Abstract
Purpose
Orteronel (TAK-700) is an investigational, nonsteroidal, reversible, selective 17,20-lyase inhibitor. This study examined orteronel in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel therapy.
Patients and Methods
In our study, 1,099 men were randomly assigned in a 2:1 schedule to receive orteronel 400 mg plus prednisone 5 mg twice daily or placebo plus prednisone 5 mg twice daily, stratified by region (Europe, North America [NA], and non-Europe/NA) and Brief Pain Inventory–Short Form worst pain score. Primary end point was overall survival (OS). Key secondary end points (radiographic progression-free survival [rPFS], ≥ 50% decrease of prostate-specific antigen [PSA50], and pain response at 12 weeks) were to undergo statistical testing only if the primary end point analysis was significant.
Results
The study was unblinded after crossing a prespecified OS futility boundary. The median OS was 17.0 months versus 15.2 months with orteronel-prednisone versus placebo-prednisone (hazard ratio [HR], 0.886; 95% CI, 0.739 to 1.062; P = .190). Improved rPFS was observed with orteronel-prednisone (median, 8.3 v 5.7 months; HR, 0.760; 95% CI, 0.653 to 0.885; P < .001). Orteronel-prednisone showed advantages over placebo-prednisone in PSA50 rate (25% v 10%, P < .001) and time to PSA progression (median, 5.5 v 2.9 months, P < .001) but not pain response rate (12% v 9%; P = .128). Adverse events (all grades) were generally more frequent with orteronel-prednisone, including nausea (42% v 26%), vomiting (36% v 17%), fatigue (29% v 23%), and increased amylase (14% v 2%).
Conclusion
Our study did not meet the primary end point of OS. Longer rPFS and a higher PSA50 rate with orteronel-prednisone indicate antitumor activity.
INTRODUCTION
Testosterone is essential for prostate-tumor cell growth and perpetuation.1 Lowering testosterone levels can reduce prostate cancer growth, improve patients' symptoms in metastatic disease, and improve survival rates in patients with high-risk localized disease that is treated with radiotherapy.2,3 However, over time prostate cancer invariably evolves to a castration-resistant state.2,4 Several mechanisms of castration resistance are known, including aberrant androgen-receptor signaling, androgen-receptor mutations or splicing variants, intracrine androgen synthesis, activation of parallel pathways, and cell-cycle activation.5–8
A current therapeutic approach in metastatic castration-resistant prostate cancer (mCRPC) is inhibition of CYP17A1, a key enzyme in androgen synthesis with both 17α-hydroxylase and 17,20-lyase activities. 17α-hydroxylase is responsible for generating steroidal precursors necessary for androgen and cortisol syntheses, which then yield androgens through 17,20-lyase conversion.9,10 17,20-lyase converts 17-OH-pregnenolone to dehydroepiandrosterone and androstenedione, and its activity is upregulated in mCRPC.8,9
In mCRPC, abiraterone acetate with prednisone is established as first-line therapy for chemotherapy-naive patients and as therapy for patients with up to two prior chemotherapeutic regimens.11,12 Abiraterone-prednisone demonstrated overall survival (OS) improvements over placebo-prednisone in chemotherapy-naive patients (hazard ratio [HR], 0.75; P = .01) and in postdocetaxel mCRPC (HR, 0.74; P < .001).11–14 Abiraterone-prednisone was generally well-tolerated, with increased frequency of adverse events (AEs) associated with adrenocorticotropic hormone–driven mineralocorticoid excess due to CYP17 blockade compared with placebo in both settings.10–12
Orteronel (TAK-700) is an investigational, nonsteroidal, reversible, 17,20-lyase inhibitor.15,16 In preclinical studies, orteronel demonstrated selectivity for 17,20-lyase over 17α-hydroxylase inhibition (IC50: 139 v 760 nmol/L).16 Phase II experience indicates that orteronel (with or without prednisone) inhibits testosterone and dehydroepiandrosterone-sulfate production, consistent with 17,20-lyase inhibition, and reduces prostate-specific antigen (PSA) levels in mCRPC patients.17,18 Prednisone was administered to maximize antitumor activity in patients with advanced mCRPC and to minimize the likelihood of adrenal insufficiency owing to 17α-hydroxylase inhibition with orteronel 400 mg twice daily.17
In this article, we report a phase III randomized, double-blind, multicenter study of orteronel plus prednisone in men with mCRPC that has progressed after docetaxel chemotherapy.
PATIENTS AND METHODS
Patients
Patients were enrolled onto study from 260 study centers in 42 countries. The study was conducted in accordance with the Helsinki Declaration and Good Clinical Practice; institutional review boards approved all aspects of the study. All participants provided written informed consent.
Eligible patients were at least 18 years old and had histologically or cytologically confirmed adenocarcinoma of the prostate and radiographically documented metastatic disease with evidence of disease progression (per RECIST 1.119 for soft tissue lesions or Prostate Cancer Working Group criteria20 for bone disease, and/or PSA increase) after receiving docetaxel (≥ 360 mg/m2 within a 6-month period). Patients intolerant to docetaxel or who had progressive disease before receiving ≥ 360 mg/m2 were eligible if they received ≥ 225 mg/m2 of docetaxel within a 6-month period and met other entry criteria. In addition, patient eligibility required surgical/medical castration with testosterone less than 50 ng/dL; PSA ≥ 2 ng/mL; Eastern Cooperative Oncology Group performance status of 0 to 2; and adequate renal, hematologic, cardiovascular, and hepatic function.
Exclusion criteria included prior orteronel, ketoconazole, aminoglutethimide, or abiraterone acetate therapy at any time; radioisotope/external-beam radiation therapy within 4 weeks of first dose, investigational drugs within 30 days, or other prostate cancer therapies within 2 weeks; or documented CNS metastasis.
Study Design and Interventions
Patients were randomly assigned at a 2:1 schedule to receive oral orteronel 400 mg plus prednisone 5 mg or placebo plus prednisone twice daily, without food restrictions, in 28-day treatment cycles (continuous dosing).17 Patients in Japan received orteronel 300 mg following a protocol amendment based on preliminary safety results from an ongoing phase I study (Japan, data on file) that suggested generally elevated rates of AEs compared with the phase II study in the United States.
Patients were stratified by region (North America [NA; USA/Canada], Europe, and non-Europe/NA) and Brief Pain Inventory-Short Form (BPI-SF) worst pain score at screening (≤ 4 v > 4).21 Patients could continue treatment until receipt of subsequent antineoplastic therapy or unacceptable AE. An independent data monitoring committee (IDMC) regularly reviewed safety data and results of interim analyses (IAs). After the first IA, the IDMC recommended continuing to the second IA for OS. Following the second IA, with crossing of prespecified futility boundary and per IDMC recommendation, the trial was unblinded.
End Points and Assessments
The primary end point was OS. Key secondary end points were radiographic progression-free survival (rPFS), PSA ≥ 50% decrease (PSA50) at 12 weeks, and pain response at 12 weeks. Other secondary end points included response by RECIST 1.1, time to PSA progression, duration of pain response, time to pain progression, and safety. Radiographic changes were assessed by independent central review, per RECIST 1.1 and Prostate Cancer Working Group criteria.
Pain response was defined as a reduction of at least two points from baseline in BPI-SF worst pain score without an increase in analgesic use or ≥ 25% reduction in analgesic use from baseline without an increase in worst pain score from baseline, confirmed by an additional assessment 3 to 5 weeks later. Toxicity was evaluated according to National Cancer Institute Common Terminology Criteria for AEs version 4.02.22
Statistical Methods
Assuming an exponential distribution for OS, 639 OS events were calculated to provide approximately 90% power to detect an HR of 1.32 (median OS, 15.8 v 12.0 months, orteronel-prednisone v placebo-prednisone) using a two-sided log-rank test at a 5% overall significance level; 1,083 patients were planned for random assignment. Two formal IAs were planned at 320 OS events (50% expected events) and 426 OS events (67% expected events; futility boundary P ≥ .4275), with the actual second IA conducted at 507 events (futility boundary P ≥ .1775).
Randomization and stratification were undertaken centrally using an interactive voice response system. A stratified log-rank test was used to compare OS between treatment groups, stratified by the randomization stratification factors. HRs and 95% CIs were estimated using the stratified Cox model with treatment as the explanatory variable. Kaplan-Meier survival curves and medians, with two-sided 95% CIs, are provided.
PSA50 and pain response rates were compared using the Cochran-Mantel-Haenszel χ2 test stratified by randomization stratification factors. rPFS (time from randomization to centrally confirmed radiographic disease progression or death from any cause) was analyzed per OS. Regardless of discontinuation reasons, all patients with protocol-specified radiographic progressive disease (rPD) or who died before data cutoff were included as rPFS events.
RESULTS
Patients and Disposition
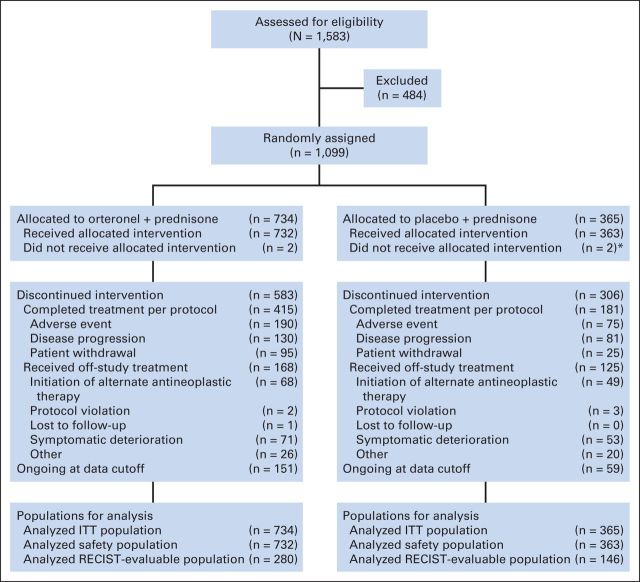
In our study, 1,099 patients were randomly assigned (orteronel-prednisone, n = 734; placebo-prednisone, n = 365; Fig 1). Patient demographics and disease characteristics were generally balanced between treatment groups (Table 1), and across regions (Appendix Table A1, online only), except for BPI-SF worst pain score, two or more prior chemotherapies, PSA, and lactate dehydrogenase.
Fig 1.
CONSORT diagram. (*) One patient allocated to placebo-prednisone received orteronel plus prednisone. This patient is included in the orteronel plus prednisone safety population. ITT, intent to treat.
Table 1.
Patient Demographics and Disease Characteristics
| Characteristic | Orteronel Plus Prednisone (n = 734) |
Placebo Plus Prednisone (n = 365) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 69.5 | 70.0 | ||
| Range | 43-89 | 48-87 | ||
| Age ≥ 70 | 367 | 50 | 194 | 53 |
| Race | ||||
| White | 620 | 84 | 305 | 84 |
| Black/African American | 18 | 2 | 9 | 2 |
| Asian | 77 | 10 | 48 | 13 |
| American Indian or Alaskan Native | 4 | < 1 | 1 | < 1 |
| Other/not reported | 15 | 2 | 2 | < 1 |
| Region | ||||
| Europe | 394 | 54 | 196 | 54 |
| Non-Europe/North America | 265 | 36 | 132 | 36 |
| North America | 75 | 10 | 37 | 10 |
| Time since initial diagnosis, years | ||||
| Median | 5.5 | 5.7 | ||
| Range | 0-22 | 0.1-29 | ||
| ECOG PS, % | ||||
| 0 | 42 | 40 | ||
| 1 | 50 | 53 | ||
| 2 | 9 | 7* | ||
| BPI-SF worst pain score | ||||
| Median | 3.0 | 3.0 | ||
| Range | 0-10 | 0-10 | ||
| PSA at baseline, ng/mL | ||||
| Median | 122.5 | 134.0 | ||
| Range | 0-8,456 | 1-19,009 | ||
| Testosterone at baseline, ng/dL | ||||
| Median | 4.65 | 4.2 | ||
| Range | 0.2-99.9 | 0.2-138.9 | ||
| Gleason score at diagnosis | ||||
| ≤ 6 | 102 | 14 | 62 | 17 |
| 7 | 213 | 29 | 105 | 29 |
| 8-10 | 372 | 51 | 171 | 47 |
| Unknown/missing | 47 | 6 | 27 | 7 |
| Extent of disease at baseline | ||||
| Bone metastases | 699 | 95 | 340 | 93 |
| Lymph node metastases | 344 | 47 | 171 | 47 |
| Lung metastases | 90 | 12 | 39 | 11 |
| Liver metastases | 64 | 9 | 44 | 12 |
| Other metastases† | 124 | 17 | 60 | 16* |
| Visceral disease‡ | 197 | 27 | 99 | 27 |
| Prior chemotherapy regimens | ||||
| 1 | 574 | 78 | 263 | 72 |
| ≥ 2 | 160 | 22 | 101 | 28* |
| Prior radiation therapy | 490 | 67 | 224 | 61 |
| Prior surgery | 390 | 53 | 190 | 52 |
| Prior ADT | 702 | 96 | 347 | 95 |
Abbreviations: ADT, androgen deprivation therapy; BPI-SF, Brief Pain Inventory–Short Form; ECOG PS, Eastern Cooperative Oncology Group performance status; PSA, prostate-specific antigen.
One missing value.
Other metastases include patients with metastases identified in locations other than the bone, lymph node, lung, and liver.
Visceral disease includes patients with metastases identified in one of the following locations: abdomen, adrenal gland, bladder, bowel, colon, kidney, liver, lung, pancreas, peritoneum, pleura, spleen, and ureter.
Median treatment duration was 6.2 cycles (range, 0 to 26.4 cycles) and 5.7 months (range, 0.03 to 24.3 months) with orteronel-prednisone versus 5.0 cycles (range, 0.3 to 29.4 cycles) and 4.6 months (range, 0.3 to 27.0 months) with placebo-prednisone. Median follow-up at data cutoff (May 16, 2013) was 10.6 months (range, 0.2 to 29.5 months) and 10.7 months (range, 0.4 to 27.1 months) in the orteronel-prednisone and placebo-prednisone groups. Overall, 583 patients (79%) and 306 patients (84%) discontinued treatment in the orteronel-prednisone and placebo-prednisone groups, respectively, with the primary reasons recorded as AEs (26% v 21%), disease progression (18% v 22%), and patient withdrawal (13% v 7%; Fig 1).
Study Unblinding
At the second IA (507 deaths; 79% of 639 deaths required for final analysis), the IDMC determined that the futility boundary (P ≥ .1775) had been crossed, indicating that the orteronel-prednisone group would likely not meet the primary end point of improved OS versus the placebo-prednisone group if continued to final analysis. This led to the recommendation that the study be unblinded. Patients randomly assigned to orteronel-prednisone were allowed to continue therapy.
Efficacy
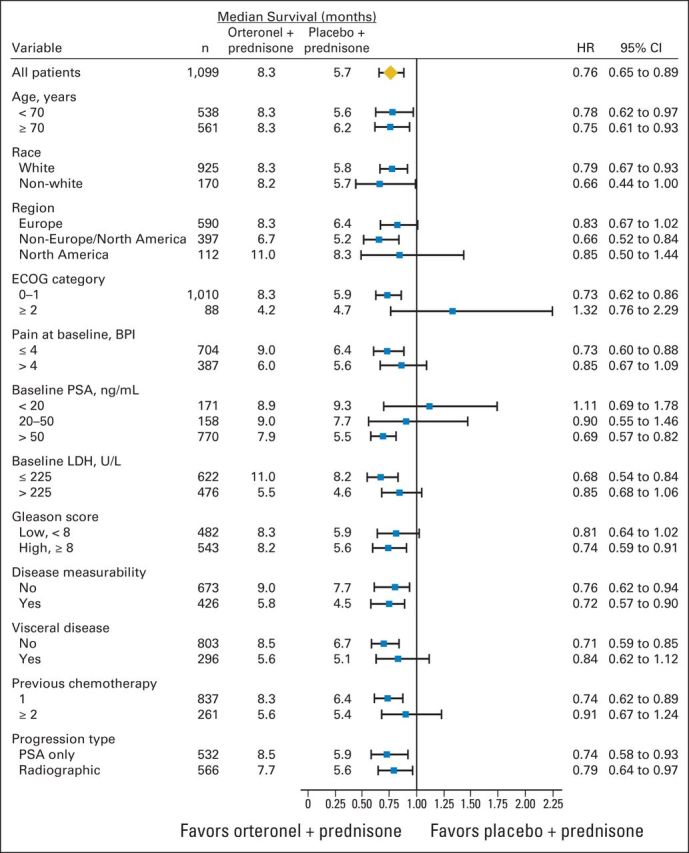
At data cutoff, 512 patients died (Fig 2A). The OS HR was 0.886 (95% CI, 0.739 to 1.062; P = .190; Fig 2A); median OS was 17.0 months versus 15.2 months with orteronel-prednisone versus placebo-prednisone, respectively. The treatment effect across protocol-specified subgroups seemed consistent with overall findings, with observed variances including baseline pain and PSA levels (Fig 3). Differences in treatment effects were seen between subgroups with (HR, 1.104; P = .928) or without baseline visceral disease (HR, 0.821; P = .084) and between regions (Fig 3; Appendix Fig A1, online only). Regional differences in OS were seen between Europe (HR, 1.048; P = .721), non-Europe/NA (HR, 0.709; P = .019), and NA (HR, 0.889; P = .680).
Fig 2.
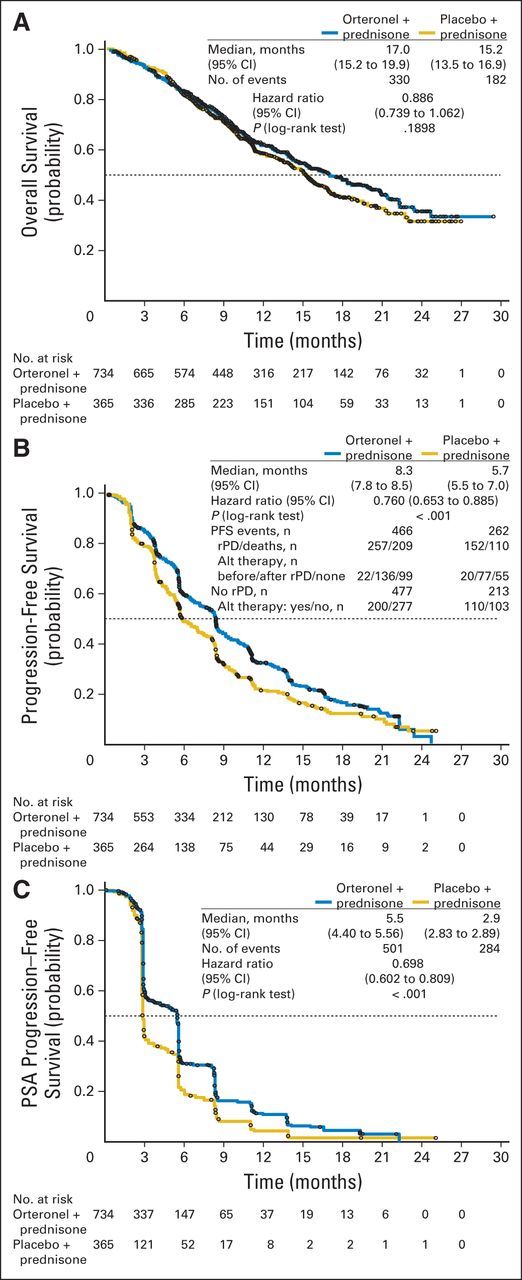
Kaplan-Meier estimates of (A) overall survival, (B) radiographic progression-free survival (PFS), and (C) time to prostate-specific antigen (PSA) progression. (B) Radiographic progressive disease (rPD) was determined by a central imaging center based on protocol definition, not per identification by investigator. Alt, alternate.
Fig 3.
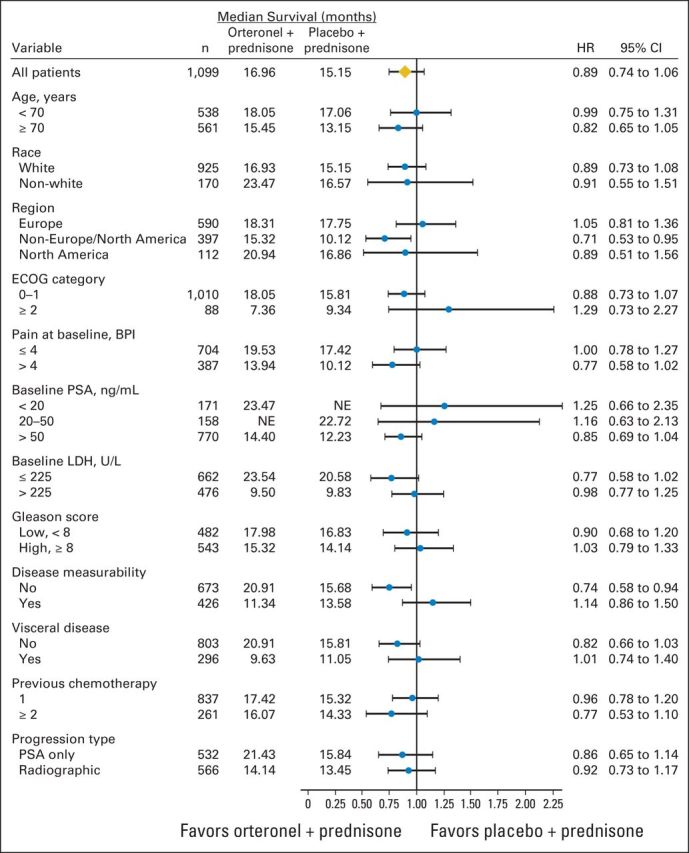
Overall survival by subgroups. BPI, Brief Pain Inventory; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; NE, not estimable; PSA, prostate-specific antigen.
Secondary efficacy end points were only to be analyzed by a sequential testing procedure if the primary end point comparison was significant. Because the study did not meet the OS end point, formal hypothesis testing could not be performed for the ranked secondary end points. Results, observed P values, and HRs are for descriptive purposes.
In the analysis of rPFS, 466 and 262 patients in the orteronel-prednisone and placebo-prednisone groups, respectively, had events of rPD (orteronel-prednisone, n = 257; placebo-prednisone, n = 152) or death (orteronel-prednisone, n = 209; placebo-prednisone, n = 110); 268 and 103 patients, respectively, were censored. Within the rPD events, 22 of 257 orteronel-prednisone patients and 20 of 152 placebo-prednisone patients received alternate therapy before rPD (Fig 2B). In patients without rPD, 200 of 477 orteronel-prednisone patients and 110 of 213 placebo-prednisone patients received alternate therapy. Numerically longer rPFS was seen with orteronel-prednisone patients (HR, 0.760; 95% CI, 0.653 to 0.885; P < .001; Fig 2B); median rPFS was 8.3 months with orteronel-prednisone versus 5.7 months with placebo-prednisone. Regional differences in rPFS subgroup analyses were noted between Europe (orteronel-prednisone v placebo-prednisone: median, 8.3 v 6.4 months; HR, 0.827; P = .075), non-Europe/NA (orteronel-prednisone v placebo-prednisone: median, 6.7 v 5.2 months; HR, 0.660; P < .001), and NA (orteronel-prednisone v placebo-prednisone: median, 11.0 v 8.3 months; HR, 0.849; P = .539; Appendix Fig A2, online only). Exploratory analysis in all patients suggested longer rPFS in the orteronel-prednisone group was observed only in patients without (HR, 0.712; P < .001) but not with (HR, 0.837; P = .232), visceral disease at baseline (Appendix Fig A2).
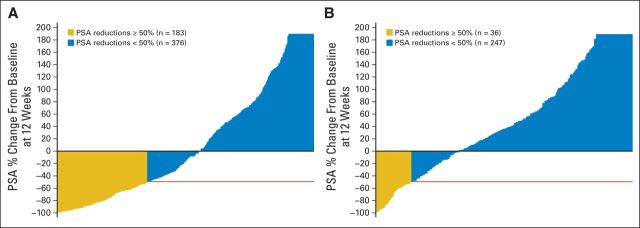
Median time to PSA progression was 5.5 months versus 2.9 months with orteronel-prednisone versus placebo-prednisone (HR, 0.698; P < .001; Fig 2C). PSA50 responses at 12 weeks were 25% v 10% with orteronel-prednisone versus placebo-prednisone (P < .001; Table 2; Fig 4). In RECIST-evaluable patients, response rates were 17% versus 3%, respectively (P < .001; Table 2). No differences in pain response were observed (Table 2).
Table 2.
Secondary Efficacy End Points
| End Point | Orteronel Plus Prednisone (n = 734) |
Placebo Plus Prednisone (n = 365) |
Log-Rank P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| PSA50 response at 12 weeks | 183 | 25 | 36 | 10 | < .001 |
| Absolute difference | 15.1 | ||||
| 95% CI | 10.5 to 19.7 | ||||
| Odds ratio | 2.93 | < .001 | |||
| 95% CI | 1.97 to 4.37 | ||||
| Duration of PSA50 response, months | |||||
| Median | 5.6 | 5.7 | |||
| Range | 0-19.5 | 0-22.6 | |||
| 95% CI | 5.5 to 5.6 | 4.9 to 6.5 | |||
| Pain response at 12 weeks* | 89 | 12 | 33 | 9 | .128 |
| Absolute difference | 3.1 | ||||
| 95% CI | −0.9 to 7.1 | ||||
| Odds ratio | 1.5 | .092 | |||
| 95% CI | 0.9 to 2.3 | ||||
| Duration of pain response, months | |||||
| Median | 13.0 | 12.6 | |||
| Range | 0-23.3 | 0-24.3 | |||
| 95% CI | 11.0 to 14.6 | 6.9 to 16.8 | |||
| Pain response for patients with significant pain at baseline† | |||||
| Total No. of patients | 336 | 171 | |||
| Patients with significant pain | 66 | 20 | 24 | 14 | .134 |
| Absolute difference | 5.6 | ||||
| 95% CI | −1.6 to 12.8 | ||||
| Odds ratio | 1.5 | .124 | |||
| 95% CI | 0.9 to 2.7 | ||||
| Time to pain progression, months | .327 | ||||
| Median | 24.2 | 22.0 | |||
| Range | 0-24.2 | 0-25.1 | |||
| 95% CI | 18.2 to 24.2 | 20.5 to NE | |||
| HR | 0.885 | ||||
| 95% CI | 0.693 to 1.131 | ||||
| Response by RECIST | |||||
| Total No. of patients | 280 | 146 | |||
| Complete response | 1 | 0.4 | 0 | ||
| Partial response | 47 | 17 | 4 | 3 | |
| Overall response rate (CR plus PR) | 48 | 17 | 4 | 3 | < .001 |
| Absolute difference | 14.4 | ||||
| 95% CI | 8.7 to 20.1 | ||||
| Odds ratio | 11.2 | < .001 | |||
| 95% CI | 3.3 to 37.8 | ||||
Abbreviations: BPI-SF, Brief Pain Inventory–Short Form; CR, complete response; HR, hazard ratio; NE, not estimable; PR, partial response; PSA50, prostate-specific antigen decrease of ≥ 50% from baseline.
Defined as the occurrence of one of the following and confirmed by an additional assessment at least 3 weeks but not more than 5 weeks later: a ≥ two-point reduction from baseline in BPI-SF worst pain score without an increase in analgesic use or a 25% or more reduction in analgesic use from baseline without an increase in worst pain score from baseline.
Defined as baseline pain score ≥ 4.
Fig 4.
Waterfall plots of prostate-specific antigen (PSA) response at 12 weeks in the evaluable patients with baseline and postbaseline assessments in the (A) orteronel-prednisone group (n = 559) and the (B) placebo-prednisone group (n = 283). Plots are truncated at 200%.
Safety
The most common all-cause, all-grade AEs were nausea (orteronel-prednisone v placebo-prednisone, 42% v 26%), vomiting (orteronel-prednisone v placebo-prednisone, 36% v 17%), and fatigue (orteronel-prednisone v placebo-prednisone, 29% v 23%; Table 3). Other AEs included worsening hypertension (orteronel-prednisone v placebo-prednisone, 11% v 6%), hypokalemia (orteronel-prednisone v placebo-prednisone, 6% v 4%), overall adrenal insufficiency (orteronel-prednisone v placebo-prednisone, 2% v < 1%), and congestive heart failure (16% each). Most of these events were grade 1 or 2.
Table 3.
Safety Profiles and Summaries of the Most Common All-Cause Adverse Events (any grade in > 10% of patients overall and grade ≥ 3 in ≥ 2%) and Other Adverse Events of Interest
| Adverse Events | Orteronel Plus Prednisone (n = 732) |
Placebo Plus Prednisone (n = 363) |
||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade ≥ 3 |
Any Grade |
Grade ≥ 3 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | ||
| Any AE | 717 | 98 | 506 | 69 | 345 | 95 | 199 | 55 |
| Any drug-related AE | 571 | 78 | 269 | 37 | 217 | 60 | 67 | 18 |
| Any serious AE | 351 | 48 | 143 | 39 | ||||
| Any drug-related serious AE | 113 | 15 | 27 | 7 | ||||
| Most common AEs | ||||||||
| Nausea | 304 | 42 | 23 | 3 | 93 | 26 | 5 | 1 |
| Vomiting | 261 | 36 | 27 | 4 | 61 | 17 | 8 | 2 |
| Fatigue | 215 | 29 | 42 | 6 | 82 | 23 | 17 | 5 |
| Constipation | 212 | 29 | 11 | 2 | 67 | 18 | 5 | 1 |
| Decreased appetite | 200 | 27 | 17 | 2 | 67 | 18 | 10 | 3 |
| Diarrhea | 194 | 27 | 26 | 4 | 54 | 15 | 5 | 1 |
| Back pain | 125 | 17 | 24 | 3 | 65 | 18 | 13 | 4 |
| Increased lipase* | 120 | 16 | 98 | 13 | 6 | 2 | 3 | < 1 |
| Decreased weight | 107 | 15 | 4 | < 1 | 32 | 9 | 4 | 1 |
| Muscle spasms | 107 | 15 | 1 | < 1 | 26 | 7 | 1 | < 1 |
| Arthralgia | 105 | 14 | 19 | 3 | 55 | 15 | 8 | 2 |
| Asthenia | 105 | 14 | 18 | 2 | 42 | 12 | 11 | 3 |
| Increased amylase† | 103 | 14 | 61 | 8 | 6 | 2 | 1 | < 1 |
| Anemia | 102 | 14 | 52 | 7 | 64 | 18 | 37 | 10 |
| Bone pain | 87 | 12 | 32 | 4 | 59 | 16 | 22 | 6 |
| Pain in extremity | 80 | 11 | 10 | 1 | 44 | 12 | 10 | 3 |
| Dizziness | 76 | 10 | 3 | < 1 | 16 | 4 | 2 | < 1 |
| Additional AEs of interest | ||||||||
| Hypertension | 83 | 11 | 24 | 3 | 21 | 6 | 6 | 2 |
| Peripheral edema | 72 | 10 | 5 | <1 | 46 | 13 | 1 | < 1 |
| Hot flashes | 63 | 9 | 0 | 20 | 6 | 0 | ||
| Hypokalemia | 44 | 6 | 16 | 2 | 14 | 4 | 1 | < 1 |
| Decreased blood potassium | 1 | < 1 | 1 | < 1 | 2 | < 1 | 0 | |
| ALT increased | 23 | 3 | 6 | < 1 | 9 | 2 | 2 | < 1 |
| AST increased | 17 | 2 | 2 | < 1 | 9 | 2 | 1 | < 1 |
Abbreviation: AE, adverse event.
Elevations in lipase levels were observed in 12% of patients (88 of 732 patients) in the orteronel plus prednisone group during cycles 1-3, 8% of patients (46 of 585 patients) during cycles 4-7, < 1% of patients (three of 412 patients) during cycles 8-12, and in two patients beyond cycle 13.
Increase in amylase levels were seen in 10% of patients (74 of 732 patients) in the orteronel plus prednisone group during cycles 1-3, 6% of patients (38 of 585 patients) during cycles 4-7, and < 1% of patients (three of 412 patients) during cycles 8-12, and in two patients beyond cycle 13.
Common grade ≥ 3 AEs included lipase increases (orteronel-prednisone v placebo-prednisone, 13% v < 1%), amylase increases (orteronel-prednisone v placebo-prednisone, 8% v < 1%), and anemia (orteronel-prednisone v placebo-prednisone, 7% v 10%; Table 3). Most events of lipase or amylase elevations were transient and subsided by cycle 7 of the study period. Seven patients (< 1%) in the orteronel-prednisone group experienced pancreatitis (five serious events requiring dose modifications). The most common serious AEs with orteronel-prednisone were pulmonary embolism (n = 19), general physical health deterioration (n = 17), pneumonia (n = 16), urinary tract infection (n = 16), vomiting (n = 16), spinal cord compression (n = 16), anemia (n = 15), sepsis (n = 13), increased lipase (n = 12), nausea (n = 11), dehydration (n = 11), and urosepsis and urinary retention (n = 11). Regional differences in safety profiles were observed in non-Europe/NA versus Europe and NA, including numerically higher rates of grade ≥ 3 AEs, serious AEs, AEs resulting in discontinuation, and on-study deaths in both treatment groups, plus relatively higher rates of serious AEs, AEs resulting in discontinuation, and on-study deaths in the placebo-prednisone versus orteronel-prednisone groups (Appendix Table A2, online only).
Incidence of AEs resulting in treatment discontinuation was similar between the orteronel-prednisone and placebo-prednisone groups (30% and 24%, respectively). In addition to disease progression recorded as an AE (n = 13 in each group), the most common AEs resulting in treatment discontinuation were vomiting (n = 19 v n = 2) and other gastrointestinal disorders, nausea (n = 14 v n = 1), and diarrhea (n = 8 v n = 0). Dose modifications because of AEs were required by 43% and 23% of patients in the orteronel-prednisone and prednisone groups, respectively, the most common AEs being gastrointestinal disorders (vomiting [7% v < 1%], nausea [7% v 2%], diarrhea [5% v 2%]), increased lipase (9% v < 1%), and increased amylase (7% v < 1%).
Overall, 47% of patients died (orteronel-prednisone, 45% v placebo-prednisone, 50%); most deaths (orteronel-prednisone, 226 patients; 69%; v placebo-prednisone, 127 patients; 70%) were related to prostate cancer and/or its complications.
Subsequent Therapies
Overall, 45% (n = 326) and 54% (n = 197) of patients in the orteronel-prednisone and placebo-prednisone groups, respectively, received alternate therapy for prostate cancer. Patients in the orteronel-prednisone and placebo-prednisone groups subsequently received abiraterone acetate (20% v 21%), cabazitaxel (15% v 18%), docetaxel (5% v 9%), or enzalutamide (5% v 4%) therapies. Types of subsequent therapies varied across the protocol-specified regional subgroups (Appendix Table A3, online only).
DISCUSSION
This phase III study of orteronel in patients with mCRPC who had received prior chemotherapy did not meet the primary end point of OS in the overall population. However, longer rPFS (P = .0004) with orteronel-prednisone suggests antitumor activity. Furthermore, patients receiving orteronel-prednisone had delays in PSA progression and a higher rate of ≥ 50% PSA decrease, but no improvement in pain, versus patients receiving placebo-prednisone. Similar to other studies of endocrine therapies for mCRPC, these results support androgen signaling pathway inhibition for reducing disease symptoms and progression.12,13,23
Orteronel had limited activity in terms of prolonging OS in patients with mCRPC that progressed after prior chemotherapy. However, numerical improvements in secondary end points and activity seen in phase II investigation suggest that orteronel seems to have antitumor activity in mCRPC alone or with prednisone.17,18 OS may have been negatively affected by short treatment duration (orteronel-prednisone v placebo-prednisone, 5.7 v 4.6 months). The abiraterone study in a similar mCRPC setting reported treatment durations of 8 months versus 4 months with abiraterone-prednisone versus placebo-prednisone.12 The short treatment duration in our study may be correlated with high rates of study discontinuations owing to various factors, including AEs (orteronel-prednisone v placebo-prednisone, 30% v 24%), notably gastrointestinal-related AEs, and initiation of subsequent therapy (45% v 54%). In the abiraterone study, AEs resulting in treatment discontinuation occurred in 19% and 23% of the abiraterone-prednisone and placebo-prednisone groups, respectively.12 At the time the abiraterone trial was initiated, there was limited survival-prolonging therapy available for men with mCRPC after docetaxel therapy.
During the course of this trial, the US and European treatment landscape included two novel androgen-directed agents, abiraterone and enzalutamide, and a new taxane, cabazitaxel (either as approved agents or through an expanded access program).13,23,24 These agents have demonstrated OS benefit in mCRPC and could have been received subsequent to study treatment, and may thus have affected OS comparisons. Furthermore, the impact of prior orteronel treatment followed by another CYP17A1 inhibitor (such as, abiraterone) on efficacy outcomes in mCRPC is unknown.
An OS advantage with orteronel-prednisone observed in non-Europe/NA (P = .019) but not in Europe or NA could be associated with the lower rate of subsequent novel therapy use (Fig 3; Appendix Table A3). Similarly, a numerical rPFS benefit was observed with orteronel-prednisone in non-Europe/NA (P = .0008) but not in Europe or NA (Appendix Fig A2); this end point is less affected by subsequent therapy. Though there were overall differences between regions, subsequent therapy use was generally similar between treatment groups and within regions. Furthermore, the less favorable baseline characteristics (higher BPI-SF worst pain score, more patients with two or more prior chemotherapies, and higher baseline PSA and lactate dehydrogenase) in patients in non-Europe/NA versus Europe or NA may indicate that these patients were sicker at baseline, and possibly more susceptible to AEs or more likely to discontinue treatment (Appendix Table A1). These factors (regional differences, use of subsequent therapy, and baseline characteristics) represent possible limitations of the study.
In a recent phase III trial of orteronel in chemotherapy-naive patients with mCRPC (ELM-PC 4), rPFS was prolonged with orteronel-prednisone versus placebo-prednisone (13.8 months v 8.7 months; HR, 0.71; P < .001).25 However, there was no improvement in the primary end point of OS (31.4 months with orteronel-prednisone v 29.5 months with placebo-prednisone; HR, 0.92; P = .314).25 Though the enrollment period for the ELM-PC 4 trial overlapped with this study, there were no regional differences observed in the prespecified subgroup analyses for either primary end point (OS and rPFS) in the ELM-PC 4 trial. Thus, the actual impact of regional differences is unclear and the failure to prolong OS in mCRPC after docetaxel-based chemotherapy may result from insufficient clinical activity of orteronel in this patient population.
In this study, the safety profile observed with orteronel-prednisone consisted of predominantly grade 1 or 2 AEs, except for amylase elevations, lipase elevations, and anemia. Notably, there was a higher rate of grade ≥ 3 gastrointestinal-related toxicities (lipase and amylase elevations) with limited clinical symptoms, consistent with observations from the phase I/II trial.17 However, these laboratory elevations were transient, often resolving by cycle 7. Furthermore, gastrointestinal-related AEs most commonly contributed to the increased frequencies of dose discontinuations and modifications in the orteronel-prednisone group. All-cause, all-grade gastrointestinal-related AEs were observed in the abiraterone study, including diarrhea (abiraterone-prednisone v placebo-prednisone, 20% v 15%), nausea (abiraterone-prednisone v placebo-prednisone, 33% v 33%), and vomiting (abiraterone-prednisone v placebo-prednisone, 24% v 26%).13
As the treatment landscape for prostate cancer continues to expand, OS alone may no longer be a fair indicator of treatment efficacy because it is not only confounded by other causes of mortality but also the impact of effective post-trial therapy. Time to disease progression and rPFS may contribute significant insight as primary efficacy parameters, as these data are available earlier than OS, are less influenced by competing causes of death, and are not affected by alternative treatments administered after progression. When several effective therapies are available in clinical practice, as for mCRPC, multiple parameters should be considered in public health decisions as long as the novel agents display favorable safety profiles.26–28
In conclusion, there was no statistically significant improvement in OS with orteronel-prednisone versus placebo-prednisone. Furthermore, the longer rPFS and higher rate of ≥ 50% PSA decrease suggest that orteronel may have antitumor activity in mCRPC after docetaxel therapy.
Acknowledgment
We thank the patients who participated in this study and their families, as well as staff at all investigational sites. We acknowledge Catherine Crookes and Dawn L. Lee of FireKite, part of KnowledgePoint360, an Ashfield Company, who provided medical writing assistance during the development of this article, which was funded by Millennium: The Takeda Oncology Company and complied with Good Publication Practice 2 guidelines (Graf C, et al: BMJ 339:b4330, 2009).
Glossary Terms
- metastatic castration-resistant prostate cancer (mCRPC):
progressive disease despite surgical castration or ongoing use of gonadotropin-releasing hormone agonists with confirmed castrate levels of testosterone.
Appendix
Full List of ELM-PC 5 Trial Investigators
The following investigators, listed in alphabetical order per country, participated in the ELM-PC 5 study. (Investigators at study sites with zero subjects enrolled are not listed, unless they were a Steering Committee member.)
Argentina: M. Brown Arnold (Rosario, Santa Fe), M.A. Cuevas (Bahia Blanca, Buenos Aires), F. Palazzo (San Miguel de Tucumán, Tucumán), M. Richardet (Cordoba, Cordoba); Australia: K. Bigby (Recliffe, QLD), H. Gurney (Westmead, NSW), E. Korbenfeld (Ciudad Autonoma de Buenos Aires), G. Mallesara (Waratah, NSW), G. Marx (Wahroonga, NSW), T. Michele (North Adelaide, SA), S. Ng (Nedlands, WA), L. Nott (Hobart, TAS), F. Parnis (Kurralta Park, SA), K. Pittman (Woodville South/Elizabeth Vale, SA), D. Pook (Clayton, VIC), J. Shapiro (Malvern/Brighton, VIC), A. Stevanovic (Kingswood, NSW), S. Troon (Perth, WA), C. Underhill (Wodonga, VIC); Austria: C. Dittrich (Wien), W. Loidl (Linz); Belarus: V. Belyakovskiy (Gomel), S. Polyakov (Minsk); Belgium: J. Goeminne (Namur), D. Luyten (Hasselt), J. Machiels (Brussels), P. Werbrouck (Kortrijk); Brazil: A. Azambuja (Porto Alegre/RS), C. Barrios (Porto Alegre/RS), L. Brust (Lajeado/RS), F. Carcano (Barretos/SP), D. Castro Jr. (Salvador/BA), A. Coradazi (Jau/SP), R. Damiao (Rio de Janeiro/RJ), K. de Carvalho Emerenciano (Natal/RN), G. Delgado Luchezi (Sorocaba/SP), A. Faccio (Preto/SP), A. Faulhaber (Santo André/SP), U. Ferreira (Campinas/SP), F. Franke (Ijui/RS), A. Gemeinder de Moraes Jr. (Piracicaba/SP), G. Girotto (Preto/SP), D. Herchenhorn (Rio de Janeiro/RJ), A. Kann (São Paulo), W. Koff (Porto Alegre/RS), C. Kussumoto (Joinville/SC), M. Liberatti (Londrina/PR), A. Malzyner (São Paulo/SP), A. Notari (Porto Alegre/RS), S. Padilha (Curitiba/PR), A. Porto Rocha Lima (Santo Andre/SP), A. Reiriz (Cazias do Sul/RS), F. Silva Melo Cruz (Santo Andre/SP), J. Yamaguchi (São Paulo), H. Zanoni Fernandes (Campos/SP); Bulgaria: B. Dimitrov (Sofia), A. Dudov (Sofia), D. Kalev (Varna); Canada: H. Assi (Moncton, NB), T. Cheng (Calgary, AB), K. Chi (Vancouver, BC), A. Jacobson (Pointe-Claire, QC), F. Saad (Montreal, QC), P. Venner (Edmonton, AB); Chile: O. Arén Frontera (Independencia Santiago), L. Soto Diaz (Las Condes Santiago), E. Yáñez Ruiz (Temuco); China: Q. Ding (Shanghai), Y. Tian (Beijing), D. Ye (Shanghai); Colombia: L. Neira Reyes (Bogota, Cundinamarca), J.G. Restrepo (Cali), C. Vargas Baez (Bogota); Croatia: A. Budisavljevic (Pula), M. Grgic (Zagreb); Czech Republic: I. Andel (Zlin), J. Jansa (Hradec Kralove), I. Pavlik (Praha); Estonia: J. Kahu (Tartu), T. Tamm (Tallin); Finland: T. Marttila (Seinajoki), N. Paunu (Tampere), M. Vaarala (Oulu), J. Viitanen (Joensuu); France: E. Bompas (St Herbalain Cedex), G. Deplanque (Paris), K. Fizazi (Villejuif Cedex), J. Giroux (Paris), I. Krakowski (Vandoeuvre Les Nancy), E. Lechevallier (Marseille), S. Oudard (Paris), F. Priou (La Roche-sur-Yon), S. Ropert (Paris), D. Spaeth (Nancy), J. Tourani (Poitiers), S. Vignot (Paris); Germany: S. Feyerabend (Nürtingen), G. Geiges (Berlin), J. Gleißner (Wuppertal), P. Hammerer (Braunschweig), T. Klotz (Weiden), M. Kuczayk (Hannover), J. Marin (Kempen), A. Stenzl (Tübingen), M. Wirth (Dresden); Greece: E. Efstathiou (Athens), G. Fountzilas (Thessaloniki), V. Georgoulias (Crete), H. Kalofonos (Patras), C. Papandreou (Larissa), K. Syrigos (Athens), A. Thanos (Athens); Hungary: G. Böszörményi-Nagy (Budapest), L. Farkas (Pecs), Z. Máté (Miskolc), J. Pinter (Miskolc); Ireland: P. Donnellan (Galway), R. McDermott (Dublin); Israel: R. Berger (Ramat-Gan), A. Gabizon (Jerusalem), E. Gez (Tel Aviv), W. Mermershtain (Beer-Sheva), O. Nativ (Haifa), A. Peer (Haifa), E. Tavdy (Holon), J. Zidan (Safed); Italy: O. Alabiso (Novara), P. Bassi (Rome), L. Ciuffreda (Torino), L. Fratino (Aviano), B. Martoni (Malpighi), C. Ortega (Candiolo TO), C. Sternberg (Rome); Japan: S. Egawa (Tokyo), T. Ichikawa (Chiba), H. Kitamura (Sapporo Hokkaido), N. Miyanaga (Ibaraki), H. Nishiyama (Ibaraki), H. Suzuki (Chiba), Y. Tomita (Yamagata), T. Ueda (Chiba), H. Fujimoto (Tokyo), T. Kosaka (Tokyo), K. Akakura (Tokyo), R. Yamaguchi (Tokyo), S. Takahashi (Tokyo), H. Uemura (Kanagawa), A. Mizokami (Ishikawa), M. Nakagawa (Kagoshima), T. Nakatani (Osaka), K. Nishimura (Osaka), M. Niwakawa (Shizuoka), F. Sato (Yufu), M. Sugimoto (Kagawa), T. Takayama (Shizuoka), A. Yokomizo (Fukuoka), K. Yoshimura (Osaka); Republic of Korea: J.B. Ahn (Seoul), B.H. Chung (Seoul), T.W. Kang (Gwangju), C. Kwak (Seoul), C. Kim (Seoul), S.W. Kim (Seoul), K.H. Lee (Goyang); Lithuania: F. Jankevicius (Vilnius), G. Jocys (Klaipeda), D. Milonas (Kauans), A. Ulys (Vilnius); Mexico: G. Garcia Jaliffe (Colonia Santa Cruz Atoyac), J.A. Rodriguez Rivera (Jalisco); the Netherlands: R. de Wit (Rotterdam), A.P. Hamberg (Rotterdam), I.M. Van Oort (Nijmegan), J.J.E.J. Vrijhof (Eindhoven); New Zealand: P.C.C. Fong (Auckland); Poland: T. Demkow, Z. Jablonska (Wroclaw), E. Kalinka-Warzocha (Lodz), R. Kmieciak (Wroclaw); Portugal: J. Coelho (Lisboa), G. Sousa (Coimbra), N. Sousa (Porto); Romania: C.L. Cebotaru (Cluj-Napoca), T. Ciuleanu (Cluj-Napoca), D. Lungulescu (Craiova), S. Mihutiu (Oradea); Russia: S. Ivanov (Moscow), A. Plekhanov (St Petersburg); Serbia: N. Babovic (Belgrade); Singapore: T.M. Hiang; Slovak Republic: J. Mardiak (Bratislava); South Africa: R. De Bruyne (Overport Durban), L. Dreosti (Pretoria), A.W. Dreyer (Western Cape), S. Fourie (Westdene Bloemfontein), G.J. Hart (Rondebosch), P. Kraus (George), G. Landers (Overport Durban), J. Malan (Port Elizabeth); Spain: J. Carles Galceran (Barcelona), D. Castellano (Madrid), M. Climent Duran (Valencia), O. Donnay (Madrid), B. Mellado Gonzalez (Barcelona), J. Perez Gracia (Navarra), B. Pérez Valderrama (Sevilla), F. Vazquez Mazon (Elche, Alicante); Sweden: O. Andrén (Orebro), L. Beckman (Sundsvall), T. Björk (Malmo), J. Damber (Gothenburg), L. Franzén (Umea), M. Seke (Vaxjo), J. Yachnin (Uppsala); Switzerland: R. Cathomas (Chur), F. Stenner (Zurich); Taiwan: Y. Chang (Taipei), C. Chen (Puzi City), P. Chiang (Kaohsiung), Y. Ou (Taichung), Y. Tsai (Taipei), H. Wu (Taichung), T. Wu (Kaohsiung); United Kingdom: A. Bahl (Bristol), S. Chowdhury (London), J. De Bono (Sutton), S. Dixit (Grimsby), P. Elliott (Manchester), J. Graham (Somerset), P. Hoskin (Northwood), R. Jones (Glasgow), A. MacDonald (Aberdeen), Z. Malik (Wirral), D. McLaren (Edinburgh), J. O'Sullivan (Belfast), H. Payne (London); United States: N. Agarwal (Salt Lake City, UT), D. Agus (Los Angeles, CA), R. Alter (Hackensack, NJ), J. Bailen (Jeffersonville, IN), J. Bellmunt (Boston, MA), W. Berry (Raleigh, NC), K. Chang (Anchorage, AK), W. Clark (Anchorage, AK), C. Cowey (Corona, CA), R. Dreicer (Cleveland, OH), M. Fleming (Canadaigua, NY), L. Forero (East Syracuse, NY), S. Goel (San Diego, CA), J. Haluschak (Cleveland, OH), L. Hart (San Juan, Puerto Rico), E. Heath (Pittsburgh, PA), P. Lara Jr. (Sacramento, CA), S. Mao (Pittsburgh, PA), L. Norquist (New Orleans, LA), D. Petrylak (Los Angeles, CA), T. Rado (Sacramento, CA), D. Richards (Riverside, CA), A. Rodney (Highland, CA), A. Sartor (New Orleans, LA), I. Schnadig (Hackensack, NJ), P. Sieber (Lancaster, PA), R. Singal (Deerfield Beach, FL), B. Somer (Memphis, TN), G. Srkalovic (Dickson, TN), M. Wertheim (Port St Lucie, FL), N. Vogelzang (Jeffersonville, IN).
Table A1.
Patient Demographics and Disease Characteristics by Region
| Characteristic | Europe |
Non-Europe/NA |
NA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orteronel Plus Prednisone (n = 394) |
Placebo Plus Prednisone (n = 196) |
Orteronel Plus Prednisone (n = 265) |
Placebo Plus Prednisone (n = 132) |
Orteronel Plus Prednisone (n = 75) |
Placebo Plus Prednisone (n = 37) |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||||||||
| Median | 69.0 | 69.0 | 70.0 | 71.0 | 71.0 | 72.0 | ||||||
| Range | 45-89 | 50-86 | 43-87 | 50-87 | 48-84 | 48-85 | ||||||
| ≥ 70 | 187 | 47 | 92 | 47 | 138 | 52 | 75 | 57 | 42 | 56 | 27 | 73 |
| Race | ||||||||||||
| White* | 388 | 98 | 193 | 98 | 169 | 64 | 79 | 60 | 63 | 84 | 33 | 89 |
| Black/African American | 3 | < 1 | 2 | 1 | 8 | 3 | 5 | 4 | 7 | 9 | 2 | 5 |
| Asian* | 1 | < 1 | 1 | < 1 | 72 | 27 | 46 | 35 | 4 | 5 | 1 | 3 |
| American Indian or Alaskan Native | 0 | 0 | 4 | 2 | 1 | < 1 | 0 | 0 | ||||
| Other/not reported | 2 | < 1 | 0 | 12 | 5 | 1 | < 1 | 1 | 1 | 1 | 3 | |
| Time since initial diagnosis, years* | ||||||||||||
| Median | 5.3 | 5.7 | 5.3 | 5.6 | 7.5 | 7.3 | ||||||
| Range | 0-22 | 0.2-28.8 | 0.1-16.8 | 0.1-20.7 | 1.4-19.6 | 1.6-20 | ||||||
| ECOG PS, %† | ||||||||||||
| 0 | 43 | 43 | 41 | 37 | 35 | 35 | ||||||
| 1 | 49 | 52 | 49 | 52 | 57 | 62 | ||||||
| 2 | 7 | 5 | 11 | 11 | 8 | 3 | ||||||
| BPI-SF worst pain score* | ||||||||||||
| Median | 3.0 | 3.0 | 4.0 | 3.5 | 2.0 | 2.5 | ||||||
| Range | 0-10 | 0-10 | 0-10 | 0-10 | 0-10 | 0-10 | ||||||
| PSA at baseline, ng/mL* | ||||||||||||
| Median | 138.0 | 127.5 | 131.0 | 169.0 | 53.6 | 51.6 | ||||||
| Range | 3-7,992 | 3-9,263 | 1-8,456 | 2-19,009 | 0-3,534 | 1-1,164 | ||||||
| Testosterone at baseline, ng/dL | ||||||||||||
| Median | 4.7 | 5.0 | 4.7 | 3.7 | 4.2 | 4.5 | ||||||
| Range | 0.2-99.9 | 0.2-36.1 | 0.2-99.9 | 0.2-138.9 | 0.3-60.7 | 0.4-19.7 | ||||||
| Gleason score at diagnosis | ||||||||||||
| ≤ 6 | 55 | 14 | 39 | 20 | 37 | 14 | 19 | 14 | 10 | 13 | 4 | 11 |
| 7 | 108 | 27 | 56 | 29 | 82 | 31 | 34 | 26 | 23 | 31 | 15 | 41 |
| 8-10 | 191 | 48 | 81 | 41 | 142 | 54 | 74 | 56 | 39 | 52 | 16 | 43 |
| Unknown/missing | 40 | 10 | 20 | 10 | 4 | 2 | 5 | 4 | 3 | 4 | 2 | 5 |
| Extent of disease at baseline | ||||||||||||
| Bone metastases | 379 | 96 | 184 | 94 | 249 | 94 | 123 | 93 | 71 | 95 | 33 | 89 |
| Lymph node metastases | 185 | 47 | 90 | 46 | 126 | 48 | 60 | 45 | 33 | 44 | 21 | 57 |
| Lung metastases | 44 | 11 | 22 | 11 | 36 | 14 | 12 | 9 | 10 | 13 | 5 | 14 |
| Liver metastases | 37 | 9 | 24 | 12 | 19 | 7 | 17 | 13 | 8 | 11 | 3 | 8 |
| Other metastases/missing | 71 | 18 | 36 | 18 | 38 | 14 | 21 | 16‡ | 15 | 20 | 3 | 8 |
| Visceral disease | 106 | 27 | 55 | 28 | 69 | 26 | 35 | 27 | 22 | 29 | 9 | 24 |
| Prior chemotherapy regimens* | ||||||||||||
| 1 | 325 | 82 | 149 | 76 | 179 | 68 | 87 | 66 | 70 | 93 | 27 | 73 |
| ≥ 2 | 69 | 18 | 46 | 23 | 86 | 32 | 45 | 34 | 5 | 7 | 10 | 27 |
| Prior radiation therapy | 257 | 65 | 118 | 60 | 181 | 68 | 82 | 62 | 52 | 69 | 24 | 65 |
| Prior surgery | 184 | 47 | 96 | 49 | 152 | 57 | 75 | 57 | 54 | 72 | 19 | 51 |
| Prior ADT | 380 | 96 | 185 | 94 | 249 | 94 | 126 | 95 | 73 | 97 | 36 | 97 |
Abbreviations: ADT, androgen-deprivation therapy; BPI-SF, Brief Pain Inventory–Short Form; ECOG PS, Eastern Cooperative Oncology Group performance status; NA, North America; PSA, prostate-specific antigen.
Differences in baseline characteristics across regions.
Percentages may not add up to 100% because of rounding.
One missing.
Table A2.
Summary of Adverse Events by Regional Subgroup Analyses
| Category | Europe |
Non-Europe/NA |
NA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orteronel Plus Prednisone (n = 392) |
Placebo Plus Prednisone (n = 194) |
Orteronel Plus Prednisone (n = 265) |
Placebo Plus Prednisone (n = 132) |
Orteronel Plus Prednisone (n = 75) |
Placebo Plus Prednisone (n = 37) |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| All TEAEs | 382 | 97 | 183 | 94 | 261 | 98 | 125 | 95 | 74 | 99 | 37 | 100 |
| Grade ≥ 3 TEAEs | 263 | 67 | 89 | 46 | 196 | 74 | 91 | 69 | 47 | 63 | 19 | 51 |
| Drug-related TEAEs | 298 | 76 | 115 | 59 | 210 | 79 | 76 | 58 | 63 | 84 | 26 | 70 |
| Grade ≥ 3 drug-related TEAEs | 126 | 32 | 28 | 14 | 115 | 43 | 32 | 24 | 28 | 37 | 7 | 19 |
| Serious AEs | 190 | 48 | 57 | 29 | 134 | 51 | 75 | 57 | 27 | 36 | 11 | 30 |
| AEs leading to discontinuation | 108 | 28 | 35 | 18 | 49 | 18 | 15 | 11 | 9 | 12 | 3 | 8 |
| On-study deaths | 37 | 9 | 15 | 8 | 86 | 32 | 46 | 35 | 22 | 29 | 5 | 14 |
Abbreviations: AE, adverse event; NA, North America; TEAEs, treatment-emergent adverse events.
Table A3.
Subsequent Therapies Received, Overall and Across Regions
| Therapy | Global |
Europe |
non-Europe/NA |
NA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orteronel Plus Prednisone (n = 732) |
Placebo Plus Prednisone (n = 363) |
Orteronel Plus Prednisone (n = 392) |
Placebo Plus Prednisone (n = 194) |
Orteronel Plus Prednisone (n = 265) |
Placebo Plus Prednisone (n = 132) |
Orteronel Plus Prednisone (n = 75) |
Placebo Plus Prednisone (n = 37) |
|||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Patients with one or more subsequent therapies, % | 326 | 45 | 197 | 54 | 192 | 49 | 119 | 61 | 94 | 35 | 58 | 44 | 40 | 53 | 20 | 54 |
| Abiraterone | 146 | 20 | 78 | 21 | 103 | 26 | 62 | 32 | 23 | 9 | 7 | 5 | 20 | 27 | 9 | 24 |
| Cabazitaxel | 107 | 15 | 66 | 18 | 65 | 17 | 42 | 22 | 28 | 11 | 16 | 12 | 14 | 19 | 8 | 22 |
| Dexamethasone | 85 | 12 | 52 | 14 | 38 | 10 | 16 | 8 | 39 | 15 | 32 | 24 | 8 | 11 | 4 | 11 |
| Docetaxel | 40 | 5 | 32 | 9 | 22 | 6 | 21 | 11 | 14 | 5 | 9 | 7 | 4 | 5 | 2 | 5 |
| Enzalutamide | 35 | 5 | 13 | 4 | 24 | 6 | 11 | 6 | 0 | 0 | 11 | 15 | 2 | 5 | ||
| Abiraterone, cabazitaxel, or enzalutamide | 222 | 30 | 129 | 36 | 149 | 38 | 93 | 48 | 41 | 15 | 21 | 16 | 33 | 44 | 15 | 41 |
Abbreviation: NA, North America.
Fig A1.
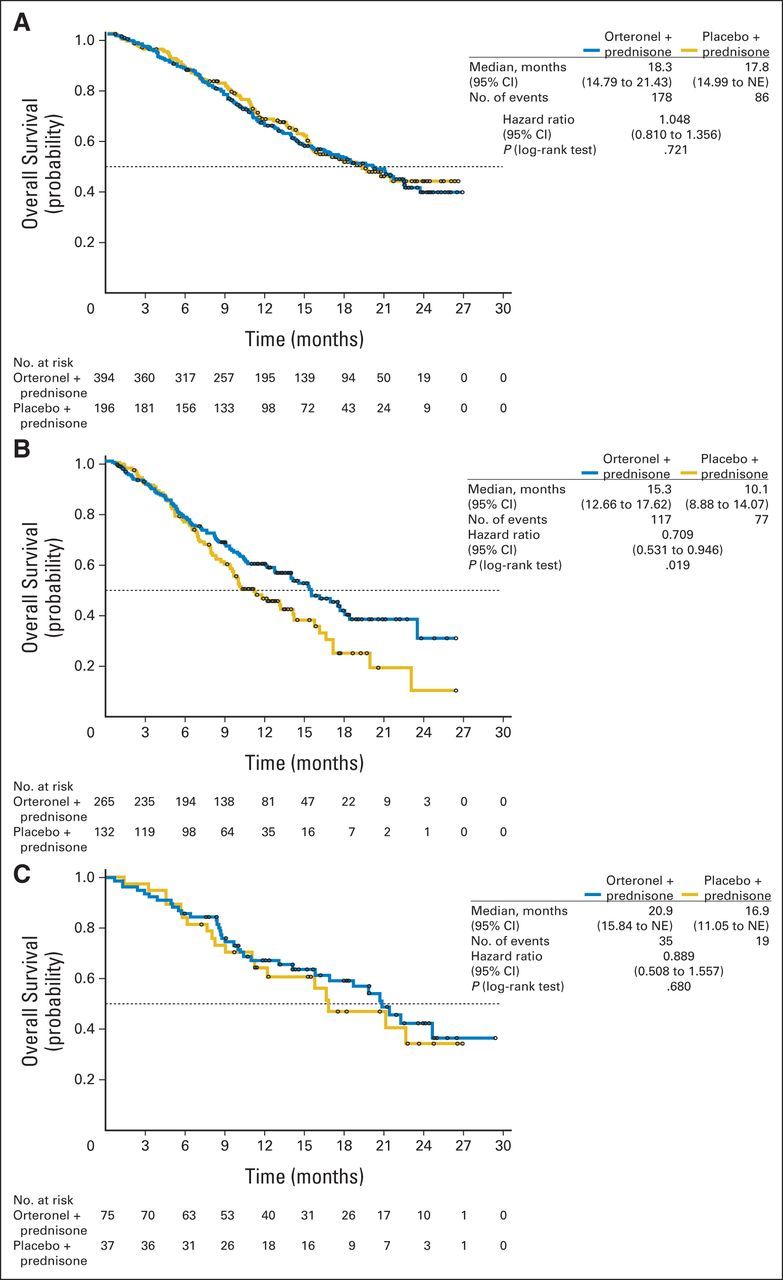
Kaplan-Meier estimates of overall survival in (A) Europe, (B) non-Europe/North America, and (C) North America. NE, not evaluable.
Fig A2.
Radiographic progression-free survival by subgroups. BPI, brief pain inventory; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
Footnotes
See accompanying editorial on page 679
Written on behalf of all ELM-PC 5 study investigators. (Full list is in the Appendix, online only.)
Supported by Takeda Pharmaceuticals International.
Presented at the 2014 American Society of Clinical Oncology Genitourinary Cancers Symposium, San Francisco, CA, January 30-February 1, 2014, and the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01193257; EudraCT information: 2010-018662-23.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Karim Fizazi, Fred Saad, Ronald de Wit, Johann De Bono, David Agus, Joaquim Bellmunt, Daniel P. Petrylak, Iain J. Webb, Bindu Tejura, Niels Borgstein, Robert Dreicer
Provision of study materials or patients: Karim Fizazi, Robert Jones, Stephane Oudard, Eleni Efstathiou, Fred Saad, Ronald de Wit, Johann De Bono, George Fountzilas, Albertas Ulys, Flavio Carcano, Neeraj Agarwal, David Agus, Joaquim Bellmunt
Collection and assembly of data: Karim Fizazi, Robert Jones, Stephane Oudard, Eleni Efstathiou, Fred Saad, Ronald de Wit, Felipe Melo Cruz, George Fountzilas, Albertas Ulys, Flavio Carcano, Neeraj Agarwal, David Agus, Joaquim Bellmunt, Bindu Tejura, Robert Dreicer
Data analysis and interpretation: Karim Fizazi, Stephane Oudard, Eleni Efstathiou, Fred Saad, Ronald de Wit, Johann De Bono, David Agus, Joaquim Bellmunt, Daniel P. Petrylak, Shih-Yuan Lee, Bindu Tejura, Niels Borgstein, Robert Dreicer
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III, Randomized, Double-Blind, Multicenter Trial Comparing Orteronel (TAK-700) Plus Prednisone With Placebo Plus Prednisone in Patients With Metastatic Castration-Resistant Prostate Cancer That Has Progressed During or After Docetaxel-Based Therapy: ELM-PC 5
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Karim Fizazi
Honoraria: Dendreon
Consulting or Advisory Role: Millennium Pharmaceuticals, sanofi-aventis, Janssen, Astellas, Bayer, Bristol-Meyers Squibb, Amgen, Dendreon, Orion
Speakers' Bureau: sanofi-aventis, Janssen, Astellas, Amgen, Dendreon
Robert Jones
Honoraria: Millennium Pharmaceuticals, sanofi-aventis, Janssen-Cilag, Astellas Pharma, GlaxoSmithKline, Bayer
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Bristol-Myers Squibb, Pfizer, Novartis, AstraZeneca
Research Funding: Pfizer (Inst), Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Roche (Inst), Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst)
Stephane Oudard
Honoraria: sanofi-aventis, Roche, Keocyt, Janssen Pharmaceuticals, Bayer, Millennium Pharmaceuticals
Consulting or Advisory Role: sanofi-aventis, Roche, Keocyt, Janssen Pharmaceuticals, Bayer, Millennium Pharmaceuticals
Travel, Accommodations, Expenses: Novartis, Roche, Bayer, Sanofi, Bristol-Myers Squibb, Janssen, Pfizer
Eleni Efstathiou
Honoraria: Millennium Pharmaceuticals
Consulting or Advisory Role: Millennium Pharmaceuticals
Fred Saad
Consulting or Advisory Role: Millennium Pharmaceuticals
Research Funding: Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst)
Ronald de Wit
Honoraria: Janssen, sanofi-aventis
Consulting or Advisory Role: Millennium Pharmaceuticals, Janssen Pharmaceuticals, Astellas Pharma, sanofi-aventis
Research Funding: Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst), sanofi-aventis (Inst)
Johann De Bono
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GlaxoSmithKline, Medivation, Millennium Pharmaceuticals, Novartis, Otsuka, Pfizer, sanofi-aventis
Research Funding: AstraZeneca (Inst), Genentech (Inst), BiPar/sanofi-aventis (Inst), Taiho Pharmaceutical (Inst)
Felipe Melo Cruz
Consulting or Advisory Role: Roche, Janssen Oncology
George Fountzilas
No relationship to disclose
Albertas Ulys
No relationship to disclose
Flavio Carcano
No relationship to disclose
Neeraj Agarwal
Honoraria: Dendreon
Research Funding: Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), ImClone Systems (Inst), Medivation (Inst), Novartis (Inst), Pfizer (Inst)
David Agus
Consulting or Advisory Role: Millennium Pharmaceuticals
Joaquim Bellmunt
Consulting or Advisory Role: Millennium Pharmaceuticals, Astellas Pharma, Janssen-Cilag
Research Funding: Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst)
Daniel P. Petrylak
Consulting or Advisory Role: Millennium Pharmaceuticals, Janssen Pharmaceuticals, Bayer, Bellicum Pharmaceuticals, Dendreon, Ferring, Exelixis
Research Funding: Millennium Pharmaceuticals (Inst), Janssen Pharmaceuticals (Inst), BiPar/sanofi-aventis (Inst)
Travel, Accommodations, Expenses: Millennium Pharmaceuticals, Johnson & Johnson, Celgene, Progenics
Shih-Yuan Lee
Employment: Takeda Pharmaceutical
Iain J. Webb
Employment: Millennium Pharmaceuticals
Bindu Tejura
Employment: Takeda Pharmaceutical, Summit Therapeutics
Stock or Other Ownership: Takeda Pharmaceutical, Summit Therapeutics
Niels Borgstein
Employment: Takeda Pharmaceutical
Stock or Other Ownership: Takeda Pharmaceutical
Robert Dreicer
Consulting or Advisory Role: Millennium Pharmaceuticals, Dendreon, Janssen Oncology, Medivation, Roche, Merck, Bind Pharma
Research Funding: Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical (Inst), Bind, Dendreon
REFERENCES
- 1.Huggins C, Hodges CV. Studies on prostatic cancer, I: The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Horwich A, Hugosson J, de Reijke T, et al. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24:1141–1162. doi: 10.1093/annonc/mds624. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 5.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K, Martinez LA, Sikes CR, et al. The association of p21((WAF-1/CIP1)) with progression to androgen-independent prostate cancer. Clin Cancer Res. 2002;8:775–781. [PubMed] [Google Scholar]
- 7.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart P. Chapter 14: The adrenal cortex. In: Kronenberg HM, Melmed S, Polonsky KS, et al., editors. Williams Textbook of Endocrinology. St Louis, MO: Elsevier Saunders; 2013. pp. 446–452. [Google Scholar]
- 11.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 14.Kluetz PG, Ning YM, Maher VE, et al. Abiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: US Food and Drug Administration drug approval summary. Clin Cancer Res. 2013;19:6650–6656. doi: 10.1158/1078-0432.CCR-13-2134. [DOI] [PubMed] [Google Scholar]
- 15.Kaku T, Hitaka T, Ojida A, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka M, Hara T, Hitaka T, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: Effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol. 2012;129:115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Dreicer R, Maclean D, Suri A, et al. Phase I/II trial of orteronel (TAK-700)—an investigational 17,20-lyase inhibitor—in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2014;20:1335–1344. doi: 10.1158/1078-0432.CCR-13-2436. [DOI] [PubMed] [Google Scholar]
- 18.Hussain M, Corn PG, Michaelson MD, et al. Phase II study of single agent orteronel (TAK-700) in patients with nonmetastatic castration-resistant prostate cancer and rising prostate-specific antigen. Clin Cancer Res. 2014;20:4218–4227. doi: 10.1158/1078-0432.CCR-14-0356. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.02. http://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02_2009-09-15_QuickReference_8.5x11.pdf.
- 23.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 25.De Wit R, Fizazi K, Jinga V, et al. Phase 3, randomized, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients (pts) with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC) (ELM-PC 4 trial) J Clin Oncol. 2014;32(suppl 15s):325s. abstr 5008. [Google Scholar]
- 26.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 27.Saad ED, Buyse M. Overall survival: Patient outcome, therapeutic objective, clinical trial end point, or public health measure? J Clin Oncol. 2012;30:1750–1754. doi: 10.1200/JCO.2011.38.6359. [DOI] [PubMed] [Google Scholar]
- 28.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]