Abstract
Purpose
Five or more circulating tumor cells (CTCs) per 7.5 mL of blood predicts for poorer progression-free survival (PFS) in patients with metastatic breast cancer (MBC). We conducted a prospective study to demonstrate that CTC results correlate strongly with radiographic disease progression at the time of and in advance of imaging.
Patients and Methods
Serial CTC levels were obtained in patients starting a new treatment regimen for progressive, radiographically measurable MBC. Peripheral blood was collected for CTC enumeration at baseline and at 3- to 4-week intervals. Clinical outcomes were based on radiographic studies performed in 9- to 12-week intervals.
Results
Sixty-eight patients were evaluable for the CTC-imaging correlations, and 74 patients were evaluable for the PFS analysis. Median follow-up was 13.3 months. A statistically significant correlation was demonstrated between CTC levels and radiographic disease progression in patients receiving chemotherapy or endocrine therapy. This correlation applied to CTC results obtained at the time of imaging (odds ratio [OR], 6.3), 3 to 5 weeks before imaging (OR, 3.1), and 7 to 9 weeks before imaging (OR, 4.9). Results from analyses stratified by type of therapy remained statistically significant. Shorter PFS was observed for patients with five or more CTCs at 3 to 5 weeks and at 7 to 9 weeks after the start of treatment.
Conclusion
We provide, to our knowledge, the first evidence of a strong correlation between CTC results and radiographic disease progression in patients receiving chemotherapy or endocrine therapy for MBC. These findings support the role of CTC enumeration as an adjunct to standard methods of monitoring disease status in MBC.
INTRODUCTION
Breast cancer is the most frequently diagnosed malignancy and is the second leading cause of cancer-related deaths in women. Despite the number of effective treatment regimens for metastatic disease, response to therapy is generally transient, and few randomized clinical trials demonstrate statistically significant overall survival (OS) benefits. Nonetheless, 5-year survival rates in the setting of metastatic breast cancer (MBC) improved from 22% in the year 20001 to 27% in the year 2008,2 which demonstrates that therapeutic advances do affect mortality from this disease. More substantial improvements are needed, and recent advances in molecular diagnostics should translate into more personalized treatment plans and improved clinical outcomes.
Circulating tumor cells (CTCs) are known to circulate in the peripheral blood of patients with breast, colorectal, prostate, and lung carcinoma.3–10 They are rare in patients who are healthy or have nonmalignant disease.3,11 The first report on CTCs was published in 1869,12 and subsequent case reports of carcinocythemia or carcinoma cell leukemia correlate the identification of CTCs in epithelial malignancies with poor clinical outcomes.13–20 Immunomagnetic platforms now are available for the reproducible enumeration of CTCs at low frequencies and in relatively small blood volumes.21,22
Increasing evidence correlates CTCs with progression-free survival (PFS) and OS in MBC.11,23–30 The strongest data are provided by analyses from a single, prospective, multi-institutional study that used the US Food and Drug Administration–approved CellSearch technology (Veridex; Raritan, NJ).11,23,24,28 The detection of five or more CTCs per 7.5 mL of blood, versus less than five CTCs per 7.5 mL of blood, before and after the initiation of a new systemic treatment regimen was associated with shorter median PFS and OS. In addition, the enumeration of CTCs before and several weeks after initiating the new treatment regimen was informative, as maintaining or decreasing the number of CTCs to less than five was indicative of treatment response and predictive of improved PFS and OS. These observations led us to conduct a validation study with the hypothesis that the number of CTCs relative to a threshold of five correlates strongly with radiographic determinations of disease progression, both at the time and before the imaging studies are obtained.
PATIENTS AND METHODS
Study Design
We conducted a prospective, longitudinal, clinical study to demonstrate a correlation between serial CTC results and disease progression in patients starting a new systemic treatment regimen for progressive, radiographically measurable MBC. The primary objectives of this study were as follows: to demonstrate equivalence between CTC enumeration and standard radiographic studies as a means of assessing disease status; to determine whether CTC levels are indicative of treatment benefit in advance of radiographic changes; and to better define the appropriate time at which to enumerate CTCs as a reliable predictor of PFS.
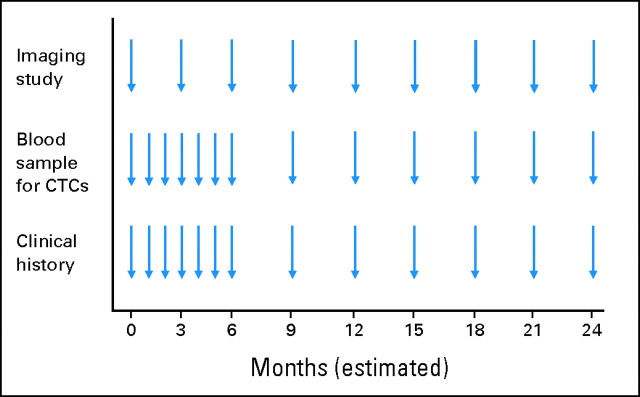
The study design is summarized in Figure 1. Before initiating a new line of systemic therapy, eligible patients had a 10-mL blood sample collected for CTC enumeration and completed an imaging evaluation; these baseline studies were completed within 14 and 28 days of starting treatment, respectively. Serial blood samples were collected at the start of each chemotherapy cycle or at the start of each month of endocrine therapy, for a total of six additional evaluations. Thereafter, blood samples were obtained in 9- to 12-week intervals in conjunction with the same type of imaging studies performed at baseline. On disease progression, all patients were observed for OS and were offered the opportunity to continue CTC testing with future therapy. CTC results were provided to clinicians and patients with the understanding that decisions regarding patient care would be based on standard clinical and radiographic evaluations.
Fig 1.
Study design. Eligible patients completed a baseline imaging evaluation and blood collection for circulating tumor cell (CTC) enumeration before starting a new line of systemic therapy (time = 0). Imaging studies and blood collection for CTCs were repeated at strictly defined intervals to perform the most rigorous analysis for correlation.
The study was independently designed by the lead investigator (M.C.L.). Patient confidentiality was maintained per the policies and procedures of the National Institutes of Health Office for Protection from Research Risks. The sponsor did not have access to the data.
Patients
Principal eligibility criteria included stage IV, histologically proven, invasive breast carcinoma; recent disease progression; radiographically measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST)31; and plans to initiate a new systemic treatment regimen. Estrogen receptor, progesterone receptor, and HER2/neu status were reported per the local guidelines of each participating institution (ie, Georgetown University Medical Center, Northern Virginia Medical Oncology and Hematology Associates, and Franklin Square Hospital Center). No restrictions were imposed with respect to the type or line of therapy. Conduct of this study was approved by the local institutional review boards in accordance with an assurance filed with and approved by the Department of Health and Human Services. All patients provided written informed consent.
Patient accrual began in June 2005, and data were collected on 81 patients as of August 8, 2008. Sixty-eight patients were evaluable for the correlation between CTC enumeration and radiographic imaging (ie, they had at least one pair of results after the baseline visit). Six additional patients lacked paired results but had initial CTC assessments and subsequent clinical and/or radiographic documentation of disease status, so they were included in the PFS analysis. The remaining seven patients did not fit either of these criteria.
Enumeration of CTCs
CTC isolation and enumeration were performed by using the US Food and Drug Administration–approved CellSearch technology (Veridex), which allows for the immunomagnetic selection, fluorescence staining, concentration, and sample enrichment of CTCs. A total of 7.5 to 10 mL of blood was collected in a CellSave Preservation Tube (Veridex), which contains an optimized preservative that stabilizes cells at room temperature for up to 72 hours. Immunomagnetic enrichment was achieved by using the anti-EpCAM (ie, epithelial cell adhesion molecule) Ferrofluid (Veridex). The isolated cells then were fluorescently labeled with the 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid dye and with monoclonal antibodies specific for epithelial cells (ie, anticytokeratin-phycoerythrin to cytokeratins 8/18/19) and leukocytes (ie, anti-CD45-allophycocyanin). CTCs were identified as cells with the appropriate morphology as cytokeratin positive, DAPI positive, and CD45 negative. Quantitative results were reported as the number of CTCs per 7.5 mL of blood with a specificity of 99.7%.3 All CTC assessments were performed in a Clinical Laboratory Improvement Amendments (CLIA) –approved laboratory at Georgetown University Medical Center by technicians trained and certified in the CellSearch technology (Veridex). All CTC results were reviewed by an experienced investigator (M.C.L. or P.G.S.).
Definition of Response by Radiographic Imaging
All imaging studies were performed per standard procedures. Radiologists were blinded with respect to the CTC results and the nature of the study to eliminate a biased interpretation of the radiographic findings. Responses were delineated as disease progression or as no disease progression (ie, stable disease, partial response, complete response) by using RECIST.
Statistical Design
The sensitivity and specificity of CTC enumeration as a test of disease progression were computed by using radiographic evaluations as the gold standard by which disease progression is defined. Because repeated observations (ie, the pairs of CTC results and radiographic findings) from the same patient were correlated, the 95% CIs were estimated with the ratio estimator for the variance of clustered binary data.32,33
The association between CTC levels and radiographic findings was assessed with logistic regression. We expected that pairs of test results from the same patient would be correlated, so generalized estimating equations were used to capture this correlation.34 An exchangeable correlation structure was assumed; if the intrapatient correlation was estimated to be less than 0, the pairs of results within a patient were treated as independent. This association was evaluated overall and by stratifying for treatment type at the time of CTC enumeration by using the results obtained at the time of radiographic imaging and at 3 to 5 weeks (±5 days) and 7 to 9 weeks (±5 days) before imaging.
PFS was measured as the time between the baseline CTC assessment (ie, the initiation of treatment) and the documentation of first radiographic disease progression or death. Patients who were alive and progression free at the time of analysis were censored by using the time between the baseline CTC assessment and their most recent follow-up evaluations. The PFS between groups defined by less than five or five or more CTCs was compared with the Kaplan-Meier method, and differences were tested with the log-rank test. Analyses were done on the basis of CTC results at baseline and at 3 to 5 weeks (±5 days) and 7 to 9 weeks (±5 days) after the initiation of therapy.
The time intervals for each analysis were strictly defined and were selected to include various definitions of treatment cycles per routine clinical care. Patients were excluded if they did not have CTC assessments within the prespecified interval.
RESULTS
Patient Characteristics
Table 1 provides demographic information for the 74 evaluable patients. Hormone receptor and HER2/neu results were based on biopsies of metastatic sites whenever available; results from the breast primary were used otherwise. Given the sample size, analyses by treatment type compared chemotherapy (alone or in combination) with endocrine therapy (alone or in combination). Fifty-three patients (72%) started second-line or greater therapy at study entry.
Table 1.
Patient Demographic and Clinical Characteristics
| Variable | Patients |
|
|---|---|---|
| No. | % | |
| Total No. of evaluable patients* | 74 | |
| Age at study entry, years | ||
| Median | 51.5 | |
| Range | 33-88 | |
| Ethnicity | ||
| White | 55 | 74 |
| Black | 19 | 26 |
| Hispanic | 1 | 1 |
| Non-Hispanic | 73 | 99 |
| Receptor status | ||
| ER and/or PR positive | 51 | 69 |
| HER2/neu positive | 19 | 26 |
| ER/PR/HER2 negative | 15 | 20 |
| Sites of metastasis at study entry† | ||
| Visceral | 55 | 74 |
| Nonvisceral | 19 | 26 |
| Type of systemic therapy at study entry | ||
| Chemotherapy | 20 | 27 |
| Chemotherapy plus biologic agent | 19 | 25 |
| Endocrine therapy | 22 | 30 |
| Endocrine therapy plus biologic agent | 11 | 15 |
| Biologic agent alone | 2 | 3 |
| Line of systemic therapy at study entry | ||
| First-line | 21 | 28 |
| Second-line or greater | 53 | 72 |
| Median progression-free survival, months | 4.5 | |
| Median follow-up, months | 13.3 | |
| Patients who continued beyond first progression | 30 | 41 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
No. of patients evaluable for the correlation of circulating tumor cell enumeration and radiographic imaging = 68.
Visceral sites included the lungs, liver, brain, adrenal glands, pancreas, and pleura (with or without effusions). Nonvisceral sites were defined as the breast, lymph nodes, chest wall, bone, and skin.
The median time from the baseline to last CTC result was 6.6 months (mean, 10.0 months; range, 0.69 to 39 months), with a range of two to 27 CTC results. Four patients had less than 2 months between the baseline and last CTC result after experiencing rapid disease progression. Forty-one patients (60%) had three or more radiographic evaluations (range, two to 15 evaluations). Thirty patients (41%) continued study participation beyond first progression, such that the timing of CTC enumeration and radiographic studies restarted with each line of therapy (Fig 1). These patients are represented through multiple lines of therapy in the CTC-imaging correlation, but the PFS analysis is based only on progression relative to treatment at study entry.
CTC Results
The CTC findings are listed in Table 2. A total of 684 blood draws were performed, 22 (3%) of which did not result in CTC enumeration for one of the following reasons: insufficient blood volume (n = 9); hemolyzed specimens (n = 2); clotted specimens (n = 1); technical issues (n = 6); and missed time points (n = 4). The sensitivity and specificity of CTC counts relative to the determination of radiographic disease progression are reported in Table 3.
Table 2.
Summary of CTC Results
| Variable | Cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| CTC Imaging |
PFS |
|||||||
| Baseline |
All Time Points |
Baseline |
All Time Points |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| CTCs per 7.5 mL of blood | ||||||||
| Mean | 12.3 | 15.8 | 11.7 | 15.5 | ||||
| SD | 31.8 | 51.1 | 30.8 | 50.7 | ||||
| Median | 2 | 1 | 2 | 1 | ||||
| Range | 0-236 | 0-580 | 0-236 | 0-580 | ||||
| No. of CTC results | ||||||||
| 0 | 23 | 34 | 289 | 43 | 24 | 32 | 292 | 43 |
| < 5* | 43 | 63 | 461 | 69 | 47 | 64 | 470 | 69 |
| ≥ 5 | 24 | 35 | 188 | 28 | 25 | 34 | 191 | 28 |
| No result | 1 | 1 | 22 | 3 | 2 | 3 | 22 | 3 |
NOTE. No. of patients in CTC imaging cohort = 68. No. of CTC results in CTC imaging cohort = 671. No. of patients in PFS cohort = 74. No. of CTC results in PFS cohort = 684.
Abbreviations: CTC, circulating tumor cells; PFS, progression-free survival; SD, standard deviation.
Includes results of zero CTCs. Seven patients had zero as their only results.
Table 3.
Sensitivity and Specificity of CTC Enumeration at Various Time Points Relative to the Determination of Disease Progression by Radiographic Imaging
| No. of CTCs | No. of Patients | Paired CTC-Imaging Results | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
|---|---|---|---|---|---|---|
| At the time of imaging | 68 | 233 | 46.5 | 35.2 to 57.7 | 88.1 | 80.0 to 96.1 |
| 3-5 weeks before imaging | 62 | 159 | 43.9 | 29.8 to 58.1 | 79.6 | 70.8 to 88.3 |
| 7-9 weeks before imaging | 59 | 176 | 39.0 | 25.8 to 52.1 | 87.9 | 80.0 to 95.7 |
Abbreviation: CTC, circulating tumor cell.
Correlation Between CTCs and Radiographic Response
Sixty-eight patients had radiographic assessments of disease response and had corresponding CTC results, with an average of 4 days between imaging and the blood draw. Forty patients (59%) had more than one pair of test results, with a maximum of 14 pairs. The matched CTC result was statistically significantly associated with disease progression for all patients (P < .001). That is, patients who had five or more CTCs had 6.3 times the odds of radiographic disease progression compared with patients who had less than five CTCs. This association remained strong for patients treated with either chemotherapy or endocrine therapy (Table 4).
Table 4.
Summary of Paired CTC Results and Imaging Results by Treatment Type for Patients With Less Than Five Versus Five or More CTCs at Baseline
| Radiographic Progression According to Treatment at Time of CTC Result | No. of CTCs |
Statistical Analysis |
|||||
|---|---|---|---|---|---|---|---|
| < 5 |
≥ 5 |
||||||
| No. | % | No. | % | OR | 95% CI | P | |
| Overall | |||||||
| No | 118 | 69 | 16 | 26 | 6.3 | 3.2 to 13 | < .001 |
| Yes | 53 | 31 | 46 | 74 | |||
| Chemotherapy | |||||||
| No | 81 | 71 | 13 | 29 | 6.3 | 2.9 to 14 | < .001 |
| Yes | 33 | 29 | 32 | 71 | |||
| Endocrine therapy | |||||||
| No | 37 | 67 | 3 | 19 | 8.9 | 2.2 to 35 | .002* |
| Yes | 18 | 33 | 13 | 81 | |||
NOTE. Three pairs were obtained from patients on biologic therapy alone and were not included in this analysis. These results were unchanged when the baseline pair of CTC results and radiographic studies were included in the analysis (total 295 pairs).
Abbreviations: CTC, circulating tumor cell; OR, odds ratio.
Intrapatient correlation was estimated as less than zero.
Sixty-two patients had radiographic assessments of disease response preceded by CTC results obtained 3 to 5 weeks before imaging. Thirty-six patients (58%) had more than one pair of test results, with a maximum of eight pairs. The CTC result 3 to 5 weeks before imaging was statistically significantly associated with disease progression for all patients, for those treated with chemotherapy, and for those treated with endocrine therapy (Table 5).
Table 5.
Summary of Paired CTC Results and Imaging Results by Treatment Type for Patients With Less Than Five Versus Five or More CTCs at 3 to 5 Weeks Before Radiographic Imaging
| Radiographic Progression According to Treatment at Time of CTC Result | No. of CTCs |
Statistical Analysis |
|||||
|---|---|---|---|---|---|---|---|
| < 5 |
≥ 5 |
||||||
| No. | % | No. | % | OR | 95% CI | P | |
| Overall | |||||||
| No | 74 | 67 | 19 | 40 | 3.1 | 1.6 to 5.8 | .001 |
| Yes | 37 | 33 | 29 | 60 | |||
| Chemotherapy | |||||||
| No | 45 | 66 | 15 | 42 | 2.7 | 1.2 to 6.3 | .02* |
| Yes | 23 | 34 | 21 | 58 | |||
| Endocrine therapy | |||||||
| No | 29 | 69 | 3 | 30 | 5.2 | 1.2 to 23 | .03* |
| Yes | 13 | 31 | 7 | 70 | |||
NOTE. Three pairs were obtained from patients on biologic therapy alone and were not included in this analysis.
Abbreviations: CTC, circulating tumor cell; OR, odds ratio.
Intrapatient correlation estimated as less than zero.
Fifty-nine patients had radiographic assessments of disease response preceded by CTC results obtained 7 to 9 weeks before imaging. Thirty-six patients (61%) had more than one pair of test results, with a maximum of 12 pairs. The CTC result 7 to 9 weeks before imaging was statistically significantly associated with disease progression for all patients and for those receiving chemotherapy. The association was positive, albeit not statistically significant, for patients treated with endocrine therapy (Table 6).
Table 6.
Summary of Paired CTC Results and Imaging Results by Treatment Type for Patients With Less Than Five Versus Five or More CTCs at 7 to 9 Weeks Before Radiographic Imaging
| Radiographic Progression According to Treatment at Time of CTC Result | No. of CTCs |
Statistical Analysis |
|||||
|---|---|---|---|---|---|---|---|
| < 5 |
≥ 5 |
||||||
| No. | % | No. | % | OR | 95% CI | P | |
| Overall | |||||||
| No | 87 | 65 | 12 | 29 | 4.9 | 2.2 to 11 | < .001 |
| Yes | 47 | 35 | 30 | 71 | |||
| Chemotherapy | |||||||
| No | 54 | 66 | 8 | 27 | 6.9 | 3.0 to 16 | < .001 |
| Yes | 28 | 34 | 22 | 73 | |||
| Endocrine therapy | |||||||
| No | 32 | 63 | 4 | 33 | 2.9 | 0.6 to 14 | .20 |
| Yes | 19 | 37 | 8 | 67 | |||
NOTE. One pair was obtained from a patient on biologic therapy alone and was not included in this analysis.
Abbreviations: CTC, circulating tumor cell; OR, odds ratio.
PFS
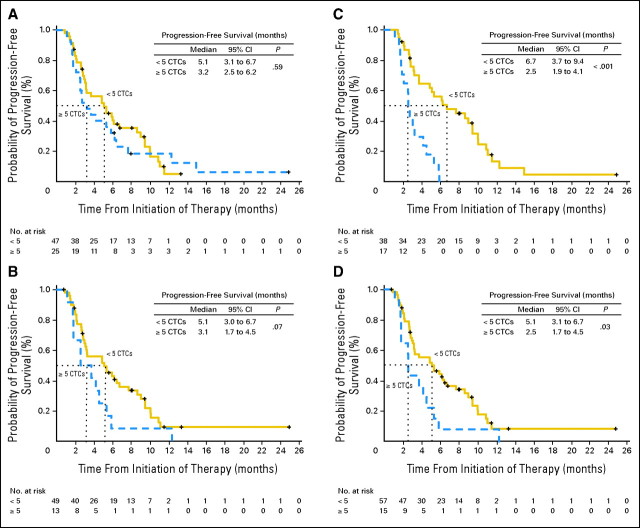
PFS was calculated for groups defined by the threshold of five CTCs at baseline, 3 to 5 weeks, and 7 to 9 weeks after the start of treatment (Figs 2A to C). Shorter median PFS was observed for patients who had five or more CTCs compared with patients who had less than five CTCs at all time points. This was marginally significant for CTCs drawn 3 to 5 weeks after the initiation of therapy (3.1 v 5.1 months; P = .07) but was highly statistically significant for CTCs drawn 7 to 9 weeks after the initiation of therapy (2.5 v 6.7 months; P < .001) in this largely pretreated patient population.
Fig 2.
Progression-free survival (PFS) for patients with less than five versus five or more circulating tumor cells (CTCs) at (A) baseline, (B) 3 to 5 weeks after initiating therapy, (C) 7 to 9 weeks after initiating therapy, and (D) the first blood draw after initiating therapy. PFS was measured as the time between the baseline CTC assessment and documentation of first radiographic evidence of disease progression or death.
Some patients did not have blood drawn within the strictly defined time intervals, so an analysis was performed with the first CTC result after baseline irrespective of timing (Fig 2D). The mean number of weeks between the baseline and first follow-up CTC was 4.6 weeks (standard deviation, ±2.1 weeks; median, 4 weeks; maximum, 15 weeks); this is similar to the first follow-up time point used by Cristofanilli et al.11,23 A statistically significant difference in median PFS was observed for patients who had five or more CTCs compared with patients who had less than five CTCs at the first follow-up time point (2.5 v 5.1 months; P = .03). The predictive value of CTC enumeration appears to be independent of the site of metastasis (visceral v nonvisceral; data not shown).
DISCUSSION
We provide, to our knowledge, the first clear evidence of a strong correlation between CTC enumeration and radiographic determinations of disease progression in patients receiving chemotherapy or endocrine therapy for MBC. This correlation applies not only to CTC results obtained at the time of imaging but, more importantly, also to CTC results obtained as far in advance as 7 to 9 weeks before imaging in patients receiving chemotherapy and 3 to 5 weeks before imaging in patients receiving chemotherapy or endocrine therapy. We also provide independent data to support observations that the enumeration of CTCs relative to a threshold of five after the initiation of systemic therapy is a reliable predictor of PFS with MBC. These findings support the role of CTC enumeration as an adjunct to standard clinical and radiographic methods of monitoring disease status in MBC.
Tumor responsiveness determined by radiographic imaging is an assumed surrogate for clinically meaningful benefit from systemic therapy in metastatic disease. The standard approach is to continue treatment in the absence of unacceptable toxicity and radiographic evidence of disease progression. However, imaging studies are subject to a considerable degree of intraobserver and interobserver variability,28,35 and it is not uncommon to find radiographically stable or responsive disease on initial follow-up and then observe rapid clinical decline shortly thereafter. Serum-based assays for carcinoembryonic antigen and the MUC1 antigens (ie, CA 15.3 and CA 27.29) may be used, but their reliability is affected by limited specificity and the tumor-spike phenomenon. Assays that more accurately reflect underlying tumor biology are needed for use in conjunction with radiology studies. Immunomagnetic labeling and immunofluorescent identification of CTCs provide for a reproducible assay28 on the basis of an easily accessible body fluid (ie, peripheral blood). Our findings demonstrate that documentation of five or more CTCs after the initiation of a chemotherapy-based treatment regimen is highly suggestive of impending clinical disease progression. This predictive capability extends to endocrine-based treatment regimens as well.
An algorithm for timing CTC enumeration relative to the start of treatment has been lacking, but our findings suggest that serial assessments taken between and at the time of imaging studies will be most useful. Patients who persistently achieve less than five CTCs are predicted to have prolonged benefit with their current therapies, whereas patients who persistently have five or more CTCs are predicted to experience only short-term benefits (if any). When used in conjunction with imaging, CTC enumeration would help confirm evidence of tumor response or reconcile discrepant findings. Consider, for example, patients who are clinically asymptomatic and are tolerating treatment with minimal toxicity but have radiographic evidence of stable disease or minimal disease progression. Those who persistently have less than five CTCs are likely to derive continued benefit from their current therapies and would avoid changing treatment too soon. Those who persistently have five or more CTCs are unlikely to derive meaningful benefit from their current therapies and should seek alternate treatment regimens. This last concept is the focus of a Southwest Oncology Group study, in which patients receiving first-line chemotherapy for MBC are randomly assigned to continue current therapy or to start a new treatment regimen if they have five or more CTCs at baseline and after the first treatment cycle.
Our study has two potential limitations. First, the study population includes patients receiving various lines and types of therapy. This heterogeneity may be a confounding variable, but the similarity between our findings about PFS and those of other studies with similar patient populations11,23,25,30 is encouraging. Second, the subgroup analysis for the CTC-imaging correlation was performed by including biologic agents with either chemotherapy or endocrine therapy. Although we preferred to group patients by chemotherapy, chemotherapy and biologic agents, endocrine therapy, or endocrine therapy and biologic agents, the numbers of patients in each group was too small when they were additionally subdivided by CTC results. This is particularly relevant for the patients who received an endocrine-based treatment regimen, as there is some debate about the utility of CTC analysis in that patient population. Nonetheless, endocrine therapy was administered only for hormone receptor–positive MBC, whether alone or in combination with a biologic agent, and only two patients received trastuzumab with endocrine therapy. The remaining patients were HER2/neu-negative and were treated on an institutional protocol of anastrozole and sorafenib. Therefore, the results of the CTC-imaging correlation for patients on endocrine therapy–containing regimens are not driven by the efficacy of HER2/neu-directed therapies and should be considered valid for patients with hormone receptor–positive disease.
In conclusion, our data support the clinical utility of serial CTC enumeration in conjunction with standard radiographic imaging to improve our ability to accurately assess treatment benefit and to expedite the identification of effective treatment regimens for individual patients with measurable disease. CTC analysis is subject to less observer variability than radiographic evaluations, has relatively high specificity, reflects a lack of treatment benefit in advance of radiographic findings, and is less costly and time consuming for patients. Collecting a single, 10-mL blood sample at various time points relative to treatment might, therefore, enable clinicians to more accurately predict for treatment benefit, limit patient exposure to ineffective agents with unnecessary toxicity, assist in the identification of patients most likely to benefit from clinical trials of novel therapeutics, and improve patient survival and quality of life. It also may be possible to limit the number of follow-up radiology studies required; that is, asymptomatic patients with stable or responsive disease and serial documentation of less than five CTCs could avoid repetitive scans in the absence of a shift to five or more CTCs. The results described here represent a final analysis for the stated end points. Continued data collection with a focus on the correlation between CTC findings and OS and on the value of serial CTC enumeration beyond first progression through multiple lines of therapy are ongoing.
Acknowledgment
We thank the patients who kindly participated in this study; Shiva Krishnan and Rong Shen for sample processing; Ann Gallagher for trial coordination; and Ihsan Abdur-Rahman for data management.
Footnotes
Supported by Veridex, Raritan, NJ.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Minetta C. Liu, Veridex(C) Stock Ownership: None Honoraria: Minetta C. Liu, Veridex Research Funding: Minetta C. Liu, Veridex Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Minetta C. Liu
Administrative support: Minetta C. Liu, Peter G. Shields
Provision of study materials or patients: Minetta C. Liu, Robert D. Warren, Philip Cohen, Mary Wilkinson, Yvonne L. Ottaviano, Suman B. Rao, Jennifer Eng-Wong, Claudine Isaacs
Collection and assembly of data: Minetta C. Liu, Peter G. Shields, Robert D. Warren, Philip Cohen, Mary Wilkinson, Yvonne L. Ottaviano, Suman B. Rao, Jennifer Eng-Wong, Claudine Isaacs
Data analysis and interpretation: Minetta C. Liu, Francoise Seillier-Moiseiwitsch, Anne-Michelle Noone
Manuscript writing: Minetta C. Liu, Claudine Isaacs
Final approval of manuscript: Minetta C. Liu, Peter G. Shields, Robert D. Warren, Philip Cohen, Mary Wilkinson, Yvonne L. Ottaviano, Suman B. Rao, Jennifer Eng-Wong, Francoise Seillier-Moiseiwitsch, Anne-Michelle Noone, Claudine Isaacs
REFERENCES
- 1.Greenlee RT, Murray T, Bolden S, et al. Cancer statistics 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 4.Böckmann B, Grill HJ, Giesing M. Molecular characterization of minimal residual cancer cells in patients with solid tumors. Biomol Eng. 2001;17:95–111. doi: 10.1016/s1389-0344(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Fetsch PA, Cowan KH, Weng DE, et al. Detection of circulating tumor cells and micrometastases in stage II, III, and IV breast cancer patients utilizing cytology and immunocytochemistry. Diagn Cytopathol. 2000;22:323–328. doi: 10.1002/(sici)1097-0339(200005)22:5<323::aid-dc13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Loberg RD, Fridman Y, Pienta BA, et al. Detection and isolation of circulating tumor cells in urologic cancers: A review. Neoplasia. 2004;6:302–309. doi: 10.1593/neo.03484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma PC, Blaszkowsky L, Bharti A, et al. Circulating tumor cells and serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2003;23:49–62. [PubMed] [Google Scholar]
- 8.Molnar B, Sipos F, Galamb O, et al. Molecular detection of circulating cancer cells: Role in diagnosis, prognosis and follow-up of colon cancer patients. Dig Dis. 2003;21:320–325. doi: 10.1159/000075355. [DOI] [PubMed] [Google Scholar]
- 9.Pelkey TJ, Frierson HF, Jr, Bruns DE. Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin Chem. 1996;42:1369–1381. [PubMed] [Google Scholar]
- 10.Zippelius A, Pantel K. RT-PCR–based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors: An overview. Ann N Y Acad Sci. 2000;906:110–123. doi: 10.1111/j.1749-6632.2000.tb06600.x. [DOI] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 12.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–149. [Google Scholar]
- 13.Carey RW, Taft PD, Bennett JM, et al. Carcinocythemia (carcinoma cell leukemia): An acute leukemia-like picture due to metastatic carcinoma cells. Am J Med. 1976;60:273–278. doi: 10.1016/0002-9343(76)90437-x. [DOI] [PubMed] [Google Scholar]
- 14.Engell HC. Cancer cells in the circulating blood: A clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir Scand Suppl. 1955;201:1–70. [PubMed] [Google Scholar]
- 15.Gallivan MV, Lokich JJ. Carcinocythemia (carcinoma cell leukemia): Report of two cases with English literature review. Cancer. 1984;53:1100–1102. doi: 10.1002/1097-0142(19840301)53:5<1100::aid-cncr2820530514>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Myerowitz RL, Edwards PA, Sartiano GP. Carcinocythemia (carcinoma cell leukemia) due to metastatic carcinoma of the breast: Report of a case. Cancer. 1977;40:3107–3111. doi: 10.1002/1097-0142(197712)40:6<3107::aid-cncr2820400653>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Salas N, Jimenez-Gordo AM, Gonzalez E, et al. Circulating cancer cells in peripheral blood: A case report. Acta Cytol. 2000;44:237–241. doi: 10.1159/000326367. [DOI] [PubMed] [Google Scholar]
- 18.Seronie-Vivien S, Mery E, Delord JP, et al. Carcinocythemia as the single extension of breast cancer: Report of a case and review of the literature. Ann Oncol. 2001;12:1019–1022. doi: 10.1023/a:1011184706281. [DOI] [PubMed] [Google Scholar]
- 19.Sile CC, Perry DJ, Nam L. Small cell carcinocythemia. Arch Pathol Lab Med. 1999;123:426–428. doi: 10.5858/1999-123-0426-SCC. [DOI] [PubMed] [Google Scholar]
- 20.Yam LT, Janckila AJ. Immunocytodiagnosis of carcinocythemia in disseminated breast cancer. Acta Cytol. 1987;31:68–72. [PubMed] [Google Scholar]
- 21.Racila E, Euhus D, Weiss AJ, et al. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terstappen LW, Rao C, Gross S, et al. Peripheral blood tumor cell load reflects the clinical activity of the disease in patients with carcinoma of the breast. Int J Oncol. 2000;17:573–578. doi: 10.3892/ijo.17.3.573. [DOI] [PubMed] [Google Scholar]
- 23.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 24.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 25.Nolé F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: Prognostic and therapeutic implications. Ann Oncol. 2008;19:891–897. doi: 10.1093/annonc/mdm558. [DOI] [PubMed] [Google Scholar]
- 26.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 27.Bauernhofer T, Zenahlik S, Hofmann G, et al. Association of disease progression and poor overall survival with detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer. Oncol Rep. 2005;13:179–184. [PubMed] [Google Scholar]
- 28.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 29.Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107:984–990. doi: 10.1002/ijc.11479. [DOI] [PubMed] [Google Scholar]
- 30.Beveridge R. Circulating tumor cells in the management of metastatic breast cancer patients. Commun Oncol. 2007;4:79–82. [Google Scholar]
- 31.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Cochran W. Sampling Techniques. ed 3. New York, NY: Wiley; 1977. [Google Scholar]
- 33.McCarthy WF, Guo N. The estimation of sensitivity and specificity of clustered binary data. Proceedings of the 31st SAS Users Group International Conference; March 26-29, 2006; San Francisco, CA. abstr 206. [Google Scholar]
- 34.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73:13–22. [Google Scholar]
- 35.Thiesse P, Ollivier L, Stefano-Louineau D, et al. Response rate accuracy in oncology trials: Reasons for interobserver variability—Groupe Francais d'Immunotherapie of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 1997;15:3507–3514. doi: 10.1200/JCO.1997.15.12.3507. [DOI] [PubMed] [Google Scholar]