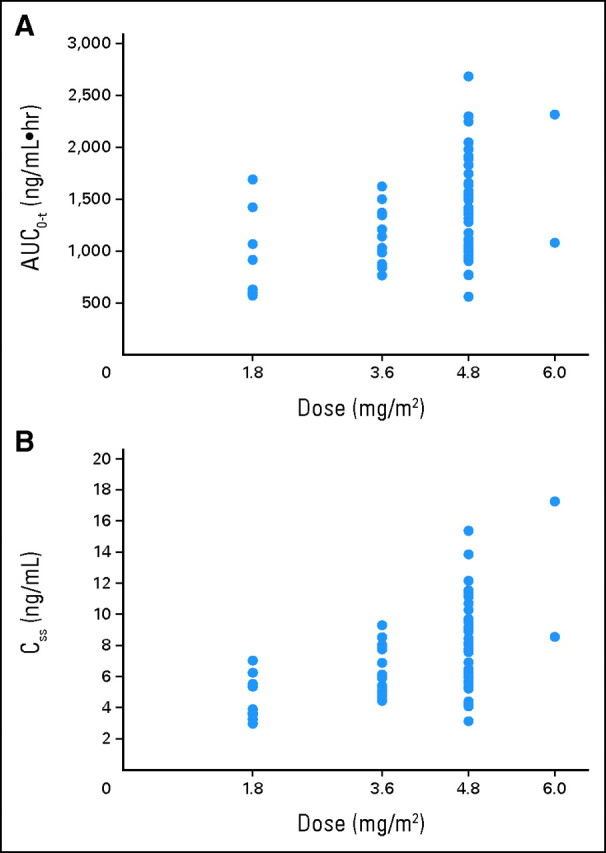
Relationship of YM155 dose to (A) area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0-t); and (B) steady-state concentration (Css).
YM155 concentrations seemed to reached steady-state between 12 and 24 hours after BOI, and after EOI, the concentrations declined in a biphasic pattern. The volume of distribution at steady-state was large, with the value at the MTD averaging 1,763 L, reflecting significant tissue distribution. At the MTD, clearance averaged 47.7 L/h, and the mean terminal elimination half-life β was 26 hours. The median fraction of YM155 excreted in the urine increased with increasing dose level and was 18.3%, 25.4%, and 28.6% at the 1.8, 3.6, and 4.8 mg/m2/d dose levels, respectively.
The relationships between the pharmacokinetic parameters that reflect YM155 exposure and renal function and hematologic and nonhematologic toxicities were also explored. There was no linear or nonlinear relationship that could be determined between YM155 peak plasma concentration observed, Css, and AUC0-∞ and toxicity (data not shown).
Antitumor Activity
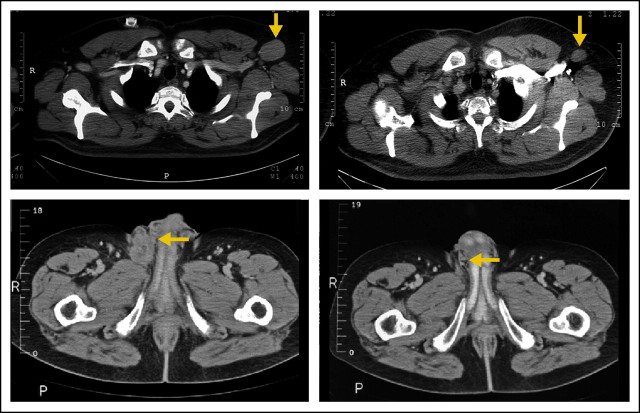
Three of five patients with chemotherapy-refractory non-Hodgkin's lymphoma had responses at the 4.8 mg/m2/d dose level. A 58-year-old male patient with biopsy-proven recurrent diffuse large B-cell lymphoma (DLBCL) despite rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone followed by rituximab plus ifosfamide, carboplatin, and etoposide chemotherapy had a partial response after two cycles of YM155 and no evidence of disease by cycle 6 (investigator complete response). Because the patient had not entered a complete response previously, he underwent high-dose chemotherapy and peripheral stem-cell transplantation (HD-PBSCT) and remains free of disease without further treatment at 4+ years. A second patient with recurrent DLBCL after cyclophosphamide, doxorubicin, vincristine, and prednisone, rituximab, and HD-PBSCT achieved a partial response after 16 cycles (12 months) on YM155 that lasted 8 months. One patient with a follicular large B-cell lymphoma recurrent to multiple agents and HD-PBSCT had a partial response documented on cycle 8 and remains in partial response on treatment at 2+ years (extension study). Representative images of responses are depicted in Appendix Figure A2 (online only).
Two of nine patients with hormone-refractory and docetaxel-treated prostate cancer had prostate-specific antigen (PSA) responses. One patient had a 50% decline in PSA after cycle 2 at the 1.8 mg/m2/d dose level but experienced disease progression after the fourth cycle. An additional patient with hormone-refractory prostate cancer treated initially at 6.0 mg/m2/d and then dose reduced to 4.8 mg/m2/d on cycle 2 had a PSA response after nine cycles of YM155 therapy. The patient was further dose reduced during cycles 11 to 13 before experiencing disease progression after cycle 13. The first patient entered at 6.0 mg/m2/d had a minor response in non–small-cell lung cancer lesions but experienced disease progression after the fourth cycle.
DISCUSSION
YM155 represents the first in a series of survivin-targeting therapies currently in early clinical development. Survivin represents an important strategic target in human malignancies: it is differentially expressed in malignancies compared with normal adult tissues and has a critical role in abrogating apoptosis signaling at the late stage in the apoptotic pathways that regulate cell life/death decisions.15 As such, inhibition of survivin may induce tumor regressions in those malignancies that exist in a pro-apoptotic state or whose tumor cells are restrained from undergoing apoptosis because ofuuu survivin expression. Furthermore, in malignancies in which survivin diminishes apoptosis induction after chemotherapy, radiation therapy, hormone ablation, or growth factor withdrawal, inhibition by YM155 may increase tumor-cell kill and the effectiveness of therapy.
The starting dose for this phase I study, 1.8 mg/m2/d, represented half of 1/10 the severe toxic dose (3.6 mg/m2d) in rats. This conservative starting dose was based on the observation of reversible acute renal tubular necrosis in both rats and dogs in toxicologic studies. In the current study, the appearance of dose-limiting impairment in renal function was concordant both in character and at Css from preclinical toxicology. At lower doses, a constellation of mild or modest nonhematologic adverse events, including fever, stomatitis, arthralgias, fatigue, and nausea, were observed during the YM155 infusion. Although the stomatitis was severe in three patients, the majority of patients who reported this toxicity characterized it as a sensation of mucosal roughness. Hematologic toxicity was rare and apparent at the highest doses.
Although it is not possible at this time to predict whether the observed toxicities represent on- or off-target effects, survivin is expressed in CD34+ cells as well as progenitor cells in the crypts of the GI tract. Nevertheless, both hematologic and nonhematologic toxicities were reversible and recovered rapidly. Therefore, alternative schedules of more frequent YM155 administration (eg, dosing every 14 days) using the 7-day infusion schedule may be feasible, and based on the longer than anticipated half-life, shorter infusion schedules should be explored.
The volume of distribution at steady-state of YM155 is extremely large and consistent with preclinical models that demonstrate wide tissue distribution. Furthermore, in experimental models, YM155 tumor tissue concentrations were 20-fold higher than the values in plasma.14 The predicted tissue concentrations, therefore, are well within the levels anticipated to significantly suppress survivin expression in tumor cells and induce apoptosis in preclinical models.
The antitumor activity observed in this phase I study is provocative. Three of five recurrent and refractory non-Hodgkin's lymphoma patients had major durable responses and two of nine patients with hormone-refractory prostate cancer had response by PSA criteria. In both these malignancies, survivin expression has been described extensively. In DLBCL, survivin is expressed in approximately 43% of patients and is concurrent with Bcl-2 overexpression in 25% patients, whereas mutually exclusive expression of these two antiapoptotic proteins occurs in the majority of patients.16 Molecular mechanisms that abrogate apoptosis seem to mediate resistance to current DLBCL therapy with both Bcl-2 and survivin being independent negative prognostic factors for patients treated with curative intent.
Tumor regression occurred slowly in three of the patients who achieved response. This observation is uncharacteristic of most anticancer agents and should provide caution in the assessment of activity in future phase II studies. Although durable stable disease is increasingly recognized as clinical benefit, this is not yet universal. With YM155, the absence of a response after several cycles should not precipitate discontinuation, as this may be premature, and response may be missed.
In conclusion, YM155 can be safely administered to patients with advanced malignancies at doses of 4.8 mg/m2/d by 168-hour CIVI every 3 weeks, attains plasma concentrations that exceed those that predict antitumor activity in preclinical models, and has promising single-agent activity. Studies are currently underway to fully evaluate the role of YM155 to enhance apoptosis in tumors either as a single agent in disease-directed studies or in combination with currently available therapies.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Pamela Bartels, Astellas Pharma US (C); Anne Keating, Astellas Pharma US (C) Consultant or Advisory Role: Anthony W. Tolcher, Astellas Pharma US Inc (C) Stock Ownership: None Honoraria: Anthony W. Tolcher, Astellas Pharma US Inc Research Funding: Lionel D. Lewis, Astellas Pharma US; Kyri Papadopoulos, Astellas Pharma US Inc; Scott Antonia, Astellas Pharma US Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Anthony W. Tolcher, Scott Antonia
Financial support: Pamela Bartels, Anne Keating
Provision of study materials or patients: Anthony W. Tolcher, Alain Mita, Lionel D. Lewis, Christopher R. Garrett, Adil I. Daud, Amita Patnaik, Kyri Papadopoulos, Chris Takimoto, Scott Antonia
Collection and assembly of data: Anthony W. Tolcher, Alain Mita, Lionel D. Lewis, Christopher R. Garrett, Elizabeth Till, Pamela Bartels, Anne Keating, Scott Antonia
Data analysis and interpretation: Anthony W. Tolcher, Alain Mita, Pamela Bartels, Anne Keating, Scott Antonia
Manuscript writing: Anthony W. Tolcher, Alain Mita, Lionel D. Lewis, Pamela Bartels, Anne Keating, Scott Antonia
Final approval of manuscript: Anthony W. Tolcher, Christopher R. Garrett, Chris Takimoto, Lionel D. Lewis, Pamela Bartels, Anne Keating, Scott Antonia
Appendix
Fig A1.
The chemical structure of YM155.
Fig A2.
Representative computed tomography images illustrating slow (10 months) and fast (6 weeks) response in patients with diffuse large B-cell non-Hodgkin's lymphoma: (A) right axillary lymph node February 9, 2005, and (B) December 29, 2005; (C) left inguinal mass October 22, 2004, and (D) December 3, 2004.
Footnotes
published online ahead of print at www.jco.org on September 29, 2008.
Supported by Astellas Pharma US.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Ambrosini G, Adida C, Altieri DC: A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917-921, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Li F, Altieri DC: The cancer antiapoptosis mouse survivin gene: Characterization of locus and transcriptional requirements of basal and cell cycle-dependent expression. Cancer Res 59:3143-3151, 1999 [PubMed] [Google Scholar]
- 3.Muchmore SW, Chen J, Jakob C, et al: Crystal structure and mutagenic analysis of the inhibitor-of-apoptosis protein survivin. Mol Cell 6:173-182, 2000 [PubMed] [Google Scholar]
- 4.Blanc-Brude OP, Mesri M, Wall NR, et al: Therapeutic targeting of the survivin pathway in cancer: Initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res 9:2683-2692, 2003 [PubMed] [Google Scholar]
- 5.Dohi T, Okada K, Xia F, et al: An IAP-IAP complex inhibits apoptosis. J Biol Chem 279:34087-34090, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Nettesheim D, Liu Z, et al: Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry 44:11-17, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Altieri DC: Molecular circuits of apoptosis regulation and cell division control: The survivin paradigm. J Cell Biochem 92:656-663, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, Altieri DC, Lu CD, et al: Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58:5071-5074, 1998 [PubMed] [Google Scholar]
- 9.Adida C, Haioun C, Gaulard P, et al: Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood 96:1921-1925, 2000 [PubMed] [Google Scholar]
- 10.Takai N, Miyazaki T, Nishida M, et al: Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett 184:105-116, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Span PN, Sweep FCGJ, Wiegerinck ETG, et al: Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem 50:1986-1993, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ferrandina G, Legge F, Martinelli E, et al: Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. Br J Cancer 92:271-277, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JP, Chang KH, Han JH, et al: Survivin, a novel anti-apoptosis inhibitor, expression in uterine cervical cancer and relationship with prognostic factors. Int J Gynecol Cancer 15:113-119, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Nakahara T, Takeuchi M, Kinoyama I, et al: YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res 67:8014-8021, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Altieri, Altieri DC: Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8:61-70, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Tracey L, Perez-Rosado A, Artiga MJ, et al: Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Path 206:123-134, 2005 [DOI] [PubMed] [Google Scholar]