Abstract
Background
Tuberculosis remains an important cause of death among patients infected with the human immunodeficiency virus (HIV). Robust data are lacking with regard to the timing for the initiation of antiretroviral therapy (ART) in relation to the start of antituberculosis therapy.
Methods
We tested the hypothesis that the timing of ART initiation would significantly affect mortality among adults not previously exposed to antiretroviral drugs who had newly diagnosed tuberculosis and CD4+ T-cell counts of 200 per cubic millimeter or lower. After beginning the standard, 6-month treatment for tuberculosis, patients were randomly assigned to either earlier treatment (2 weeks after beginning tuberculosis treatment) or later treatment (8 weeks after) with stavudine, lamivudine, and efavirenz. The primary end point was survival.
Results
A total of 661 patients were enrolled and were followed for a median of 25 months. The median CD4+ T-cell count was 25 per cubic millimeter, and the median viral load was 5.64 log10 copies per milliliter. The risk of death was significantly reduced in the group that received ART earlier, with 59 deaths among 332 patients (18%), as compared with 90 deaths among 329 patients (27%) in the later-ART group (hazard ratio, 0.62; 95% confidence interval [CI]; 0.44 to 0.86; P = 0.006). The risk of tuberculosis-associated immune reconstitution inflammatory syndrome was significantly increased in the earlier-ART group (hazard ratio, 2.51; 95% CI, 1.78 to 3.59; P<0.001). Irrespective of the study group, the median gain in the CD4+ T-cell count was 114 per cubic millimeter, and the viral load was undetectable at week 50 in 96.5% of the patients.
Conclusions
Initiating ART 2 weeks after the start of tuberculosis treatment significantly improved survival among HIV-infected adults with CD4+ T-cell counts of 200 per cubic millimeter or lower. (Funded by the French National Agency for Research on AIDS and Viral Hepatitis and the National Institutes of Health; CAMELIA ClinicalTrials.gov number, NCT01300481.)
Tuberculosis is a major cause of death in persons infected with the human immunodeficiency virus (HIV), especially in resource-limited settings.1,2 Despite effective tuberculosis therapy, mortality is particularly high among patients with severe immunosuppression.3,4 Although mortality among HIV-infected patients has been reported to be approximately 30% within the first 2 months of tuberculosis treatment if antiretroviral therapy (ART) is withheld,5 the timing for starting ART in patients with tuberculosis has remained unclear.
Arguments that support delayed initiation of ART include concern about the combined toxic effects of drugs, an increased risk of the immune reconstitution inflammatory syndrome (IRIS), and poor adherence to a regimen that involves an increased pill burden. In contrast, arguments that support earlier initiation of ART include more rapid restoration of the immunocompetence needed to cure the tuberculosis and the enhancement of immune responses to other specific pathogens, thus reducing the risk of opportunistic infections.6,7 The Starting Antiretroviral Therapy at Three Points in Tuberculosis (SAPIT) study (ClinicalTrials.gov number, NCT00398996) showed that initiating ART during tuberculosis therapy provided a significant survival advantage in patients with CD4+ T-cell counts lower than 500 per cubic millimeter.8 The retrospective Multicenter Cohort of Patients with HIV Infection in the Madrid South-Eastern Metropolitan Crown (COMESEM) study showed that survival was further increased when ART was started within 2 months after the start of tuberculosis treatment.9 On the basis of these data, the World Health Organization (WHO) guidelines recommend that ART be started as soon as possible within the first 8 weeks after tuberculosis therapy is begun.10 However, there has been no specific guidance regarding timing within this critical period, owing to the lack of evidence-based research addressing this point.
To determine whether the earlier initiation of ART (2 weeks after the onset of tuberculosis treatment), as compared with later initiation (8 weeks afterward), could reduce mortality among patients with advanced immunodeficiency, we designed the Cambodian Early versus Late Introduction of Antiretrovirals (CAMELIA) trial.
METHODS
STUDY DESIGN AND OVERSIGHT
CAMELIA was a prospective, randomized, multicenter, open-label superiority trial (with no placebo) designed to determine the effect of earlier versus later initiation of ART on mortality among HIV-infected adults with no previous exposure to antiretroviral drugs who had a CD4+ T-cell count of 200 per cubic millimeter or less and had received a new diagnosis of tuberculosis, as confirmed by any clinical sample that was smear-positive for acid-fast bacilli.
Inpatients and outpatients were recruited between January 31, 2006, and May 27, 2009, from five hospitals in Cambodia. (For detailed inclusion criteria, see the Supplementary Appendix, available with the full text of this article at NEJM.org.) After providing written informed consent, patients were randomly assigned to begin ART either earlier (2 weeks [±4 days]) or later (8 weeks [±4 days]) after the start of treatment for tuberculosis. Randomization was performed with the use of a computer-generated, per-block, random-numbers list in a 1:1 ratio and was stratified according to study site and CD4+ T-cell count at enrollment (≤50 or 51 to 200 per cubic millimeter). The study was conducted in accordance with the protocol, which is available at NEJM.org. The funding agencies had no role in the study design, data collection and analysis, or decision to submit the manuscript for publication.
The trial was approved by the ethics review boards of the Cambodian government, the Immune Disease Institute, and Médecins sans Frontières. Patient representatives and community advisory boards participated in the implementation and review of the trial.
STUDY INTERVENTION
Patients were evaluated by on-site clinicians at 2, 4, 8, 10, 14, 18, 22, 26, 34, 42, and 50 weeks after the initiation of antituberculosis therapy, again at weeks 58 and 78, and every 6 months thereafter until the end of data collection, which was 50 weeks after the last patient was enrolled (May 13, 2010). Tuberculosis treatment consisted of a standard daily regimen of isoniazid, rifampin, ethambutol, and pyrazinamide during the first 2 months, followed by daily administration of isoniazid and rifampin during the ensuing 4 months. Mycobacterial culture and drug-susceptibility testing were systematically performed at the time of enrollment and in the event of treatment failure or recurrence of tuberculosis. When drug resistance was documented, tuberculosis treatment was modified on the basis of the WHO guidelines11 or the results of testing for susceptibility to second-line drugs.
All patients received counseling regarding adherence to the treatment regimen, as well as prophylaxis with trimethoprim–sulfamethoxazole and fluconazole (if the CD4+ T-cell count was below 100 per cubic millimeter). ART consisted of stavudine, lamivudine, and efavirenz, in accordance with the Cambodian national guidelines. After 1 year of follow-up, recommendations were given to consider switching from stavudine to zidovudine to minimize the risk of mitochondrial toxic effects and switching from efavirenz to nevirapine to reduce costs in accordance with Cambodian national guidelines and to avoid the risk of injury to the fetus in case of pregnancy.
STUDY END POINTS
The primary study end point was survival. Secondary end points included tuberculosis outcome, CD4+ T-cell count, viral load below the detection threshold (2.4 log10 [250] copies per milliliter), side effects of the drugs, and the occurrence of tuberculosis-associated IRIS, which was defined as the worsening or emergence of tuberculosis symptoms after the initiation of ART in any patient who had no evidence of newly acquired infection, evolution of drug-resistant tuberculosis, infection with a previously recognized pathogen, or side effects of antiretroviral therapy. The adjudication of IRIS was not blinded.
HIV LABORATORY MONITORING
CD4+ T-cell counts and plasma HIV RNA viral loads (Biocentric)12,13 were measured at weeks 8, 26, 50, and 78 and then every 6 months. Bulk sequencing of reverse transcriptase, protease, or both was performed to detect resistance mutations in all patients with a detectable viral load at week 50 and thereafter.
STATISTICAL ANALYSIS
On the basis of empirical observations and the available literature,14,15 mortality in the reference group (i.e., patients receiving ART later, at 8 weeks) was expected to be 35%. Assuming that mortality would be reduced by 10% in the earlier-ART group (hazard ratio, 1.5), with a two-sided type I error rate of 5%, a power of 80%, and use of a log-rank test, we calculated that we would need to enroll 330 patients in each of the two treatment groups.
A data and safety monitoring board reviewed interim analyses that were not shared with the study team. The prespecified rule for stopping the study was based on the boundary for significance, as described by Peto et al.16 and Slutsky and Lavery.17 There were six board meetings between February 2006 and September 2009, during which early termination of the study was not considered. On November 7, 2006, the board recommended that all patients be followed to the calendar-date end of the study (50 weeks after enrollment of the last patient) rather than for only 50 weeks, as originally scheduled. The study protocol was amended accordingly.
Analyses were based on the intention-to-treat principle. Between-group comparisons of characteristics of the patients were performed with the use of Student’s t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The primary end point, survival, was described with the use of Kaplan–Meier estimates and was compared between groups with the use of the log-rank test, as specified in the protocol.18 The Cox proportional-hazards model was used to identify factors associated with an increased risk of death. Factors associated with mortality with a P value of less than 0.20 in the univariate analysis were entered in the multivariate model, and nonsignificant factors were removed by means of a backward-selection procedure. The proportional-hazards assumption was checked with the use of a test on Schoenfeld residuals and was found to be valid for all factors investigated. Two-sided hypotheses and tests were used for all statistical inferences. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
CHARACTERISTICS OF THE PATIENTS
A total of 661 patients (236 women) were enrolled in the study: 332 in the earlier-ART group and 329 in the later-ART group (Fig. 1). The median age of those enrolled was 35 years, the median body-mass index (the weight in kilograms divided by the square of the height in meters) was 16.7, the median CD4+ T-cell count was 25 per cubic millimeter, and the median viral load was 5.64 log10 copies per milliliter. There were no significant differences between the two groups with regard to baseline characteristics (Table 1).
Figure 1. Screening, Enrollment, and Follow-up.
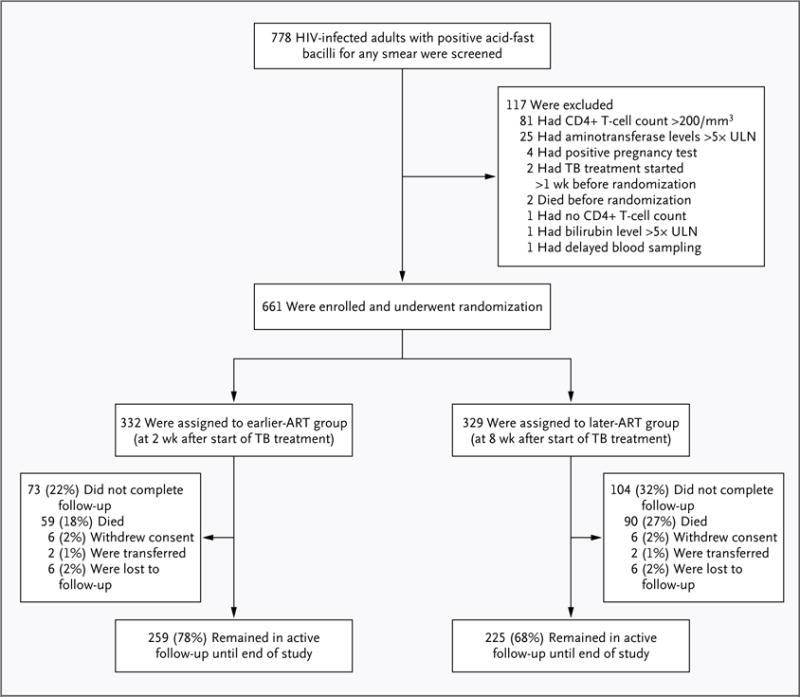
Of the 4 patients who were not enrolled because of a positive pregnancy test, 1 also had impaired liver function. Of the 81 patients who were not enrolled because their CD4+ T-cell counts exceeded 200 per cubic millimeter, 2 also had impaired liver function and 1 was pregnant. ART denotes antiretroviral therapy, TB tuberculosis, and ULN upper limit of the normal range.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Earlier ART (N = 332) |
Later ART (N = 329) |
P Value |
|---|---|---|---|
| Male sex — no. of patients (%) | 215 (64.8) | 210 (63.8) | 0.80 |
| Age — yr | 0.38 | ||
| Median | 35 | 36 | |
| Interquartile range | 30–41 | 30–42 | |
| Body-mass index† | 0.90 | ||
| Median | 16.7 | 16.8 | |
| Interquartile range | 15.3–18.3 | 15.2–18.6 | |
| Karnofsky performance score — no. of patients (%)‡ | 0.83 | ||
| ≥80 | 43 (13.0) | 44 (13.4) | |
| 50 to 70 | 259 (78.0) | 251 (76.3) | |
| ≤40 | 30 (9.0) | 34 (10.3) | |
| CD4+ T-cell count | 0.61 | ||
| Median — per mm3 | 25 | 25 | |
| Interquartile range — per mm3 | 11–56 | 10–55 | |
| CD4+ T-cell count level | 0.79 | ||
| ≤50/mm3 — no. of patients (%) | 237 (71.4) | 238 (72.3) | |
| 51–200/mm3 — no. of patients (%) | 95 (28.6) | 91 (27.7) | |
| Viral load — log10 copies/ml | 0.25 | ||
| Median | 5.60 | 5.66 | |
| Interquartile range | 5.20–6.02 | 5.25–6.00 | |
| Hemoglobin — g/dl | 0.98 | ||
| Median | 8.7 | 8.7 | |
| Interquartile range | 7.1–10.4 | 7.0–10.2 | |
| Opportunistic infections — no. of patients (%) | |||
| Pneumocystis jirovecii pneumonia | 3 (0.9) | 7 (2.1) | 0.22 |
| Esophageal candidiasis | 8 (2.4) | 6 (1.8) | 0.60 |
| Extrapulmonary cryptococcosis | 6 (1.8) | 4 (1.2) | 0.53 |
| Cryptosporidiosis, with diarrhea for >1 mo | 1 (0.3) | 1.00 | |
| Herpes simplex infection | 1 (0.3) | 1.00 | |
| Progressive multifocal leukoencephalopathy | 1 (0.3) | 1.00 |
ART denotes antiretroviral therapy.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The Karnofsky performance score is a measure of the patient’s general condition and degree of autonomy on a scale ranging from 0 to 100, with higher numbers indicating better performance.
Positive smears for acid-fast bacilli were obtained from the respiratory tract in 278 of the 332 patients (83.7%) in the earlier-ART group and in 278 of the 329 patients (84.5%) in the later-ART group (P = 0.25). (A list of positive smears obtained from other anatomical sites can be found in the Supplementary Appendix.) Mycobacterium tuberculosis was identified by means of culture in 282 patients (84.9%) and 295 patients (89.7%) in the earlier-ART and later-ART groups, respectively, and nontuberculous mycobacteria were identified in 12 patients (3.6%) and 4 patients (1.2%) in the two groups, respectively. Culture results were negative in the remaining 38 patients (11.4%) in the earlier-ART group and 30 patients (9.1%) in the later-ART group and thus did not permit mycobacterial identification. The pattern of distribution of tuberculosis-drug resistance was similar in the two treatment groups (see the Supplementary Appendix).
FOLLOW-UP
The median follow-up period was 25 months (interquartile range, 14 to 36). Of the 332 participants in the earlier-ART group, 259 (78.0%) were followed until the end of the study, 59 (17.8%) died, 6 (1.8%) withdrew, 2 (0.6%) were transferred to another facility for follow-up, and 6 (1.8%) were lost to follow-up. Of the 329 participants in the later-ART group, 225 (68.4%) were followed until the end of the study: 90 (27.4%) died, 6 (1.8%) withdrew, 2 (0.6%) were transferred, and 6 (1.8%) were lost to follow-up. The leading cause of death was tuberculosis in both groups (see the Supplementary Appendix).
SURVIVAL
Patients in the earlier-ART group had a significantly higher rate of survival than did those in the later-ART group (P = 0.004 by the log-rank test) (Fig. 2). The rate of death was 8.28 per 100 person-years (95% confidence interval [CI], 6.42 to 10.69) in the earlier-ART group and 13.77 per 100 person-years (95% CI, 11.20 to 16.93) in the later-ART group (P = 0.002). In the multivariate analysis, the adjusted hazard ratio for death in the earlier-ART group, as compared with the later-ART group, was 0.62 (95% CI, 0.44 to 0.86; P = 0.006). Even when patients who were lost to follow-up were considered to have died, survival remained significantly higher in the earlier-ART group (P = 0.005).
Figure 2.
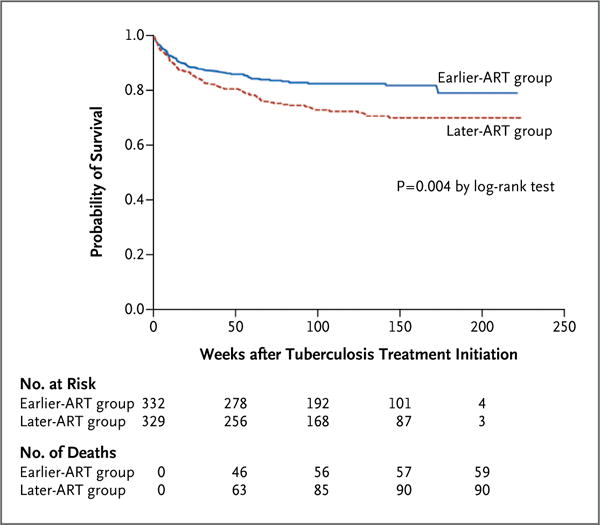
Kaplan–Meier Survival Estimates According to Study Group.
In the multivariate analysis, factors at study inclusion other than the later start of ART that were independently associated with an increased risk of death were an age of 40 years or older, a body-mass index less than 16, a Karnofsky performance score below 40 (on a scale that ranges from 0 to 100, with higher scores indicating better performance), an aspartate aminotransferase level more than 1.25 times the upper limit of the normal range, disseminated tuberculosis, nontuberculous mycobacterial disease, and multidrug-resistant tuberculosis (i.e., tuberculosis that was resistant to isoniazid and rifampin) (Table 2). When the 13 patients with multidrug-resistant tuberculosis or the 16 patients with nontuberculous mycobacterial disease were excluded, the adjusted hazard ratios for death in the earlier-ART group, as compared with the later-ART group, were 0.63 (95% CI, 0.45 to 0.88) and 0.60 (95% CI, 0.43 to 0.85), respectively. In the multivariate analysis, a CD4+ T-cell count of 50 per cubic millimeter or lower at study entry was not associated with an increased risk of death, as compared with a count of 51 to 200 per cubic millimeter (P = 0.24). To test whether the effect of initiating ART early would be similar between patients with lower CD4+ T-cell counts and those with higher counts, we introduced an interaction between the CD4+ T-cell count category (≤50 per cubic millimeter vs. 51 to 200 per cubic millimeter) and the study group: the hazard ratio associated with earlier ART did not differ significantly between patients with lower counts and those with higher counts (P = 0.49).
Table 2.
Factors Associated with Mortality.*
| Factor | Crude Hazard Ratio on Univariate Analysis (95% CI) |
P Value | Adjusted Hazard Ratio on Multivariate Analysis (95% CI)† |
P Value |
|---|---|---|---|---|
| Treatment group | 0.004 | 0.006 | ||
| Earlier ART | 0.62 (0.45–0.86) | 0.62 (0.44–0.86) | ||
| Later ART | 1.00 | 1.00 | ||
| Sex | 0.05 | |||
| Male | 1.00 | |||
| Female | 1.39 (1.00–1.92) | |||
| Age | 0.14 | 0.03 | ||
| ≤29 yr | 1.00 | 1.00 | ||
| 30–39 yr | 1.12 (0.72–1.75) | 1.26 (0.81–1.98) | ||
| ≥40 yr | 1.50 (0.96–2.33) | 1.87 (1.18–2.95) | ||
| Body-mass index | 0.001 | 0.02 | ||
| <16.0 | 1.79 (1.17–2.75) | 1.61 (1.03–2.54) | ||
| 16.0–17.0 | 0.86 (0.49–1.51) | 0.84 (0.47–1.48) | ||
| 17.1–18.5 | 0.93 (0.54–1.60) | 1.08 (0.62–1.91) | ||
| >18.5 | 1.00 | 1.00 | ||
| Karnofsky performance score | <0.001 | <0.001 | ||
| ≥80 | 1.00 | 1.00 | ||
| 50–70 | 1.78 (0.95–3.30) | 1.60 (0.83–3.09) | ||
| ≤40 | 5.23 (2.62–10.43) | 4.69 (2.17–10.11) | ||
| CD4+ T-cell count | 0.01 | 0.24 | ||
| ≤50 per mm3 | 1.60 (1.08–2.38) | 1.29 (0.84–1.98) | ||
| 51–200 per mm3 | 1.00 | 1.00 | ||
| Viral load | 0.80 | |||
| ≤5.0 log10 copies/ml | 1.00 | |||
| 5.1–6.0 log10 copies/ml | 1.15 (0.73–1.80) | |||
| >6.0 log10 copies/ml | 1.05 (0.62–1.76) | |||
| Hemoglobin level | <0.001 | |||
| ≤7.0 g/dl | 2.53 (1.59–4.01) | |||
| 7.1–8.5 g/dl | 1.65 (1.00–2.73) | |||
| 8.6–10.0 g/dl | 1.73 (1.06–2.83) | |||
| >10.0 g/dl | 1.00 | |||
| Aspartate aminotransferase level | <0.001 | 0.02 | ||
| ≤1.25× ULN | 1.00 | 1.00 | ||
| >1.25× ULN | 1.76 (1.26–2.48) | 1.53 (1.08–2.18) | ||
| Alanine aminotransferase level | 0.84 | |||
| ≤1.25× ULN | 1.00 | |||
| >1.25× ULN | 0.95 (0.61–1.50) | |||
| Mycobacterial disease pattern | <0.001 | <0.001 | ||
| Pulmonary tuberculosis | 1.00 | 1.00 | ||
| Extrapulmonary tuberculosis | 0.99 (0.51–1.91) | 1.02 (0.52–2.01) | ||
| Disseminated tuberculosis | 2.52 (1.78–3.55) | 2.55 (1.78–3.65) | ||
| Nontuberculous mycobacteria | 3.00 (1.30–6.88) | 3.21 (1.35–7.63) | ||
| Tuberculosis-drug resistance | 0.01 | <0.001 | ||
| No | 1.00 | 1.00 | ||
| Yes | 0.95 (0.60–1.50) | 1.10 (0.68–1.74) | ||
| Multidrug resistance‡ | 3.60 (1.76–7.39) | 7.65 (3.45–16.99) | ||
| Tuberculosis-associated IRIS§ | 0.37 | |||
| No | 1.00 | |||
| Yes | 0.83 (0.51–1.30) | |||
| Hospitalization | 0.85 | |||
| No | 1.00 | |||
| Yes | 0.97 (0.70–1.34) |
All factors except immune reconstitution inflammatory syndrome (IRIS) were present at study inclusion. Values were calculated with the use of Cox univariate and multivariate models. ULN deneotes upper limit of the normal range.
Values were adjusted according to two stratification factors: study site and CD4+ T-cell count at baseline.
Multidrug resistance was defined as resistance to both isoniazid and rifampin.
This factor was time-dependent, since exposure to the risk was considered to extend until week 50.
TUBERCULOSIS OUTCOMES
The median duration of tuberculosis treatment was 26.0 weeks (interquartile range, 25.8 to 26.9) in the earlier-ART group and 26.0 weeks (interquartile range, 25.7 to 26.4) in the later-ART group (P = 0.13). When tuberculosis treatment ended, there was no significant difference between the two groups with regard to tuberculosis outcomes (see the Supplementary Appendix). Recurrence of tuberculosis was observed in 22 patients: 8 in the earlier-ART group and 14 in the later-ART group (P = 0.11).
VIROLOGIC RESPONSE AND IMMUNE RECONSTITUTION
ART was initiated in 319 of 332 patients (96%) in the earlier-ART group at a median of 14 days after the onset of tuberculosis treatment (interquartile range, 14 to 15) and in 300 of 329 patients (91%) in the later-ART group at a median time of 56 days (interquartile range, 56 to 57). At week 50, the viral load was undetectable in 96.5% of patients, with no difference between the two study groups (P = 0.82), whereas the median gain in the CD4+ T-cell count was 118 per cubic millimeter (interquartile range, 67 to 191) in the earlier-ART group and 112 per cubic millimeter (interquartile range, 53 to 175) in the later-ART group (P = 0.22). At all subsequent follow-up times, there was no difference between the groups in the percentage of patients with an undetectable viral load (which remained consistently above 95%) or in the median CD4+ T-cell count (Fig. 3).
Figure 3.
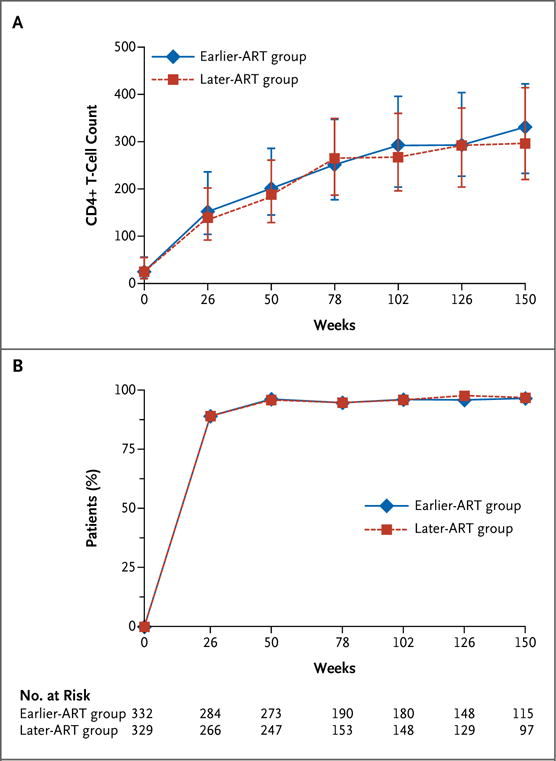
Changes in the CD4+ T-Cell Count and Percentage of Patients with Undetectable Viral Load during Follow-up.
TUBERCULOSIS-ASSOCIATED IRIS
The incidence of tuberculosis-associated IRIS within the first 50 weeks of follow-up was 3.76 cases per 100 person-months (95% CI, 3.14 to 4.47) in the earlier-ART group and 1.53 cases per 100 person-months (95% CI, 1.13 to 2.03) in the later-ART group. Thus, the risk of tuberculosis-associated IRIS was significantly increased in the earlier-ART group, with 110 events, as compared with 45 events in the later-ART group (hazard ratio, 2.51; 95% CI, 1.78 to 3.59; P<0.001). After the initiation of ART, tuberculosis-associated IRIS occurred at a median of 14 days (interquartile range, 10 to 42) in the earlier-ART group and 16 days (interquartile range, 11 to 39) in the later-ART group (P = 0.89). Six deaths were directly related to tuberculosis-associated IRIS, all occurring in the earlier-ART group.
ADVERSE EVENTS
The incidence of serious drug-related adverse events was 2.93 events per 100 person-months (95% CI, 2.58 to 3.32) in the earlier-ART group and 3.21 (95% CI, 2.83 to 3.63) in the later-ART group (P = 0.31). Hepatic toxic effects accounted for 43% of all serious drug-related adverse events (Table 3). Drug toxicity was the second most common cause of death, after tuberculosis, accounting for 5 and 12 deaths (including 4 and 6 due to lactic acidosis) in the earlier-ART and later-ART groups, respectively. (Additional information about adverse events can be found in the Supplementary Appendix.)
Table 3.
Distribution of Serious Drug-Related Adverse Events.*
| Event | Earlier ART | Later ART number of events |
Total |
|---|---|---|---|
| Hepatic toxicity | 107 | 105 | 212 |
| Anemia | 69 | 43 | 112 |
| Mitochondrial toxicity | 16 | 19 | 35 |
| Cutaneous toxicity | 10 | 22 | 32 |
| Electrolyte disorders | 12 | 14 | 26 |
| Metabolic disorders | 11 | 10 | 21 |
| Neutropenia | 7 | 14 | 21 |
| Peripheral neuropathy | 8 | 8 | 16 |
| Gastrointestinal disorders | 3 | 6 | 9 |
| Neuropsychiatric disorders | 6 | 1 | 7 |
| Ocular toxicity | 1 | 2 | 3 |
| Hypothyroidism | 0 | 1 | 1 |
| Ototoxicity | 1 | 0 | 1 |
| Total | 251 | 245 | 496 |
Serious adverse events were defined as those that were grade 3 or higher. Drugs included antiretroviral agents.
DISCUSSION
The results of the CAMELIA trial show that initiating ART 2 weeks after the start of tuberculosis therapy significantly increases survival among HIV-infected adults with newly diagnosed tuberculosis and CD4+ T-cell counts of 200 per cubic millimeter or lower, as compared with delaying the start of ART for 8 weeks. The benefit of earlier ART was observed in patients with CD4+ T-cell counts of 50 per cubic millimeter or lower, as well as in patients with counts between 51 and 200 per cubic millimeter. For such patients, these results argue strongly in favor of beginning ART earlier despite an increased risk of IRIS. This is of particular relevance in resource-limited settings where tuberculosis is the leading cause of death in HIV-infected patients.19–21
Two other studies that examined the timing of ART in patients coinfected with tuberculosis are reported by Havlir et al.22 and by Abdool Karim et al.23 in this issue of the Journal. These studies differ from ours with respect to their design and the characteristics of their patient populations (i.e., higher body-mass index22 and higher baseline CD4+ T-cell counts22,23), which may at least in part explain the markedly lower overall mortality observed in these two studies (6.6% and 7.0%, respectively), as compared with an overall mortality in our study of more than 16% at week 50. The combination of applying our investigational strategy to this patient population, together with the longer follow-up period, maximized our ability to observe a potential survival difference between the earlier and later start of ART.
We chose to enroll patients with a positive smear for acid-fast bacilli in order to confirm the diagnosis of tuberculosis. Given the high level of immunodeficiency in all the patients enrolled and the focus on detection of acid-fast bacilli in this trial, we believe that our results would have been similar in similarly immunocompromised patients with negative smears. This view is supported by an observational study that showed no difference in mortality between HIV-infected patients with smear-positive and smear-negative pulmonary tuberculosis or extrapulmonary tuberculosis.24
Initiation of ART early in the course of tuberculosis treatment is associated with an increased incidence of tuberculosis-associated IRIS, especially when the CD4+ T-cell count is 50 per cubic millimeter or lower.25,26 We found that even though the incidence of IRIS was increased by a factor of 2.5 in the earlier-ART group, as compared with the later-ART group, and six cases were fatal, the survival rate was still higher in the earlier-ART group. Most cases of IRIS developed 2 to 3 weeks after the initiation of ART, irrespective of the study group. Thus, within the first weeks after ART is begun, clinicians must anticipate the possible occurrence of IRIS, which may require aggressive management.27,28 Studies of the mechanisms underlying the development of IRIS are under way, with a focus on natural killer cells and T cells.
In CAMELIA, the efficacy of ART was excellent, irrespective of the treatment group, as evidenced by a median gain in the CD4+ T-cell count that exceeded 100 per cubic millimeter and an undetectable viral load at week 50 in more than 95% of patients, which remained undetectable until the end of the trial. Nesting the trial in established clinical networks devoted to delivery of care and support of patient adherence to treatment certainly contributed to this achievement by maintaining a low rate of loss to follow-up.29–32
The goal of shortening the time to the start of ART was to significantly reduce the time that patients had profound immunodeficiency, since increased mortality among patients with the acquired immunodeficiency syndrome is strongly associated with the length of time that the CD4+ T-cell count is below 200 per cubic millimeter, particularly when the count remains below 50 per cubic millimeter.33
The median duration of follow-up was 25 months, which allowed us to assess whether the reduction in mortality observed in the earlier-ART group was maintained for longer than 12 months after the beginning of the study. The survival benefit derived from starting ART early continued to be observed up to 3 years. This survival advantage cannot be attributed to a difference between the two treatment groups with respect to the virologic response or the gain in the CD4+ T-cell count. Although the survival advantage was presumably related to the earlier immune reconstitution that occurred in the earlier-ART group, the precise underlying mechanism remains to be determined.
In summary, the CAMELIA trial showed a significant survival benefit when ART was initiated 2 weeks after the start of tuberculosis treatment in HIV-infected patients with a CD4+ T-cell count of 200 per cubic millimeter or lower.
Acknowledgments
Supported by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS 1295) and the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS (CIPRA KH001/DAID-ES ID 10425).
Dr. Delfraissy reports serving on the international board for HIV treatment of GlaxoSmithKline, Merck Sharp & Dohme–Chibret, Gilead, and Bristol-Myers Squibb; and Dr. Goldfeld, receiving funds from the Annenberg Foundation to her laboratory and to the Cambodian Health Committee to conduct studies and serving as a consultant for the Aeras Global TB Vaccine Foundation, which provides funds to the Cambodian Health Committee to conduct studies.
We thank the patients for their participation in the trial; Françoise Barré-Sinoussi for her help with initiating the CIPRA project and the CAMELIA trial and for her support throughout the trial; H.E. Eng Huot for his early and continuing support; Philippe Glaziou for providing the initial design of the study; Charles Mayaud for his clinical mentorship; Jean-Louis Sarthou for ensuring high-quality laboratory and basic scientific support during the trial; H.E. Mean Chhivun and H.E. Mao Tan Eang for the support of the Cambodian National AIDS and Tuberculosis Programs, respectively; Takmao, Kossamak, and Kampong Trach Hospitals for referring patients to the study sites; Bart Janssens, Petros Isaakidis, and Tony Reid for the support from Médecins sans Frontières Belgium to two of our study sites (Donkeo and Siem Reap Provincial Hospitals); the data and safety monitoring board, chaired by John Modlin, and the scientific advisory board, chaired by Charles Mayaud, for their significant contributions to the study; the scientific and logistic teams of the ANRS (Brigitte Bazin, Séverine Blesson, Alpha Diallo, Annie Metro, Saphonn Vonthanak, Isabelle Fournier-Nicolle, and Michel Kazatchkine) and the National Institutes of Health (Jane E. Bupp, Ray Y. Chen, Margaret Matula, Rod Hoff, Sandra Nusinoff Lehrman, Karen Near, Barbara Laughon, Mike Ussery, Trinh Ly, Mary Fanning, and Ana Martinez) for their support and intellectual contributions; and Pierre L’Her, Etienne Leroy-Terquem, the Organisation Franco-Cambodgienne de Pneumologie, Pean Polidy, Sylvia Taylor, Joséphine Braun, Vincent Guillemet, Adrienne Shapiro, Catherine Quillet, Jean-Paul Dousset, Anne-Marie Taburet, Wayne Wilson, and Vincent Deubel for their support.
APPENDIX
The authors’ affiliations are as follows: from the Pneumology Unit, Internal Medicine Department, Bicêtre Hospital, Assistance Publique–Hôpitaux de Paris (F.-X.B., J.-F.D.), and INSERM, Unité 1012, Paris XI University (J.-F.D.) — both in Le Kremlin-Bicêtre, France; the Cambodian Health Committee (T.S., D.L., O.M., S.C., K.K.L., B.D., S.S., M.F., A.E.G.); Institut Pasteur in Cambodia (L.B., E.N., B.G., B.S., S.V.); the Departments of Infectious Diseases (N.P.) and Pneumology (C.I.S.), Khmer Soviet Friendship Hospital; and Médecins sans Frontières (C.K., B.D.), Calmette Hospital (C.H.) — all in Phnom Penh; Donkeo Provincial Hospital, Takeo (C.K.); Svay Rieng Provincial Hospital, Svay Rieng (K.K.L., S.S.); and Siem Reap Provincial Hospital, Siem Reap (B.D.) — all in Cambodia; the Clinical Immunology Department, Georges Pompidou European Hospital, Assistance Publique–Hôpitaux de Paris (D.L.); Agence Nationale de Recherche sur le Sida et les Hépatites Virales (C.R., J.-F.D.); and Unité d’Epidémiologie des Maladies Emergentes, Institut Pasteur (Y.M.) — all in Paris; the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD (L.F.); and the Immune Disease Institute and Program in Cellular and Molecular Medicine at Children’s Hospital, Harvard Medical School, Boston (A.E.G.).
Footnotes
Presented in part at the 18th International AIDS Conference, Vienna, July 18–23, 2010.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
References
- 1.Global tuberculosis control: WHO report 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–52. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 3.Koenig SP, Riviere C, Leger P, et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48:829–31. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyeyune R, den Boon S, Cattamanchi A, et al. Causes of early mortality in HIV-infected TB suspects in an East African referral hospital. J Acquir Immune Defic Syndr. 2010;55:446–50. doi: 10.1097/qai.0b013e3181eb611a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood R. HIV/TB: when is it safe to start HAART? South Afr J HIV. 2008;9(4):18–24. [Google Scholar]
- 7.Dannenberg AM., Jr Perspectives on clinical and preclinical testing of new tuberculosis vaccines. Clin Microbiol Rev. 2010;23:781–94. doi: 10.1128/CMR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velasco M, Castilla V, Sanz J, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 10.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach — 2010 revision. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 11.Treatment of tuberculosis: guidelines. 4th. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 12.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouet F, Chaix ML, Nerrienet E, et al. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J Acquir Immune Defic Syndr. 2007;45:380–8. doi: 10.1097/QAI.0b013e3180640cf5. [DOI] [PubMed] [Google Scholar]
- 14.Tamura M, Khun KE, Yuos BH, et al. High prevalence/incidence of TB and poor outcomes of TB treatment among people living with HIV/AIDS (PLWHA) in Phnom Penh, Cambodia. Presented at the XV International AIDS Conference; Bangkok, Thailand. July 11–16, 2004; abstract. ( http://www.iasociety.org/Default.aspx?pageId=11&abstractId=2173528.) [Google Scholar]
- 15.Thai S, Lynen L, Kimura K, et al. Public private partnership in tuberculosis control in Phnom Penh, Cambodia. Presented at the XV International AIDS Conference; Bangkok, Thailand. July 11–16, 2004; abstract. ( http://www.iasociety.org/Default.aspx?pageId=11&abstractId=2170940.) [Google Scholar]
- 16.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slutsky AS, Lavery JV. Data safety and monitoring boards. N Engl J Med. 2004;350:1143–7. doi: 10.1056/NEJMsb033476. [DOI] [PubMed] [Google Scholar]
- 18.Lang TA, Secic M, editors. How to report statistics in medicine. 2nd. Philadelphia: American College of Physicians; 2006. Assessing time-to-event as an endpoint: reporting survival analyses; pp. 115–24. [Google Scholar]
- 19.Cain KP, Anekthananon T, Burapat C, et al. Causes of death in HIV-infected persons who have tuberculosis, Thailand. Emerg Infect Dis. 2009;15:258–64. doi: 10.3201/eid1502.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 21.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 22.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varma JK, Nateniyom S, Akksilp S, et al. HIV care and treatment factors associated with improved survival during TB treatment in Thailand: an observational study. BMC Infect Dis. 2009;9:42. doi: 10.1186/1471-2334-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harries AD, Chimzizi R, Zachariah R. Safety, effectiveness, and outcomes of concomitant use of highly active antiretroviral therapy with drugs for tuberculosis in resource-poor settings. Lancet. 2006;367:944–5. doi: 10.1016/S0140-6736(06)68387-6. [DOI] [PubMed] [Google Scholar]
- 26.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 28.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381–90. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spire B, Carrieri P, Sopha P, et al. Adherence to antiretroviral therapy in patients enrolled in a comprehensive care program in Cambodia: a 24-month follow-up assessment. Antivir Ther. 2008;13:697–703. [PubMed] [Google Scholar]
- 30.Goldfeld AE, Corbett EL. TB/AIDS coinfection: an integrated clinical and research response. In: Kaufman S, Walker BD, editors. AIDS and tuberculosis: a deadly liaison. Weinheim, Germany: Wiley-VCH Verlag; 2009. pp. 209–52. (Infection biology series). [Google Scholar]
- 31.Thim S, Sath S, Sina M, et al. A community-based tuberculosis program in Cambodia. JAMA. 2004;292:566–8. doi: 10.1001/jama.292.5.566-c. [DOI] [PubMed] [Google Scholar]
- 32.Ferradini L, Laureillard D, Prak N, et al. Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293–301. doi: 10.1097/QAD.0b013e32828cc8b7. [DOI] [PubMed] [Google Scholar]
- 33.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]


