Abstract
Purpose
A neutropenic diet is often used to prevent infection in patients with acute myeloid leukemia (AML). Although such a diet potentially entails inconvenience, its value is uncertain.
Patients and Methods
One hundred fifty-three patients admitted to a high-efficiency particulate air–filtered room (protected environment [PE]) to receive induction therapy for newly diagnosed AML were randomly assigned to a diet containing no raw fruits or vegetables (cooked diet) or to a diet containing fresh fruit and fresh vegetables (raw diet). Stratification was based on the patients’ early risk of mortality (ERM) score. All patients received antibacterial and antifungal prophylaxis and remained on study until they were discharged from the PE. The outcomes of principal interest were major infection (pneumonia, bacteremia, or fungemia) and death; if the true probability of either event was 20% on the cooked arm and 40% on the raw arm, then the probability that the cooked arm would be selected as superior was 83%.
Results
Seventy-eight patients were randomly assigned to the cooked arm, and 75 were assigned to the raw arm. The two groups were similar with respect to age, ERM, chemotherapy received, and days at risk. Twenty-nine percent of patients in the cooked group and 35% of patients in the raw group developed a major infection (P = .60). Time to major infection and survival time were similar in the two groups. Fever of unknown origin occurred in 51% of the cooked group and 36% of the raw group.
Conclusion
In patients treated in a PE, a neutropenic diet did not prevent major infection or death.
INTRODUCTION
It is well known that neutropenia predisposes patients to infection.1 It is similarly established that foods, particularly fresh fruits and vegetables, contain Escherichia coli, Pseudomonas aeruginosa, and other Gram-negative bacilli that can cause life-threatening sepsis and pneumonia.2,3 These two facts have led to the use of the neutropenic diet. After querying 400 hospitals associated with the Association of Community Cancer Centers, Smith and Besser4 reported that 78% of the 156 responding hospitals used such a diet, typically once neutropenia (< 1,000 or 1,500/μL) was documented. A wide range of dietary exclusions fell under the rubric of a neutropenic diet. However, 98% of such diets restricted fresh vegetables, and 93% restricted fresh fruits and juices.
Despite the routine use of a neutropenic diet, there seems to be little evidence of its benefit.5 In contrast, data exist that demonstrate that at least some patients would prefer not to be limited to a neutropenic diet. Specifically, DeMille et al6 found that 30% of the 23 outpatients participating in a study of the effect of this diet on infection rate were not compliant with the mandates of the diet. Infection rates were similar in compliant and noncompliant patients. A small trial (19 patients total) in children (median age, 4 to 5 years) found no difference in rates of febrile neutropenia according to whether patients were randomly assigned to a US Food and Drug Administration–approved food safety diet or a diet that, in addition, did not allow raw fruits and vegetables, take-out food, or fast food.7 Similarly a small randomized trial (total of 20 patients, 15 with acute myeloid leukemia [AML]) found no differences in gut colonization or infection according to whether a normal hospital diet or a low bacterial diet was used.8 In each of these trials, it was unclear whether the different dietary groups were at equal underlying risk of infection.
To assess whether the benefits of a neutropenic diet justify its inconvenience, we performed a larger trial that randomly assigned patients with untreated AML or high-risk myelodysplastic syndrome (MDS) who were about to receive chemotherapy in high-efficiency particulate air–filtered rooms (protected environment [PE]) to either a diet that contained fruits and vegetables only if cooked, as per standard practice of The University of Texas M. D. Anderson Cancer Center, or to a diet that permitted fresh (ie, raw) fruits and vegetables.
PATIENTS AND METHODS
Patients admitted to the PE were eligible provided that, at the time of random assignment, they had neither pneumonia nor bacteremia and were about to receive remission induction therapy on an ongoing Leukemia Department protocol for AML or high-risk MDS (10% to 19% blasts in marrow or blood). Two hundred six patients were eligible. Fifty-three of the patients (26%) preferred to conform to the hospital's policy of eating a diet containing no raw fruits and vegetables. The remaining 153 patients consented to be randomly assigned to either the hospital's diet of eating no raw fruits or vegetables or a diet containing fresh fruits and vegetables. The Leukemia Department Data Management Office performed the random assignment using the patients’ early risk of mortality (ERM) score9 as a stratification factor. The ERM incorporates pretreatment values for performance status, bilirubin, age, albumin, fibrinogen, absolute neutrophil count, hemoglobin, and creatinine to estimate the probability of death within 4 weeks after initiation of chemotherapy. The 604 patients admitted to the PE from 2000 to 2004 had a median ERM score of 0.17 (corresponding to 17% probability of death in the 28 days after beginning chemotherapy), leading us to stratify patients according to whether their ERM was greater or less than this value. Patients randomly assigned to the diet allowing fresh fruits and vegetables were encouraged to eat at least one of these daily, with the fruits and vegetables washed with cold water for 30 seconds before eaten. Compliance with the assigned diet was facilitated by placing notices of this diet on patients’ charts and by use of diaries in which patients recorded what they ate each day. All patients remained on the correct diet while on study, although some did not eat a fresh fruit or vegetable every day as suggested.
All patients had central lines placed and received prophylaxis with levofloxacin, valacyclovir, and depending on protocol, itraconazole, voriconazole, or a lipid preparation of amphotericin B. If fever of unknown origin (FUO) or pneumonia occurred, patients received intravenous ceftazidime or equivalent; if fever did not resolve, antifungal coverage was broadened. Patients remained on study until they were discharged from the PE to the outpatient setting, usually after return of the neutrophil count to more than 500/μL or after 6 weeks in patients in whom neutrophil recovery was delayed. Granulocyte colony-stimulating factor was used only when there was a delay (eg, 6 weeks in neutrophil recovery or after a major infection developed), and its use was equally infrequent in the cooked and raw diet groups.
The principal outcomes of interest were major infection (pneumonia, bacteremia, or fungemia, or pneumonia accompanied by bacteremia or fungemia) and death; we also noted the rates of minor infections and FUO. The principal comparison was between the group randomly assigned to eat only cooked food and the group randomly assigned to eat fresh fruits and vegetables; these groups are hereafter referred to as cooked and raw, respectively. To allow the reader to evaluate whether the patients randomly assigned to the cooked group were representative, we also report the incidences in the nonrandomized group (ate only cooked food). A diagnosis of pneumonia required a compatible chest x-ray or computed tomography scan. Bronchoalveolar lavage to isolate a causative organism was performed if no resolution had occurred after 3 to 5 days. A diagnosis of bacteremia as a result of frequent contaminants such as coagulase-negative Staphylococcus required two positive blood cultures.
The statistical design was the Bayesian multiple outcome design of Thall et al10 as extended by Thall and Sung.11 The outcomes monitored were major infection and death. Specifically, if after every 20 patients were randomly assigned, the probability was more than 0.995 that the incidence of death or major infection was greater in either the raw or cooked arm, then the study would stop. Otherwise, 188 patients would be randomly assigned, with 94 patients on each arm. If the true probability of major infection was 20% on the cooked arm and 40% on the raw arm, then the probability that the cooked arm would be selected as superior at the end of the trial was 83%; this is analogous to a power of 83% using a more traditional design.12 In contrast, if the true probability of major infection was 30% on both arms, then the probability that an arm would be selected as superior was 0.07, corresponding to P = .07 in more traditional designs.12
We used the χ2 or Kruskal-Wallis test to compare various pretreatment characteristics; number of days on study and number of days with neutrophil counts less than 500/μL and less than 100/μL; and rates of diarrhea, mucositis, and infection in the cooked, raw, and nonrandomized groups. Time to major infection and time to death in the cooked and raw groups were compared using the log-rank test. We performed approximately 40 tests of statistical significance. Although a standard Bonferroni correction, which would regard a P value as significant if less than approximately .001 (0.05/40), is perhaps overly conservative given the related nature of many of the tests (eg, patients with any infection, patients with any infection or FUO), we would certainly advise regarding any P > .01 with caution. The M. D. Anderson Cancer Center Institutional Review Board approved this study, which was conducted in accordance with the Declaration of Helsinki.
RESULTS
Seventy-eight patients were randomly assigned to the cooked arm, and 75 were assigned to the raw arm; 53 patients chose not to be randomly assigned, thus eating only cooked food (Fig 1). The three groups were similar with respect to age and ERM score (Table 1). Patients in the cooked and raw groups received prophylaxis with voriconazole more often than non–randomly assigned patients, of note because we have reported that such prophylaxis is more effective than itraconazole or lipid amphotericin B in preventing fungal infection13; however, similar proportions of patients in the cooked and raw groups received voriconazole (Table 1). Patients in the cooked group were more likely than those in the raw group to receive cytarabine, but the two groups were equally likely to receive high-dose therapy of any kind as opposed to targeted therapy (eg, decitabine; Table 1). Patients were on study (ie, at risk) for 24 to 25 days in each group. Fifty-three percent of patients in the cooked group and 57% of patients in the raw group had an antecedent hematologic disorder. The number of days spent with neutrophil counts less than 500/μL and less than 100/μL were similar in the cooked and raw groups (median of 20 and 21 days at < 500/μL in the cooked and raw groups, respectively; P = .77; and median of 15 and 16 days at < 100/μL in the cooked and raw groups, respectively; P = .53). Twenty-eight patients in both the cooked and raw groups had lost weight (a median of 5 lb in each group) when they came off study. The incidence of grade 3 to 4 mucositis or diarrhea was similar in the cooked and raw groups (10% and 16%, respectively; P = .34), further suggesting that the anti-AML therapies were equitoxic in the two groups. Grade 3 to 4 diarrhea or mucositis was seen in only one of the 36 cooked or raw group patients not administered cytarabine compared with 19 of the 117 cooked or raw group patients administered cytarabine.
Fig 1.

Flow of patients admitted to protected environment.
Table 1.
Patient Characteristics
| Characteristic | Patients Randomly Assigned to No Fresh Fruits and Vegetables (cooked diet; n = 78) |
Patients Randomly Assigned to Fresh Fruits and Vegetables (raw diet; n = 75) |
Patients Not Randomly Assigned (preferred cooked diet; n = 53) |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| Age, years | .62 | |||||||||
| Median | 64 | 63 | 66 | |||||||
| Range | 17-88 | 47-84 | 49-81 | |||||||
| ERM score | ||||||||||
| 25th percentile | 0.05 | 0.05 | 0.05 | .98 | ||||||
| Median | 0.11 | 0.14 | 0.12 | |||||||
| 75th percentile | 0.25 | 0.26 | 0.32 | |||||||
| Disease | .63 | |||||||||
| AML | 75 | 69 | 51 | |||||||
| MDS | 3 | 6 | 2 | |||||||
| Voriconazole prophylaxis | 14 | 18 | 8 | 11 | 0 | .005* | ||||
| Chemotherapy | ||||||||||
| With cytarabine | 65 | 83 | 52 | 69 | 40 | 75 | .13† | |||
| Idarubicin + cytarabine ± tipifarnib | 32 | 24 | 21 | |||||||
| Clofarabine + cytarabine | 20 | 18 | 16 | |||||||
| Fludarabine + cytarabine | 10 | 9 | 3 | |||||||
| Without cytarabine | 13 | 17 | 23 | 31 | 13 | 25 | ||||
| Clofarabine | 3 | 11 | 2 | |||||||
| VNP40101M | 4 | 8 | 8 | |||||||
| High dose | 70 | 90 | 70 | 93 | 50 | 94 | .64 | |||
| Targeted | 8 | 10 | 5 | 7 | 3 | 6 | ||||
| No. of days on study | .27 | |||||||||
| Median | 24 | 24 | 24 | |||||||
| Range | 10-47 | 6-42 | 8-45 | |||||||
Abbreviations: ERM, early risk of mortality; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
P = .25 for comparison of cooked v raw groups.
P = .06 for comparison of cooked v raw groups.
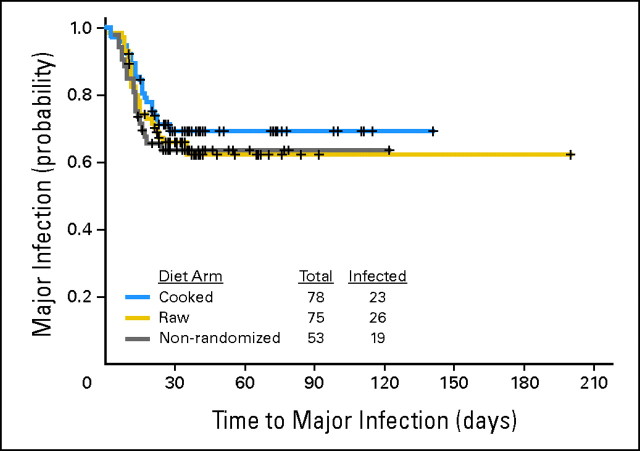
Major Infection
Twenty-nine percent and 35% of patients in the cooked and raw groups, respectively, developed a major infection (P = .60; Table 2). An exact 95% CI for the true difference in rates is −11% to 21%. The rate of major infection in the nonrandomized group (36%) was similar to the rate in the randomized cooked group. Time to development of a major infection was likewise similar in the three groups (Fig 2). Excluding coagulase-negative Staphylococcus and α-hemolytic Streptococcus, the rates of major infection were 27% in the cooked group and 24% in the raw group (P = .71); when excluding patients administered monotherapy with clofarabine or VNP40101M (Cloretazine; Vion Pharmaceuticals, New Haven, CT), major infections occurred in 22 (31%) of 72 and 21 (34%) of 61 patients in the cooked and raw groups, respectively (P = .71).
Table 2.
Incidence of Infection or FUO
| Infection and FUO | Patients Randomly Assigned to No Fresh Fruits and Vegetables (cooked diet; n = 78) |
Patients Randomly Assigned to Fresh Fruits and Vegetables (raw diet; n = 75) |
P (cooked v raw) | Patients Not Randomly Assigned (preferred cooked diet; n = 53) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| Patients with any major infection | 23 | 29 | 26 | 35 | .60 | 19 | 36 | |||
| Patients with pneumonia | 12 | 15 | 4 | 5 | .06 | 12 | 23 | |||
| Patients with bacteremia or fungemia | 7 | 9 | 17 | 23 | .03 | 4 | 8 | |||
| Patients with pneumonia accompanied by bacteremia or fungemia | 4 | 5 | 5 | 7 | .74 | 3 | 6 | |||
| Patients with any minor infection | 5 | 6 | 4 | 5 | .99 | 4 | 8 | |||
| Patients with FUO | 40 | 51 | 27 | 36 | .07 | 22 | 42 | |||
| Patients with either major or minor infection | 28 | 36 | 30 | 40 | .62 | 23 | 43 | |||
| Patients with infection or FUO | 68 | 87 | 57 | 76 | .09 | 45 | 85 | |||
Abbreviation: FUO, fever of unknown origin.
Fig 2.
Probability of major infection in cooked, raw, and nonrandomized groups; the nonrandomized group ate only cooked food (log-rank test, P = .50 for three-way comparison and P = .44 for comparison of cooked and raw groups).
Bearing in mind the multiple tests of statistical significance, the incidence of pneumonia was higher in the cooked group, whereas the incidence of bacteremia was higher in the raw group. With either type of infection, the nonrandomized group bore more resemblance to the cooked group than the raw group. In only one of the 28 patients with pneumonia unaccompanied by bacteremia or fungemia was an organism recovered (Aspergillus species in a patient in the cooked group), and there were only three other documented fungal infections (one in each of the cooked, raw, and nonrandomized groups; Table 3). Organisms resident in the gut (E coli, Enterococcus, Candida albicans, Enterobacter, Pseudomonas, and Klebsiella) were cultured from blood in 6% of the cooked group and 15% of the raw group (P = .12; exact 95% CI, −.04 to .23).
Table 3.
Isolated Organisms
| Organism | No. of Patients |
||||
|---|---|---|---|---|---|
| Cooked Group | Raw Group | Nonrandomized Group | |||
| Pneumonia | |||||
| Aspergillus | 1 | — | — | ||
| Unknown | 11 | 4 | 12 | ||
| Bacteremia/fungemia ± pneumonia | |||||
| Escherichia coli | 2 | 3 | — | ||
| Enterococcus | 2 | 5* | 1 | ||
| Staphylococcus aureus | 1 | — | — | ||
| Coagulase-negative Staphylococcus | 1 | 3 | 1 | ||
| α-Hemolytic Streptococcus | 1 | 5 | — | ||
| Enterobacter | — | 1 | 1 | ||
| Neisseria | 1 | — | — | ||
| Pseudomonas | — | 1 | 1 | ||
| Klebsiella | — | 1 | 1 | ||
| Stomatococcus | — | — | 1 | ||
| Flavimonas | 1 | — | — | ||
| Scedosporium | — | — | 1 | ||
| Alcaligenes xylosoxidans | 1 | — | — | ||
| Candida albicans | 1 | — | — | ||
| Corynebacterium | — | 1 | — | ||
| Roseomonas | — | 1 | — | ||
| Capnocytophaga | — | 1 | — | ||
| Fusarium | — | 1* | — | ||
One patient had both Enterococcus and Fusarium.
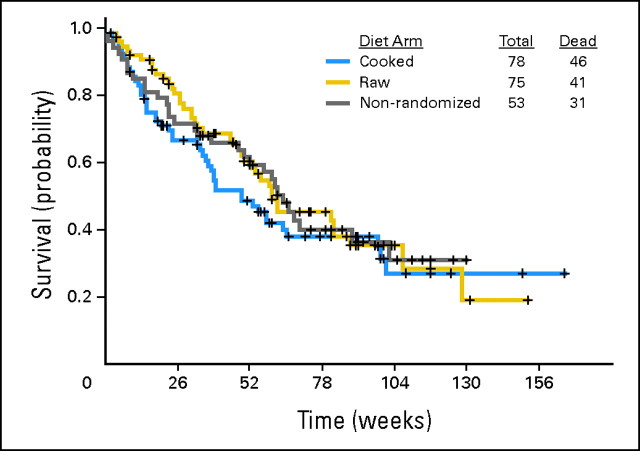
Death
There was no suggestion that patients randomly assigned to the cooked group lived longer than those assigned to the raw group (Fig 3). Survival in all three groups was as expected in newly diagnosed AML or MDS patients, as were complete response rates (56% in cooked group, 61% in raw group, and 64% in patients not randomly assigned).
Fig 3.
Probability of death in cooked, raw, and nonrandomized groups (log-rank test, P = .32 for three-way comparison and P = .36 for comparison of cooked and raw groups).
Minor Infection and FUO
Minor infections were observed in less than 10% of patients in all three groups. FUO was more common in the cooked group than the raw group. As typically occurs in treatment of AML or high-risk MDS, the great majority of patients had either an infection or an FUO (87% in cooked group, 76% in raw group, and 85% in patients not randomly assigned; Table 2).
DISCUSSION
Our results suggest that there is little to be gained from use of a neutropenic diet in patients undergoing remission induction therapy for newly diagnosed AML or high-risk MDS. In particular, rates of major infection and death, the two major end points, were similar regardless of whether patients were randomly assigned to eat a cooked or raw diet. The exact 95% CI for the difference in rates of major infection suggests that a difference of no more than 10% is as plausible as no difference because 0 and 0.10 are equidistant from the midpoint of the CI (0.05), whereas the probability of survival was slightly higher in the raw group, making it unlikely that death would in fact have been more frequent in this group than the cooked group had more patients been entered. Although the incidence of bacteremia was higher in the raw group, a substantial part of this difference reflected isolation of organisms not resident in the gut; the presence of such organisms would not be expected to be influenced by cooking of fruits and vegetables. Furthermore, the incidence of FUO and, hence, of potentially false-negative bacteremias was higher in the cooked group.
A major question is the extent to which our results can be generalized. This question arises because 26% of patients who were eligible for random assignment declined to be randomly assigned (preferring to eat only cooked food). The observation that results in nonrandomized patients were similar to those in patients randomly assigned to a cooked diet gives us some confidence that our results are not a function of selection bias. It may also be difficult to generalize our results to patients treated outside a PE or to patients not administered, as were our patients, antifungal prophylaxis with itraconazole, voriconazole, or lipid amphotericin. Nonetheless, at the least, our results suggest that a randomized trial similar to ours but conducted outside a PE would be appropriate. This is particularly the case as induction therapy changes in older patients to emphasize targeted rather than intensive therapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Alison Gardner, Elihu Estey
Provision of study materials or patients: Alison Gardner, Stefan Faderl, Gautam Borthakur, Guillermo Garcia-Manero
Collection and assembly of data: Alison Gardner
Data analysis and interpretation: Alison Gardner, Gloria Mattiuzzi, Sherry Pierce, Mark Brandt, Elihu Estey
Manuscript writing: Alison Gardner, Elihu Estey
Final approval of manuscript: Alison Gardner, Gloria Mattiuzzi, Stefan Faderl, Gautam Borthakur, Guillermo Garcia-Manero, Sherry Pierce, Mark Brandt, Elihu Estey
Footnotes
published online ahead of print at www.jco.org on October 27, 2008
Presented in part at the 32nd Annual Oncology Nursing Congress, April 24-27, 2007, Las Vegas, NV.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Bodey GP, Buckley M, Sathe YS, et al: Quantitative relationship between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328-340, 1966 [DOI] [PubMed] [Google Scholar]
- 2.Casewell M, Phillips I: Food as a source of Klebsiella species for colonization and infection of intensive care patients. J Clin Pathol 31:845-849, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright C, Kominoa SD, Yee RB: Enterobacteriaceae and Pseudomonas aeruginosa recovered from vegetable salads. Appl Environ Microbiol 31:453-454, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LH, Besser SG: Dietary restrictions for patients with neutropenia: A survey of institutional practices. Oncol Nurs Forum 27:515-520, 2000 [PubMed] [Google Scholar]
- 5.Wilson BJ: Dietary recommendations for neutropenic patients. Semin Oncol Nurs 18:44-49, 2002 [DOI] [PubMed] [Google Scholar]
- 6.DeMille D, Deming P, Lupinacci P, et al: The effect of the neutropenic diet in the outpatient setting: A pilot study. Oncol Nurs Forum 33:337-343, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Moody K, Finlay J, Mancuso C, et al: Feasibility and safety of a pilot randomized trial of infection rate: Neutropenic diet versus standard food safety guidelines. J Pediatr Hematol Oncol 28:126-133, 2006 [DOI] [PubMed] [Google Scholar]
- 8.van Tiel F, Harbers M, Terporten P, et al: Normal hospital and low-bacterial diet in patients with cytopenia after intensive chemotherapy for hematological malignancy: A study of safety. Ann Oncol 18:1080-1084, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Estey E, Smith TL, Keating MJ, et al: Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia 3:257-263, 1989 [PubMed] [Google Scholar]
- 10.Thall PF, Simon R, Estey EH: New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol 14:296-303, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Thall PF, Sung HG: Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 17:1563-1580, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Goodman S: Toward evidence-based medical statistics. 2: The Bayes factor. Ann Intern Med 130:1005-1013, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Mattiuzzi G, Estey E, Hernandez M, et al: Voriconazole and liposomal amphotericin B (Ambisome) effectively prevent mold infections in patients with acute myelogenous leukemia following remission induction chemotherapy. Blood 106:2773, 2005. (abstr) [Google Scholar]