Abstract
Purpose
The antibody-drug conjugate glembatumumab vedotin links a fully human immunoglobulin G2 monoclonal antibody against the melanoma-related glycoprotein NMB (gpNMB) to the potent cytotoxin monomethyl auristatin E. This study evaluated the safety and activity of glembatumumab vedotin in patients with advanced melanoma.
Patients and Methods
Patients received glembatumumab vedotin every 3 weeks (schedule 1) in a dose escalation and phase II expansion at the maximum-tolerated dose (MTD). Dosing during 2 of 3 weeks (schedule 2) and weekly (schedule 3) was also assessed. The primary end points were safety and pharmacokinetics. The secondary end points included antitumor activity, gpNMB expression, and immunogenicity.
Results
One hundred seventeen patients were treated using schedule 1 (n = 79), schedule 2 (n = 15), or schedule 3 (n = 23). The MTDs were 1.88, 1.5, and 1.0 mg/kg for schedules 1, 2, and 3, respectively. Grade 3/4 treatment-related toxicities that occurred in two or more patients included rash, neutropenia, fatigue, neuropathy, arthralgia, myalgia, and diarrhea. Three treatment-related deaths (resulting from pneumococcal sepsis, toxic epidermal necrolysis, and renal failure) occurred at doses exceeding the MTDs. In the schedule 1 phase II expansion cohort (n = 34), five patients (15%) had a partial response and eight patients (24%) had stable disease for ≥ 6 months. The objective response rate (ORR) was 2 of 6 (33%) for the schedule 2 MTD and 3 of 12 (25%) for the schedule 3 MTD. Rash was correlated with a greater ORR and improved progression-free survival.
Conclusion
Glembatumumab vedotin is active in advanced melanoma. The schedule 1 MTD (1.88 mg/kg once every 3 weeks) was associated with a promising ORR and was generally well tolerated. More frequent dosing was potentially associated with a greater ORR but increased toxicity.
INTRODUCTION
Despite recent successes with oncogenic pathway inhibition and immune checkpoint blockade,1–8 novel treatments for advanced melanoma are still needed. Antibody-drug conjugates (ADCs) represent one strategy with the potential to expand the armamentarium of effective agents for the treatment of melanoma.
The human 560-amino-acid type I glycoprotein NMB (gpNMB) was identified using a homology-based genomic mining process. gpNMB shows homology closest to pMEL-17, a melanocyte-specific marker that is differentially expressed in melanoma cells.9,10 Both are intracellular transmembrane proteins that transit the cell surface, representing a new class of targets for ADCs. gpNMB is expressed in subcellular compartments and on the cell surface on multiple cell types, including epithelial cells, osteoclasts, osteoblasts, macrophages, and dendritic cells (DCs).11–14 A number of tumors, including those of melanoma, breast cancer, and glioblastoma, overexpress gpNMB relative to normal tissue.10,15,16 Overexpression of gpNMB promotes invasion and metastasis of hepatocellular carcinoma, glioma, and breast cancer cells,15,17–20 decreases tumor cell apoptosis, and promotes angiogenesis20 in preclinical models.
Glembatumumab vedotin (CDX-011 or CR011-vcMMAE; Celldex Therapeutics, Hampton, NJ) was produced by covalently linking a fully human immunoglobulin G2 monoclonal antibody against gpNMB (CR011) to monomethyl auristatin E (MMAE), a potent mitotic spindle formation inhibitor.21–23 Glembatumumab vedotin is designed to bind to gpNMB on tumor cells and release MMAE via proteolytic cleavage of the valine-citrulline linker after lysosomal internalization, resulting in cell death from microtubule inhibition by free MMAE. Glembatumumab vedotin has potent antitumor activity against melanoma cell lines expressing gpNMB in vitro and in mouse xenograft models using sk-mel-2 and sk-mel-5 cells in vivo.10, 24 This phase I/II study was designed to assess the safety and activity of glembatumumab vedotin in patients with unresectable stage III or stage IV melanoma.
PATIENTS AND METHODS
Patients
Eligible patients were ≥ 18 years of age; had histologically confirmed, progressive, unresectable stage III or IV cutaneous or ocular melanoma with measurable disease according to RECIST 1.0; a life expectancy of ≥ 3 months; adequate organ function; and a Karnofsky performance score (KPS) of ≥ 70. Participants must have experienced treatment failure on no more than one line of systemic cytotoxic therapy for metastatic disease, but there were no restrictions on the number of prior treatments with biologic or immunotherapeutic agents. Selected exclusion criteria included progressive CNS metastases; cytotoxic chemotherapy, immunotherapy, biologic therapy, or radiotherapy in the 4 weeks before entry; unresolved grade 2 or higher toxicity from prior treatment; significant comorbid illness; and pregnancy or nursing.
This study was conducted at four participating institutions in accordance with the Declaration of Helsinki and good clinical practice guidelines after approval by a local human investigations committee and in accord with an assurance filed with and approved by the Department of Health and Human Services, where appropriate. All patients signed a written informed consent form before any protocol-specific procedures.
Study Design and Treatment
The primary objectives of this study were to evaluate the dose-limiting toxicity (DLT), maximum-tolerated dose (MTD), pharmacodynamics, and pharmacokinetics of glembatumumab vedotin. The secondary objectives were to assess its antitumor activity and immunogenicity and to determine its recommended phase II dose and schedule.
The study initially evaluated dosing once every 3 weeks (schedule 1; day 1 of a 21-day cycle). A phase I dose escalation to establish the MTD was followed by use of an open-label, single-arm, Simon two-stage,25 phase II expansion cohort to further assess the safety and efficacy of the MTD of glembatumumab vedotin. For the dose escalation we used a classical three-plus-three design.26 The initial dose level was 0.03 mg/kg, representing a 10-fold reduction from the highest nontoxic dose in nonhuman primates, with scaling to the human equivalent dose27 and an additional three-fold reduction for safety. Dose levels were escalated by 100% until a patient developed grade 2 or higher toxicity (excluding infusion-related and select manageable toxicities), after which the doses were to be escalated by 40% until identification of the MTD. DLTs were defined as any of the following occurring in cycle 1: grade 4 thrombocytopenia; grade 4 neutropenia lasting > 5 days or associated with fever; or grade 3 to 4 nonhematologic toxicity (excluding grade 3 nausea, vomiting, rash, arthralgia, myalgia, and fatigue resolved to grade 2 or lower within 72 hours). After completion of dose escalation, two intermediate dose levels were tested in a dose de-escalation design for potential refinement of the schedule 1 MTD.
Because pharmacokinetic extrapolation and simulation studies suggested that pharmacokinetic profiles might be optimized with more frequent dosing, the protocol was amended to include dose escalations for schedule 2 (days 1 and 8 of a 21-day cycle) and schedule 3 (days 1, 8, and 15 of a 21-day cycle). The starting dose levels were 1.25 and 0.75 mg/kg, respectively. Doses were escalated in 0.25 mg/kg increments in independent dose-escalation phases, per the criteria described for the dose-escalation part of schedule 1. Six to 15 patients were to be treated at the MTD for each dose schedule.
Glembatumumab vedotin was administered as a 90-minute intravenous infusion. Delays of up to 3 weeks and up to two dose reductions were permitted for toxicity. Dosing continued until unmanageable treatment-related toxicities, disease progression, or death occurred.
On-Study Evaluation
Safety parameters assessed at baseline and at study visits included physical examination, vital signs, KPS, hematology, blood chemistry, and urinalysis. An ECG and an ophthalmic examination were performed at baseline and at treatment end. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0. Tumor response was assessed every 6 weeks by the investigator according to RECIST 1.0. Patients who discontinued treatment for reasons other than disease progression were evaluated every 3 months until the initiation of alternative therapy, disease progression, or death.
Correlative Studies
Serum samples for assessing immunogenicity, measuring circulating gpNMB levels, and pharmacokinetic analysis were obtained before dosing on day 1 of each cycle. During the first two cycles, additional pharmacokinetic samples were taken at select time points. Intact ADC (CR011 antibody carrying at least one attached molecule of MMAE) and total CR011 antibody (TA) were quantified using enzyme-linked immunosorbent assays (ELISAs). Free MMAE was quantified using liquid chromatography/mass spectrometry. The assay sensitivities were 40, 400, and 0.05 ng/mL for ADC, TA, and free MMAE, respectively. Noncompartmental analysis was performed with WINNonlin (Scientific Consultant, Apex, NC) software version 5.3 (Pharsight, Mountain View, CA). Soluble gpNMB was measured using an ELISA with immobilized CR011 antibody to capture gpNMB and a rabbit polyclonal anti-gpNMB antibody for detection.
Immunogenicity to glembatumumab vedotin was determined with a bridging ELISA between immobilized and horseradish peroxidase–conjugated glembatumumab vedotin, with a sensitivity of 50 ng/mL. The threshold for positivity was defined by the upper 95% CI boundary for the background optical density of 31 predose patient serum samples.
Tumor gpNMB expression was assessed by central immunohistochemistry with a biotinylated CR011 monoclonal antibody (Mosaic Laboratories, Lake Forest, CA) for patients with available archived tumor specimens who consented to the optional correlative studies. To retrospectively explore the relationship between gpNMB expression and response to glembatumumab vedotin, an H-score was calculated for each patient using the equation (3 × percentage of cells staining at 3+ intensity) + (2 × percentage of cells staining at 2+ intensity) + (1 × percentage of cells staining at 1+ intensity).
Statistical Analysis
The primary objective of this study was to evaluate the safety and pharmacokinetics of glembatumumab vedotin. Secondary objectives included the determination of the objective response rate (ORR) for the schedule 1 MTD expansion cohort (the primary efficacy objective). A Simon two-stage design was used to test the null hypothesis that the ORR was ≤ 5% versus the alternative hypothesis that the ORR was ≥ 20%. This design had a type I error rate of 10% and a type II error rate of 10%.25 If one or more responses were observed among the first 18 patients, an additional 14 patients would be enrolled. If four or more responses were observed in the full cohort of 32 patients, glembatumumab vedotin would be deemed worthy of additional development.
Additional end points included time to response, duration of response, and progression-free survival (PFS). PFS was calculated from study day 1 and summarized descriptively using the Kaplan-Meier method. Disease progression was assumed on symptomatic deterioration, progression per RECIST, or death resulting from any cause. Patients who initiated alternative cancer therapy or discontinued the study without documented disease progression were censored as of therapy initiation or the last evaluable tumor assessment, respectively.
RESULTS
Patient Characteristics and Disposition
One hundred seventeen patients were enrolled between June 2006 and September 2009. Baseline characteristics of the patients are listed in Table 1.
Table 1.
Pretreatment Patient Characteristics
| Characteristics | All Treated Patients (N = 117) |
|
|---|---|---|
| No. of Patients | % | |
| Male | 75 | 64 |
| Age, years | ||
| Median | 62 | |
| Range | 36-82 | |
| KPS | ||
| 100 | 59 | 50 |
| 90 | 42 | 36 |
| 80 | 15 | 13 |
| 70 | 1 | 1 |
| Disease stage | ||
| III | 12 | 10 |
| IV* | 105 | 90 |
| M1a | 14 | 12 |
| M1b | 21 | 18 |
| M1c | 62 | 53 |
| Elevated LDH | 49 | 42 |
| Duration of metastatic disease, years | ||
| Median | 1.4 | |
| Range | 0.05-10.3 | |
| Prior therapies | ||
| Biochemotherapy | 16 | 14 |
| Chemotherapy | 59 | 50 |
| Biologic therapy | 67 | 57 |
| CTLA-4 inhibitors | 25 | 21 |
| Interferon alfa | 25 | 21 |
| Interleukin-2 | 23 | 10 |
| Other/unknown immunotherapy | 23 | 20 |
| Other/unknown biologic | 11 | 9 |
| Kinase inhibitors | 9 | 8 |
| Other therapies | 26 | 22 |
Abbreviations: CTLA-4, cytotoxic T-cell lymphocyte antigen-4; KPS, Karnofsky performance score; LDH, lactate dehydrogenase.
M1 staging was not available for eight patients with stage IV disease.
Treatment and Toxicity
All 117 patients enrolled onto the study received at least one dose of glembatumumab vedotin and were assessed for its safety. Median duration of treatment in the overall study population was 9.1 weeks (range, 1 to 58 weeks).
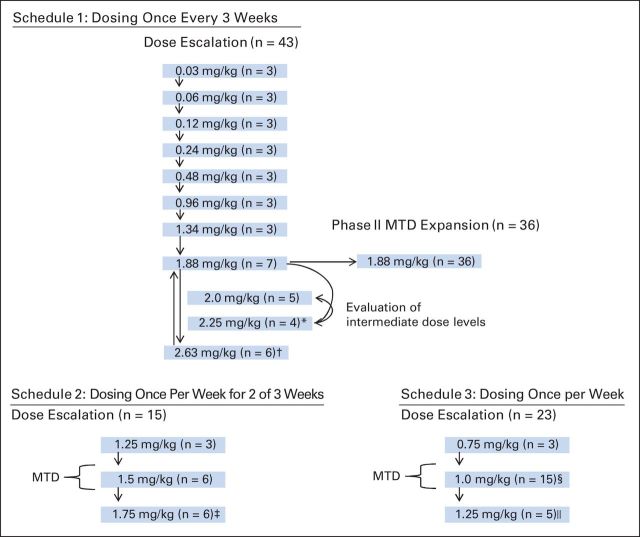
Patient dispositions to dosing schedules/levels and DLTs encountered are illustrated in Figure 1. In schedule 1 (once every 3 weeks; n = 79), the dose was initially escalated in 100% increments. Because all three patients receiving the 0.96 mg/kg dose experienced grade 1 diarrhea, subsequent dose escalations occurred in 40% increments. In the 2.63 mg/kg cohort, two patients experienced grade 3 rash that persisted for > 72 hours in cycle 1, and a third patient developed grade 2 erythema multiforme in the second cycle. Therefore, the MTD was declared to be 1.88 mg/kg. Intermediate schedule 1 dose cohorts (2.0 and 2.25 mg/kg) were subsequently explored. The 2.25 mg/kg dose was deemed to be not tolerated as a result of DLTs in two patients (grade 3 palmar-plantar erythrodysesthesia syndrome and grade 4 rash/neutropenic fever). The 2.0 mg/kg dose was tolerated for one cycle without evidence of DLTs, but this dose level was not pursued further, because it represents only a minor increase over the dose (1.88 mg/kg) that was already under evaluation in the phase II expansion. For schedule 2 (once per week for 2 of 3 weeks; n = 15), the MTD was declared to be 1.5 mg/kg because of the occurrence of DLTs in three patients (grade 3 hyperglycemia/rash, grade 3 rash, and fatal toxic epidermal necrolysis [TEN]) at the 1.75 mg/kg dose. For schedule 3 (once per week; n = 23), after one death from acute renal failure occurred at the 1.25 mg/kg dose, the MTD was declared to be 1.0 mg/kg, and this cohort was expanded to 15 patients.
Fig 1.
Patient dispositions. DLT, dose-limiting toxicity; MTD, maximum-tolerated dose. (*) Two DLTs: Grade 3 palmar-plantar erythrodysaesthesia syndrome and grade 4 rash/neutropenic fever. (†) Two events of grade 3 rash and one event of grade 2 erythema multiforme. (‡) Three DLTs: grade 3 hyperglycemia/rash, grade 3 rash, and grade 5 toxic epidermal necrolysis. (§) Grade 4 rash with grade 3 pruritus. (‖) Grade 5 renal failure.
The most significant treatment-related toxicities were rash, fatigue, alopecia, neuropathy, and neutropenia (Table 2). Rash was associated with pruritus in most cases but was not characterized by other consistent features. Rash was severe in approximately 30% of the patients treated with the schedule 1 and schedule 3 MTDs. One patient in each of these cohorts discontinued treatment because of rash. Neuropathy appeared cumulatively, with somewhat earlier onset and greater severity associated with more frequent dosing. Neuropathy required discontinuation of treatment for one patient (2%) treated with the schedule 1 MTD, compared with five patients (24%) in the more frequent dosing MTDs. Three possibly treatment-related deaths occurred at doses that exceeded the MTDs. An 80-year-old man receiving 1.75 mg/kg once per week for 2 of 3 weeks was hospitalized on day 10 with diarrhea, nausea, vomiting, fever, dyspnea, and an erythematous rash with flaccid bullae and sloughing. Skin biopsy results were consistent with TEN. Laboratory tests revealed leukopenia and renal failure. The patient then developed worsening multiorgan failure and died. A 72-year-old man receiving 1.25 mg/kg once per week was hospitalized with hypotension and acute renal failure on day 15; worsening renal and hepatic failure eventually lead to cardiopulmonary arrest and death. A 54-year-old man receiving 2.25 mg/kg once every 3 weeks was hospitalized at week 6 with a temperature of 105°F, dyspnea, and mental status changes. The work-up revealed leukopenia and pneumonia. Despite intubation, antibiotics, and vasopressor support, the patient died as a result of sepsis.
Table 2.
Treatment-Related Toxicity
| Event | Doses Below the MTD (all schedules pooled; n = 21) |
Schedule 1* MTD (1.88 mg/kg; n = 43) |
Schedule 2† MTD (1.5 mg/kg; n = 6) |
Schedule 3‡ MTD (1.0 mg/kg; n = 15) |
Doses Above the MTD (all schedules pooled; n = 32) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Grades | Grade 3 or Higher | All Grades | Grade 3 or Higher | All Grades | Grade 3 or Higher | All Grades | Grade 3 or Higher | All Grades | Grade 3 or Higher | |
| Nonhematologic toxicity | ||||||||||
| Rash | 14 | — | 74 | 30 | 67 | — | 67 | 33 | 81 | 22 |
| Fatigue | 14 | — | 65 | 7 | 67 | 17 | 53 | 13 | 63 | 13 |
| Pruritus | 10 | — | 63 | — | 33 | — | 47 | 7 | 59 | 3 |
| Alopecia | — | — | 65 | — | 33 | — | 33 | — | 41 | — |
| Diarrhea | 10 | — | 47 | 2 | 67 | — | 20 | — | 38 | 6 |
| Neuropathy | 5 | — | 33 | 7 | 33 | 17 | 60 | 27 | 34 | 3 |
| Nausea | 5 | — | 35 | — | 50 | — | 7 | — | 41 | — |
| Anorexia | — | — | 40 | — | 33 | — | 13 | — | 28 | 3 |
| Dysgeusia | — | — | 33 | — | 33 | — | 13 | — | 16 | — |
| Constipation | — | — | 30 | — | 17 | — | 20 | — | 16 | — |
| Vomiting | 5 | — | 14 | — | 17 | — | 13 | — | 25 | — |
| Myalgia | — | — | 12 | — | — | — | 27 | 13 | 19 | 6 |
| Dry skin | — | — | 12 | — | 33 | — | 13 | — | 9 | — |
| Pyrexia | — | — | 19 | — | — | — | — | — | 13 | — |
| Pain in extremity | — | — | 14 | 5 | 17 | — | 13 | — | 6 | — |
| Arthralgia | — | — | 7 | — | 17 | — | 7 | 7 | 16 | 6 |
| Mucosal inflammation | — | — | 14 | — | — | — | 7 | — | 9 | 6 |
| Palmar-plantar erythrodysesthesia syndrome | — | — | 5 | — | — | — | 7 | — | 13 | 6 |
| Hyperglycemia | — | — | — | — | — | — | — | — | 6 | 6 |
| Hematologic toxicity | ||||||||||
| Neutropenia | 5 | 5 | 28 | 19 | 33 | 17 | 13 | 13 | 22 | 19 |
| Thrombocytopenia | — | — | 5 | 2 | — | — | — | — | 6 | 3 |
| Leukopenia | 5 | 5 | 2 | 2 | — | — | — | — | — | — |
NOTE. Data are shown as the percentage of patients experiencing the event. Listed are events that occurred at any severity in > 10% of patients or at grade 3 or higher severity in ≥ 2% of patients.
Abbreviations: MTD, maximum-tolerated dose.
Schedule 1: once every 3 weeks.
Schedule 2: once per week for 2 of 3 weeks.
Schedule 3: once per week.
Activity
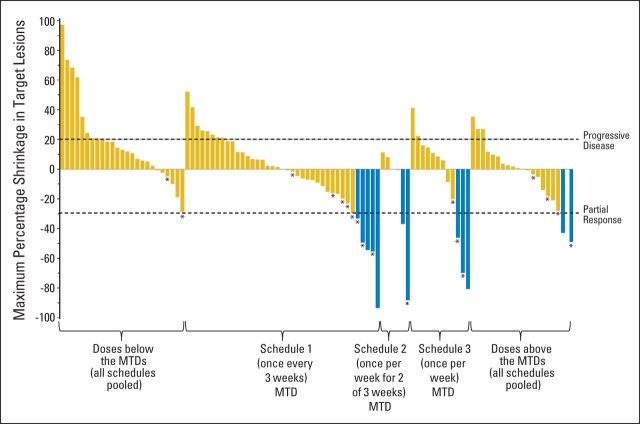
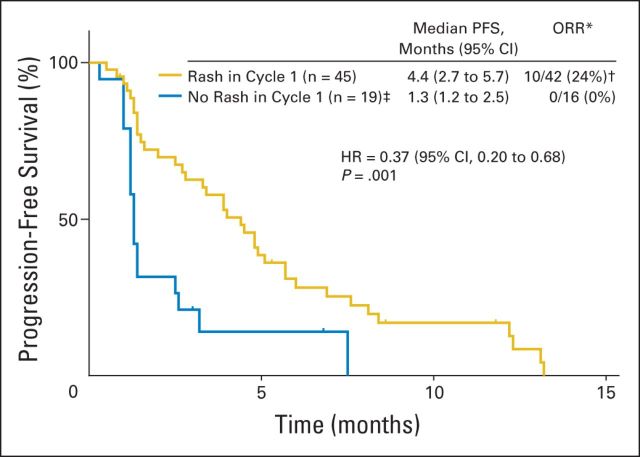
Five (15%) of the 34 assessable patients in the schedule 1 (once every 3 weeks) MTD expansion cohort experienced a partial response. Additional activity end points and waterfall plots for each dosing schedule MTD are presented in Table 3 and Figure 2, respectively. It should be noted that the development of any grade rash within the first cycle (within 21 days of the first dose) was associated with a greater ORR and prolonged PFS (Appendix Fig A1, online only).
Table 3.
Efficacy Analyses
| Schedule 1* MTD (1.88 mg/kg; n = 43†) |
Schedule 2‡ MTD (1.5 mg/kg; n = 6) |
Schedule 3§ MTD (1.0 mg/kg; n = 15) |
All MTDs Combined (N = 64) |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | No. | % | No. | % | No. | % | No. | % |
| Best response‖ | ||||||||
| PR | 5 of 40 | 13¶ | 2 of 6 | 33 | 3 of 12 | 25 | 10 of 58 | 17 |
| SD or better | 27 of 40 | 68 | 3 of 6 | 50 | 7 of 12 | 58 | 37 of 58 | 64 |
| Time to response, months | ||||||||
| Median | 1.6 | 1.3, 2.6# | 1.2, 1.5, 3.7# | 1.6 | ||||
| Range | 1.4-2.7 | 1.2-3.7 | ||||||
| Duration of response, months | ||||||||
| Median | 5.3 | 3.5, 9.0+# | 3.4, 7.3, 9.5# | 5.5 | ||||
| Range | 2.8-10.6 | 2.8-10.6 | ||||||
| Progression-free survival | ||||||||
| Median, months | 3.3 | 3.1 | 1.5 | 2.8 | ||||
| 95% CI | 1.6 to 4.4 | 0.5 to NE | 1.0 to 5.7 | 1.4 to 4.4 | ||||
| Six-month rate | 24 | 33 | 20 | 24 | ||||
| 95% CI | 20 to 27 | 21 to 46 | 16 to 24 | 21 to 26 | ||||
Abbreviations: MTD, maximum-tolerated dose; NE, not estimated; PR, partial response; SD, stable disease.
Schedule 1: once every 3 weeks.
Includes all patients who received the 1.88 mg/kg dose, including those treated in the dose-escalation and expansion phases. Five (15%) of the 34 assessable patients in schedule 1 (once every 3 weeks) MTD expansion cohort experienced a PR.
Schedule 2: once per week for 2 of 3 weeks.
Schedule 3: once per week.
Excludes patients without postbaseline assessment of measurable lesions.
Including one response (93% tumor shrinkage) that was not sustained at the subsequent assessment.
Individual patient data are listed in cases where the sample size was < 5.
Fig 2.
Antitumor responses. The best response (represented by shrinkage in RECIST target lesions) is shown for each patient with postbaseline evaluation of all target lesions. Blue bars indicate patients achieving partial response; asterisks indicate patients with response or stable disease for > 6 months. MTD, maximum-tolerated dose.
Correlative Studies
Pharmacokinetics.
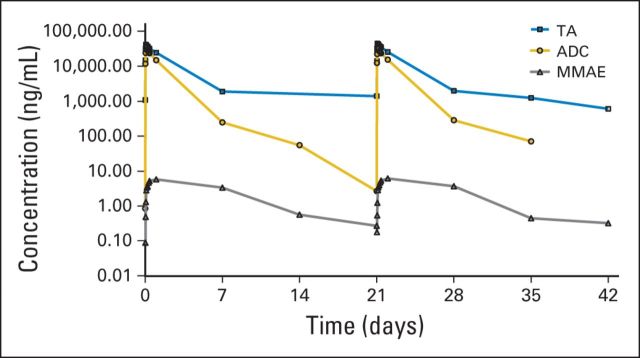
Increases in exposure to ADC, TA, and free MMAE were proportional to dose. The maximum concentration occurred immediately after infusion for the ADC and TA and from 1 to 7 days after infusion for MMAE. For the schedule 1 MTD (1.88 mg/kg; once every 3 weeks), the half-lives of ADC and TA were between approximately 1 and 2 days, consistent with the MTDs of schedules 2 and 3. No accumulation was observed from cycle 1 to cycle 2 across all doses and schedules studied. Concentration-time curves and pharmacokinetic measures are provided in Figure 3 and Appendix Table A1 (online only).
Fig 3.
Concentration-time curves for the glembatumumab vedotin antibody-drug conjugate (ADC), total CR011 antibody (TA), and free monomethyl auristatin E (MMAE) for the schedule 1 maximum-tolerated dose (1.88 mg/kg once every 3 weeks).
Immunogenicity.
Eleven of 94 patients tested developed an antidrug antibody response. However, only one antibody response was confirmed to be specific to glembatumumab vedotin, and the end of study sample showed no antibody response, suggesting that this response was transient. The development of antibodies did not seem to affect the pharmacokinetic profiles of TA, ADC, or MMAE.
Soluble and tissue gpNMB expression.
For the 38 patients tested across all dosing groups, the mean baseline soluble gpNMB concentration was 34.8 ng/mL (0.5 nmol/L; range, 10.6 to 164.9 ng/mL [0.14 to 2.2 nmol/L]). In comparison, soluble gpNMB levels in healthy volunteers range from 0.4 to 44 ng/mL (BioVendor; product insert, gpNMB ELISA).28,29 The effect of soluble gpNMB on the efficacy of glembatumumab vedotin is unknown; however, the Cmax of ADC at the MTD ranged from 125 to 377 nmol/L (mean, 245 nmol/L; Fig 3) and far exceeded the maximum baseline soluble gpNMB value found in this trial.
Of the 52 patients with an archival sample available for analysis, 40 (77%) had gpNMB expression in ≥ 5% of tumor cells. For exploratory analyses, an H-score of ≥ 100 was retrospectively defined as a functional cutoff to divide the patients treated at the MTDs with an available sample (n = 33) into low or high expression groups. A nonsignificant trend toward prolonged PFS was seen for patients with tumors expressing higher levels of gpNMB. The median PFS was 3.9 months for the 14 patients with an H-score of ≥ 100 compared with 2.7 months for the 19 patients with an H-score of < 100 (hazard ratio, 0.71;95% CI, 0.3 to 1.5). The ORRs were similar for the 11 response-evaluable patients with an H-score of ≥ 100 (27%) and the 18 response-evaluable patients with an H-score of < 100 (22%).
DISCUSSION
To our knowledge, this is the first study to evaluate an ADC for melanoma. Recently, ADCs targeting CD30 and HER2 demonstrated efficacy in Hodgkin lymphoma/adult T-cell leukemia and breast cancer, respectively.30–32 Glembatumumab vedotin is directed against gpNMB, a melanosome-associated protein expressed by melanocytes and melanoma cells (including uveal melanomas)33 and associated with melanoma development in humans. Melanocytes, macrophages, and DCs also express gpNMB on their cell surfaces, which may participate in cell-cell interactions.11,12,34,35 gpNMB contains an extracellular integrin-binding RGD motif and is thought to contribute to DC adherence to endothelial cells, as well as to melanocyte binding to keratinocytes.11,34 gpNMB expression may also be associated with downregulation of immune responses. gpNMB binding to syndecan-4 on T cells delivers a negative signal that blunts T-cell responses, including interleukin-2 production and proliferation.36–39 Syndecan-4 is expressed primarily on effector-memory T cells and not on naive T cells. Thus, preventing the gpNMB–syndecan-4 interaction may enhance antitumor responses in vitro and in vivo.
Glembatumumab vedotin was generally well tolerated at the MTD, although significant toxicity was observed at doses above the MTD. The adverse event profile was similar to that observed with the MMAE-containing ADC that targets CD30, brentuximab vedotin. However, rash appears more frequent and severe with glembatumumab vedotin. Severe rash was reported in 30% of the patients treated at the recommended phase II dose, and one patient treated with 1.75 mg/kg every 2 of 3 weeks died as a result of TEN. gpNMB expression in tumors did not correlate with the development of rash, which may have been caused, in part, by low levels of endogenous expression of gpNMB in skin melanocytes.
The prespecified threshold for promising activity was met with an ORR of five of 34 (15%) in the phase II MTD expansion. More frequent dosing schedules were potentially associated with a modest improvement in the ORR, but not prolonged PFS, in the small cohorts of patients treated at the MTDs. Because the DLTs also seemed more severe for these schedules, additional evaluation was not pursued in this study. Future trials may seek to optimize dosing.
The emergence of rash and gpNMB tumor expression may be potential biomarkers for the activity of glembatumumab vedotin. The emergence of rash during cycle 1 correlated with improved clinical outcomes (PFS and ORR). In murine xenograft models, gpNMB expression on melanoma cells is necessary for glembatumumab vedotin activity.10 In those patients whose gpNMB expression levels were measured, a trend toward prolonged PFS was seen for patients with tumors expressing higher levels of gpNMB; however, these results did not reach statistical significance. It should be noted that the immunohistochemistry method, which used a monoclonal antibody to a conformational epitope that may become compromised during tissue fixation or antigen retrieval with proteinase K, may have under-represented gpNMB expression. In comparison, a more robust immunohistochemistry assay was implemented in the phase II study in patients with heavily pretreated metastatic breast cancer, and this study demonstrated a significant association of tumor gpNMB expression with improved outcome for glembatumumab vedotin–treated patients.40
Interestingly, in vitro studies have shown that blockade of the mitogen-activated protein kinase pathway using RAF, MEK, or ERK inhibition leads to enhanced gpNMB expression in BRAF- and NRAS-mutated melanoma cells,41,42 and pretreatment with a MEK inhibitor sensitizes melanoma cell lines to glembatumumab vedotin. These results suggest that MAPK pathway inhibition may be synergistic with glembatumumab vedotin, particularly in tumors with low baseline gpNMB expression levels. Thus, it seems worthwhile to evaluate this combination in patients with advanced cutaneous and uveal melanomas. An additional combination strategy with immune checkpoint inhibitors is also of particular interest, because gpNMB-expressing cells may negatively regulate immune responses and because glembatumumab vedotin-induced tumor killing may liberate tumor antigens that could augment checkpoint inhibitor-mediated antitumor immune responses, as has been demonstrated with chemotherapy and radiation therapy.43,44 However, these combinations will need to be approached with caution, given the potential for overlapping cutaneous and GI toxicities.
In summary, the recommended phase II dose of glembatumumab vedotin identified in this trial (1.88 mg/kg once every 3 weeks) was generally well tolerated, with a promising ORR. The relationship between efficacy and tumor gpNMB expression will be prospectively explored in future trials using a revised, validated assay. Studies are planned in patients with metastatic melanoma who have experienced treatment failure with kinase inhibitor- and CTLA-4/PD-1 pathway-blocking therapies and in combination with these therapies.
Acknowledgment
Assistance with clinical trial management was provided by Anne Clarke (Celldex Therapeutics).
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010; 45th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009; 23rd Annual Meeting of the Society for Immunotherapy of Cancer, San Diego, CA, October 31, 2008; 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL; American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer Conference on Molecular Targets and Cancer Immunotherapeutics, San Francisco, CA, October 22-26, 2007.
Appendix
Table A1.
Pharmacokinetic Parameters of Glembatumumab Vedotin for the MTDs Established for Variable Dosing Frequencies
| Parameter | Schedule 1* MTD (1.88 mg/kg) |
Schedule 2† MTD (1.5 mg/kg) |
Schedule 3‡ MTD (1.0 mg/kg) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADC |
TA |
MMAE |
ADC |
TA |
MMAE |
ADC |
TA |
MMAE |
||||||||||
| Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | Mean | % CV | |
| Cycle 1 | ||||||||||||||||||
| No. of patients§ | 19 | 19 | 19 | 6 | 6 | 6 | 15 | 15 | 15 | |||||||||
| AUCinf_pred, h·ng/mL | 1,435,306 | 67 | 2,601,751 | 39 | 1,085 | 61 | 1,067,361 | 52 | 1,903,704 | 45 | 1,112 | 58 | 696,729 | 76 | 1,314,163 | 60 | 1,072 | 53 |
| Cmax, ng/mL | 36,709 | 29 | 47,438 | 43 | 5.4 | 50 | 27,471 | 18 | 32,535 | 21 | 4.8 | 63 | 19,650 | 35 | 23,475 | 25 | 4.3 | 92 |
| Tmax, hours | ||||||||||||||||||
| Median | 1.5 | 1.5 | 25.5 | 1.5 | 1.6 | 25.0 | 1.5 | 1.5 | 24.4 | |||||||||
| Range | 1.5-5.5 | 1.5-5.4 | 7.4-173 | 0.04-1.5 | 1.5-2.5 | 21.5-335.7 | 1.5-3.5 | 1.5-3.5 | 7.8-29.8 | |||||||||
| t1/2, hours | 27.4 | 47 | 38.9 | 59 | 80 | 73 | 20.6 | 27 | 36.2 | 32 | NA | 23.6 | 67 | 37.8 | 73 | NA | ||
| Clpred, mL/h/kg | 1.63 | 58 | 0.91 | 65 | NA | 1.77 | 56 | 0.97 | 56 | NA | 2.6 | 103 | 1.2 | 85 | NA | |||
| Vsspred, mL/kg | 46.95 | 46 | 34.7 | 52 | NA | 39.3 | 60 | 37.3 | 55 | NA | 52.6 | 40 | 40.4 | 42 | NA | |||
| Cycle 2 | ||||||||||||||||||
| No. of patients§ | 19 | 19 | 19 | 7 | 7 | 7 | 12 | 12 | 12 | |||||||||
| AUCinf_pred, h·ng/mL | 1,434,530 | 39 | 2,818,775 | 52 | 1,094 | 47 | 1,466,200 | 49 | 2,459,099 | 67 | 1,025.9 | 67 | 568,887 | 59 | 1,109,107 | 42 | 717 | 43 |
| Cmax, ng/mL | 36,471 | 21 | 48,118 | 43 | 5.8 | 56 | 51,058 | 114 | 32,259 | 26 | 4.5 | 38 | 16,942 | 29 | 23,519 | 25 | 6.3 | 111 |
| Tmax, hours | ||||||||||||||||||
| Median | 1.7 | 2.5 | 25.3 | 2.5 | 2.5 | 25.4 | 1.5 | 1.5 | 24.0 | |||||||||
| Range | 1.5-2.5 | 1.5-3.5 | 7.5-175 | 1.5-7.5 | 1.0-2.5 | 23.2-167.8 | 1.5-2.5 | 1.5-2.5 | 1.5-25.6 | |||||||||
| t1/2, hours | 27.41 | 34 | 35.7 | 30 | 71 | 63 | 24.3 | 24 | 47.8 | 61 | NA | 21.3 | 51 | 30.1 | 37 | NA | ||
| Clpred, mL/h/kg | 1.56 | 51 | 0.84 | 63 | NA | 1.24 | 48 | 0.88 | 66 | NA | 3.1 | 113 | 1.3 | 95 | NA | |||
| Vsspred, mL/kg | 47.8 | 49 | 30.8 | 62 | NA | 32.6 | 66 | 40.1 | 56 | NA | 54.0 | 40 | 33.7 | 40 | NA | |||
| Accumulation ratio‖ | 0.999 | 1.083 | 1.030 | 1.374 | 1.292 | 0.922 | 0.953 | 0.844 | 0.669 | |||||||||
NOTE. Data shown as mean (% CV), except No. of patients, which is an absolute value, and Tmax, which is shown as median and range. Terminal phase parameters for MMAE estimated from 2 points; estimates for total antibody and antibody-drug conjugate were estimated from at least 3 points.
Abbreviations: ADC, antibody-drug conjugate; AUCinf_pred, area under the concentration-time curve extrapolated to infinity using the last predicted concentration; Clpred, predicted clearance; Cmax, maximum concentration; CV, coefficient of variation of the geometric mean; MMAE, monomethyl auristatin E; MTD, maximum-tolerated dose; NA, not applicable (the terminal phase of MMAE disposition was inadequately defined); t1/2, half-life; TA, total CR011 antibody; Tmax, time to maximum concentration; Vsspred, predicted volume of distribution at steady state.
Schedule 1: once every 3 weeks.
Schedule 2: once per week for 2 of 3 weeks.
Schedule 3: once per week.
Includes data for evaluated patients who received the indicated dose level for either cycle 1 or 2, regardless of initial treatment assignment.
Accumulation ratios calculated from AUCinf_pred values in cycle 2 to cycle 1.
Fig A1.
Skin rash correlates with prolonged progression-free survival and greater overall response rate (ORR). The Kaplan-Meier plot includes all pooled data from patients treated at the maximum-tolerated dose (MTD) level for each dosing schedule: 1.88 mg/kg once every 3 weeks (n = 43), 1.5 mg/kg once per week for 2 of 3 weeks (n = 6), and 1.0 mg/kg once per week (n = 15). (*) Excludes patients without postbaseline assessment of measurable lesions. (†) Includes one response (93% tumor shrinkage) that was not sustained at the subsequent assessment. (‡) Three of these patients developed rash in subsequent cycles. HR, hazard ratio.
Footnotes
See accompanying article on page 3619
Supported by CuraGen (now Celldex Therapeutics).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00412828.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Elizabeth Crowley, Celldex Therapeutics (C); Jennifer A. Green, Celldex Therapeutics (C); Thomas Hawthorne, Celldex Therapeutics (C); Thomas A. Davis, Celldex Therapeutics (C) Consultant or Advisory Role: Omid Hamid, Celldex Therapeutics (U); Ronit Simantov, Celldex Therapeutics (C); Mario Sznol, Bristol-Myers Squibb (C), MedImmune (C), Amgen (C), Genentech (C), Symphogen (C), Merus (C), Anaeropharma (C), Lion Biotechnologies (C), Kyowa-Kirin (C), Immune Design (C), Nektar (C) Stock Ownership: Elizabeth Crowley, Celldex Therapeutics; Jennifer A. Green, Celldex Therapeutics; Thomas Hawthorne, Celldex Therapeutics; Thomas A. Davis, Celldex Therapeutics Honoraria: Anna C. Pavlick, Bristol-Myers Squibb Research Funding: Omid Hamid, Celldex Therapeutics; Anna C. Pavlick, Bristol-Myers Squibb, Genentech, Merck Expert Testimony: None Patents, Royalties, and Licenses: Ronit Simantov, Antibodies Directed to gpNMB and Uses Thereof, US patent 20130058948 A1 Other Remuneration: Ronit Simantov, former employee of CuraGen (now Celldex Therapeutics)
AUTHOR CONTRIBUTIONS
Conception and design: Ronit Simantov, Elizabeth Crowley, Thomas Hawthorne, Mario Sznol, Patrick Hwu
Provision of study materials or patients: Patrick A. Ott, Anna C. Pavlick, Patrick Hwu
Collection and assembly of data: Patrick A. Ott, Omid Hamid, Anna C. Pavlick, Harriet Kluger, Kevin B. Kim, Peter D. Boasberg, Ronit Simantov, Elizabeth Crowley, Jennifer A. Green, Thomas Hawthorne, Mario Sznol, Patrick Hwu
Data analysis and interpretation: Patrick A. Ott, Omid Hamid, Anna C. Pavlick, Harriet Kluger, Elizabeth Crowley, Jennifer A. Green, Thomas Hawthorne, Thomas A. Davis, Mario Sznol, Patrick Hwu
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 7.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weterman MA, Ajubi N, van Dinter IM, et al. Nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 10.Tse KF, Jeffers M, Pollack VA, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12:1373–1382. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- 11.Shikano S, Bonkobara M, Zukas PK, et al. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 12.Ripoll VM, Irvine KM, Ravasi T, et al. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 13.Selim AA, Abdelmagid SM, Kanaan RA, et al. Anti-osteoactivin antibody inhibits osteoblast differentiation and function in vitro. Crit Rev Eukaryot Gene Expr. 2003;13:265–275. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.180. [DOI] [PubMed] [Google Scholar]
- 14.Sheng MH, Wergedal JE, Mohan S, et al. Osteoactivin is a novel osteoclastic protein and plays a key role in osteoclast differentiation and activity. FEBS Lett. 2008;582:1451–1458. doi: 10.1016/j.febslet.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16:2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- 16.Kuan CT, Wakiya K, Dowell JM, et al. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res. 2006;12:1970–1982. doi: 10.1158/1078-0432.CCR-05-2797. [DOI] [PubMed] [Google Scholar]
- 17.Rose AA, Pepin F, Russo C, et al. Osteoactivin promotes breast cancer metastasis to bone. Mol Cancer Res. 2007;5:1001–1014. doi: 10.1158/1541-7786.MCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 18.Rich JN, Shi Q, Hjelmeland M, et al. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278:15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- 19.Onaga M, Ido A, Hasuike S, et al. Osteoactivin expressed during cirrhosis development in rats fed a choline-deficient, l-amino acid-defined diet, accelerates motility of hepatoma cells. J Hepatol. 2003;39:779–785. doi: 10.1016/s0168-8278(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 20.Rose AA, Annis MG, Dong Z, et al. ADAM10 releases a soluble form of the GPNMB/osteoactivin extracellular domain with angiogenic properties. PLoS One. 2010;5:e12093. doi: 10.1371/journal.pone.0012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 22.Doronina SO, Bovee TD, Meyer DW, et al. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem. 2008;19:1960–1963. doi: 10.1021/bc800289a. [DOI] [PubMed] [Google Scholar]
- 23.McDonagh CF, Turcott E, Westendorf L, et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel. 2006;19:299–307. doi: 10.1093/protein/gzl013. [DOI] [PubMed] [Google Scholar]
- 24.Pollack VA, Alvarez E, Tse KF, et al. Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol. 2007;60:423–435. doi: 10.1007/s00280-007-0490-z. [DOI] [PubMed] [Google Scholar]
- 25.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 27.DeGeorge JJ, Ahn CH, Andrews PA, et al. Regulatory considerations for preclinical development of anticancer drugs. Cancer Chemother Pharmacol. 1998;41:173–185. doi: 10.1007/s002800050726. [DOI] [PubMed] [Google Scholar]
- 28.Li YN, Zhang L, Li XL, et al. Glycoprotein nonmetastatic B as a prognostic indicator in small cell lung cancer. APMIS. 2014;122:140–146. doi: 10.1111/apm.12107. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H, Shimazawa M, Kimura M, et al. The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis. Sci Rep. 2012;2:573. doi: 10.1038/srep00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burris HA, III, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 31.Krop IE, Lorusso P, Miller KD, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30:3234–3241. doi: 10.1200/JCO.2011.40.5902. [DOI] [PubMed] [Google Scholar]
- 32.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 33.Williams MD, Esmaeli B, Soheili A, et al. GPNMB expression in uveal melanoma: A potential for targeted therapy. Melanoma Res. 2010;20:184–190. doi: 10.1097/CMR.0b013e3283364a08. [DOI] [PubMed] [Google Scholar]
- 34.Tomihari M, Hwang SH, Chung JS, et al. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp Dermatol. 2009;18:586–595. doi: 10.1111/j.1600-0625.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pahl MV, Vaziri ND, Yuan J, et al. Upregulation of monocyte/macrophage HGFIN (Gpnmb/osteoactivin) expression in end-stage renal disease. Clin J Am Soc Nephrol. 2010;5:56–61. doi: 10.2215/CJN.03390509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung JS, Sato K, Dougherty II, et al. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung JS, Dougherty I, Cruz PD, Jr, et al. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 38.Tomihari M, Chung JS, Akiyoshi H, et al. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70:5778–5787. doi: 10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung JS, Cruz PD, Jr, Ariizumi K. Inhibition of T-cell activation by syndecan-4 is mediated by CD148 through protein tyrosine phosphatase activity. Eur J Immunol. 2011;41:1794–1799. doi: 10.1002/eji.201041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yardley D, Weaver R, Melisko M, et al. A randomized phase 2 study of the antibody-drug conjugate CDX-011 in advanced GPNMB-overexpressing breast cancer: The EMERGE study. Cancer Res. 2012;72(suppl):543s. abstr P6-10-01. [Google Scholar]
- 41.Rose AAN, Siegel PM. B-raf/Mek/Erk pathway inhibition induces GPNMB expression and sensitizes melanoma cells to the antibody-drug conjugate glembatumumab vedotin. Mol Cancer Ther. 2013;12:C232. [Google Scholar]
- 42.Qian X, Mills E, Torgov M, et al. Pharmacologically enhanced expression of GPNMB increases the sensitivity of melanoma cells to the CR011-vcMMAE antibody-drug conjugate. Mol Oncol. 2008;2:81–93. doi: 10.1016/j.molonc.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8:e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]