Abstract
Purpose
Currently, treatment recommendations for small-cell urothelial cancer (SCUC) are based on anecdotal case reports and small retrospective series. We now report results from the first phase II clinical trial developed exclusively for SCUC, to our knowledge.
Patients and Methods
From 2001 to 2006, 30 patients with SCUC provided consent and were treated with alternating doublet chemotherapy. Patients with surgically resectable disease (≤ cT4aN0M0) received a total of four cycles of neoadjuvant chemotherapy, whereas those with unresectable disease (≥ cT4b, N+, or M+) received two cycles beyond maximal response.
Results
Eighteen patients with surgically resectable SCUC received neoadjuvant treatment with a median overall survival (OS) of 58 months; 13 of these patients remain alive and cancer free. For patients with cT2N0M0 SCUC, the 5-year OS rate is 80%; only one of four patients with cT3b-4aN0M0 remains alive (median OS, 37.8 months). For 12 patients with unresectable or metastatic SCUC, the median OS was 13.3 months. Chemotherapy was well tolerated, with transfusion, neutropenic fever, and infection remaining the most frequent grade 3 and 4 toxicities. There was only one postsurgical death. Brain metastases were strongly associated with more advanced-stage disease, developing in eight of 16 patients with either bulky tumors (≥ cT3b) or metastatic disease (P = .004).
Conclusion
These clinical trial results are consistent with previously reported retrospective data demonstrating long-term survival with four cycles of neoadjuvant chemotherapy for surgically resectable SCUC. Once metastases develop, the prognosis remains poor. The strong positive association between disease stage and brain metastases highlights a patient subset that may potentially benefit from prophylactic cranial irradiation.
INTRODUCTION
Historically, the prognosis for patients with small-cell urothelial cancer (SCUC) has remained uniformly poor. Given the lack of evidence to the contrary, a radical cystectomy has been considered the de facto standard for patients without evidence of metastatic disease.1 However, the lack of efficacy of this approach is readily apparent; even in recently published series, most patients die of SCUC within 2 years of cystectomy.2,3
Being an extremely rare malignancy, accounting for approximately 0.5% to 0.7% of urothelial tumors,4,5 there have been no prospective clinical trials exclusively for SCUC. On the basis of anecdotal case reports and small retrospective case series reported in the literature, management ranges from cystectomy alone,2,3,6,7 with or without adjuvant radiation therapy5 or adjuvant chemotherapy.2,3,6,8–11 Recent retrospective reviews suggest more survivors with initial chemotherapy followed by local consolidation with cystectomy6,12 or radiation.13,14
The use of preoperative chemotherapy follows from the frequent observation of rapid growth rates and typical upstaging on initial surgery, not uncommonly leading to aborted cystectomy. A retrospective review of our experience suggested the potential for improved survival with neoadjuvant chemotherapy.6 In addition, treatment with chemotherapy combinations typically used for neuroendocrine tumors of other organ sites was more likely to eradicate the small-cell component compared with typical bladder cancer regimens.6 Consequently, we designed a phase II clinical trial to study the effects of front-line systemic chemotherapy for SCUC both in the preoperative and metastatic settings.
PATIENTS AND METHODS
Patient Eligibility
A phase II trial of alternating doublet chemotherapy with ifosfamide plus doxorubicin (IA) and etoposide plus cisplatin (EP) for patients with SCUC was approved by the The University of Texas M. D. Anderson Cancer Center Institutional Review Board. From 2001 to 2006, 30 patients prospectively consented and were enrolled. All patients had proof of SCUC. In cases of mixed histology, the small-cell component was felt to be the clinically relevant component. Patients with only focal small-cell component (for example, small-cell changes in only a few clusters of cells) were not considered eligible. Patients with SCUC limited to the bladder did not require radiographic evidence of measurable disease; patients with metastases had at least one measurable site. No previous systemic chemotherapy for metastatic disease was allowed.
Patients had adequate physiologic reserve with a Zubrod performance status of ≤ 2; a performance status of 3 was allowed if it was of recent onset and entirely a result of SCUC and not a comorbid medical condition. The trial required adequate bone marrow reserves, with an absolute neutrophil count ≥ 1,800/μL, a platelet count more than 150,000/μL, and adequate liver function with transaminase ≤ 2× the upper limit of normal and conjugated bilirubin ≤ 1 mg/dl or a total bilirubin of less than 2 mg/dl. Adequate renal function requires a creatinine clearance ≥ 45 mL/min. Patients with uncontrolled brain metastases were not eligible. An abnormal ECG or a history of heart disease required an ejection fraction of ≥ 50%.
Chemotherapy
Doublet chemotherapy consisted of IA alternating with EP. The choice of IA was based on our own observations of activity in patients with relapsing SCUC and in transitional cell tumors. Both agents have good single-agent activity in small-cell tumors of the lung, with the similar alkylating agent cyclophosphamide having a long history of activity and use as treatment for small-cell lung cancer. EP is considered by many to be one of the front-line therapies for small-cell lung cancer.
Ifosfamide 2,000 mg/m2 was infused over 3 hours daily on days 1 through 4. Doxorubicin 25 mg/m2 was infused daily on days 1 through 3. Mesna 300 mg/m2 was infused at hours 0, 4, 8, and 12 daily on days 1 through 4, starting before the ifosfamide. Patients were aggressively hydrated, frequently using a sodium acetate infusion. Patients experiencing neurologic toxicity received methylene blue; ifosfamide was reduced in subsequent cycles if neurologic symptoms recurred despite scheduled methylene blue. Renal function was monitored daily, with treatment held on development of renal insufficiency. Most patients with hydronephrosis had a nephrostomy tube placed before chemotherapy, even with normal serum creatinine, to help maintain renal function. This inpatient regimen was repeated at 3-week intervals with growth factor support.
Etoposide 80 mg/m2 was infused over 2 hours daily on days 1 through 5; cisplatin 20 mg/m2 was infused in 1 L of normal saline with mannitol 20 g daily on days 1 through 5. Patients received additional intravenous hydration at the discretion of their physician. Growth factor support, although not absolutely required, could be administered as deemed necessary. This 3-week regimen was administered on an inpatient or outpatient basis.
Clinical Evaluation and Treatment Duration
Staging within 1 month of study entry consisted of an abdominal-pelvic computed tomography scan and a chest x-ray or chest computed tomography scan. Suspicious lymph nodes not considered diagnostic of metastases were recommended for biopsy. Patients without metastases received a cystoscopy and examination under anesthesia. Patients were treated on the neoadjuvant arm for surgically resectable disease (≤ cT4aN0M0) and on the metastatic arm for stage IV cancer (cT4b, N+, or M+).
For neoadjuvant patients, repeat radiographic imaging was not required unless symptoms suggested progressive cancer. After four cycles of alternating chemotherapy (two cycles of IA and two cycles of EP), patients proceeded with cystectomy and node dissection. For patients with metastatic disease, repeat imaging was obtained at 6-week intervals. In the absence of toxicity, patients were treated with two cycles beyond maximal response and observed with repeat imaging every 3 months.
Response Criteria
For neoadjuvant chemotherapy, a major response was defined as ≤ pT1N0 at cystectomy. For metastases, a complete response required disappearance of all radiographic and clinical evidence of SCUC persisting for at least 8 weeks. A partial response required a reduction in the sum of bidimensional cross products of indicator lesions by at least 50% persisting for at least 8 weeks with no new lesions and no worsening of cancer-related symptoms. Progressive disease was defined as a 25% increase in the sum of the bidimensional cross products of indicator lesions or the appearance of new lesions.
Statistical Considerations
Because patients with surgically resectable SCUC (≤ cT4aN0M0) have potentially curable disease and those with metastases are not curable, the end points for these two subgroups were different. The goals for surgically resectable disease were to estimate the rate of downstaging to ≤ pT1N0M0 after chemotherapy and to estimate the proportion of patients who were disease free at 2 years as a potential surrogate for cure. In the subset with metastases, the overall response rate was expected to be nearly 100%. More important measures of anticancer activity were the clinical complete response rate, the proportion of patients disease free at 2 years, and overall survival (OS). Association between presence of brain metastases and disease stage (II, III, or IV) was assessed by a generalized Fisher's exact test.15 OS within each disease stage subgroup was estimated using the Kaplan-Meier method.16 Cox proportional hazards regression was used to examine the effects of brain metastases and disease stage on OS, with goodness of fit assessed using the Grambsch-Therneau test.17
RESULTS
Patient Characteristics
Baseline characteristics for all patients are listed in Table 1. In all patients, SCUC was the predominant component, including those patients with mixed SCUC plus either a transitional cell component or carcinoma in situ. There was no difference in outcomes regardless of the extent of small-cell component present, although the small numbers of patients limit our ability to definitively state the prognostic significance of a pure versus mixed SCUC. Eighteen patients were eligible for neoadjuvant chemotherapy. Twelve patients had evidence of metastatic disease at diagnosis; five patients had only lymph node metastases.
Table 1.
Patient Demographics and Clinical Characteristics at Diagnosis
| Demographic/Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 66.2 | |
| Range | 43.1-81.0 | |
| Sex | ||
| Male | 28 | 93 |
| Female | 2 | 7 |
| Performance status | ||
| 0 | 18 | 60 |
| 1 | 12 | 40 |
| Site of primary tumor | ||
| Bladder | 29 | 97 |
| Renal pelvis/ureter | 1 | 3 |
| Histology at diagnosis | ||
| Small cell only | 17 | 57 |
| Small cell + CIS | 3 | 10 |
| Small cell ≥ TCC | 10 | 33 |
| Neoadjuvant | 18 | 60 |
| cT2 | 14 | 78 |
| cT3b | 4 | 22 |
| Metastatic disease at diagnosis | 12 | 40 |
| Node only | 5 | |
| Nodes + other | 4 | |
| Liver | 5 | |
| Lung | 2 | |
| Pelvic mass/peritoneal implant | 2 | |
| Bone | 1 | |
Abbreviations: CIS, carcinoma in situ; TCC, transitional cell carcinoma.
Response and Survival
Neoadjuvant chemotherapy.
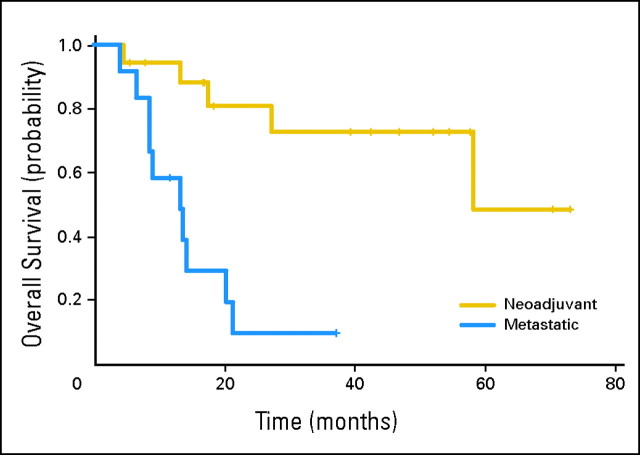
Overall, pathologic downstaging to ≤ pT1N0M0 occurred in 14 (78%) of 18 patients treated on the neoadjuvant arm (Table 2). The Kaplan-Meier estimate of median OS in patients with surgically resectable disease (≤ cT4aN0M0) was 58 months (95% CI, 58 months to not achieved; Fig 1). The lower confidence bound equals the median as a result of the small sample size, with five deaths in 18 patients. In patients with muscle-invasive bladder cancer, the median OS has not been reached; 92% and 80% of patients were alive at 2 and 5 years, respectively. In these cT2 patients, there were only two deaths; one occurred in a patient with an intraoperative diverticular abscess requiring emergency sigmoid colon resection, whereas the only other death after 2 years was in a patient who developed acute myelogenous leukemia. The median OS time for patients with cT3B SCUC was 37.8 months. The only death after 2 years in this cohort was in a patient who developed brain metastases. Currently, 13 neoadjuvant patients (72%) remain alive and cancer free.
Table 2.
Clinical Versus Pathologic Staging in Patients Treated With Neoadjuvant Chemotherapy
| Clinical Stage | Pathologic Stage (No. of patients) |
|||||
|---|---|---|---|---|---|---|
| pT0 | pTis | pT1 | pT2 | pT3/4a | pT4b, N+, or M+ | |
| cT2 | 7 | 6 | — | 1* | — | — |
| cT3/4a | 1 | — | — | 1† | — | 2‡ |
Transitional cell carcinoma.
Small-cell carcinoma only.
One small-cell carcinoma only and one small-cell and transitional cell carcinoma.
Fig 1.
Kaplan-Meier overall survival (OS). On the neoadjuvant arm, the median OS time was 58 months (95% CI, 58 months to not achieved [NA]). On the metastatic arm, the median OS time was 13.3 months (95% CI, 8.5 months to NA).
Metastatic disease.
For patients with metastases, the overall response rate was nearly 100%; eight of 12 patients had a clinical complete response, whereas three patients experienced a partial response. One patient was inassessable for response because the patient discontinued therapy after one cycle and refused further follow-up. Despite initial evidence of clinical activity, most patients with metastases experienced relapse, with a median OS time of 13.3 months (95% CI, 8.5 months to not achieved; Fig 1).
Surgical consolidation of metastases.
Three patients with lymph node metastases experienced a clinical complete response and proceeded with surgical consolidation. At resection, all three patients had negative lymph nodes with no residual SCUC. Residual transitional cell carcinoma was present in two patients. Two patients died of progressive SCUC (one in bone and one in brain). One patient remains alive and free of SCUC after more than 28 months but has required treatment for a second primary transitional cell carcinoma in the ureter.
Intracranial metastases.
Brain metastases were significantly associated with the presence of bulky, higher stage tumors (≥ T3b, N+, or M+), occurring in eight (50%) of 16 patients, compared with no brain metastases in the14 patients with ≤ cT2N0M0 disease (Table 3, P = .004). Seven of these eight patients had no progressive tumor elsewhere, with nearly all patients dying within 2 months of this diagnosis. One patient received prophylactic cranial irradiation (PCI) after preliminary review defined this high-risk cohort and has not experienced relapse to date.
Table 3.
Association Between Disease Stage and Brain Metastases
| Brain Metastasis | Disease Stage (No. of patients) |
||
|---|---|---|---|
| II | III | IV | |
| No | 14 | 2 | 6 |
| Yes | 0 | 2 | 6 |
NOTE. Generalized Fisher exact test: P = .004.
Chemotherapy-Related Toxicity
Patients received a total of 137 cycles of chemotherapy. Three patients experienced grade 4 chemotherapy-related toxicity, including low neutrophil count, catheter-related infection, and a pulmonary embolus (Table 4). The most frequent grade 3 chemotherapy-related toxicities were transfusions (37%) and neutropenic (23%) and non-neutropenic fevers (17%). Two patients were unable to complete chemotherapy secondary to toxicity, one as a result of fatigue and the second as a result of grade 3 renal insufficiency. Six patients required dose reduction (one patient in cycles 1 and 2, two patients in cycles 3 and 4, and thre patients in cycles 5 and 6).
Table 4.
Chemotherapy-Related Toxicity in All Patients
| Chemotherapy-Related Toxicity | No. of Patients (N = 30) | % |
|---|---|---|
| Grade 4 | ||
| Neutrophil count | 1 | 3 |
| Catheter-related infection | 1 | 3 |
| Pulmonary embolus | 1 | 3 |
| Grade 3 | ||
| Transfusion of packed RBC | 11 | 37 |
| Neutropenic fever | 7 | 23 |
| Non-neutropenic infection | 5 | 17 |
| Nausea/vomiting | 3 | 12 |
| Platelet transfusion | 2 | 7 |
| Fatigue | 1 | 3 |
| Hyperglycemia | 1 | 3 |
| Renal insufficiency | 1 | 3 |
NOTE. Two patients were unable to complete chemotherapy as a result of toxicity; one patient had fatigue, and the other patient had acute renal insufficiency.
Surgical Toxicity
The median hospital stay for patients undergoing surgery was 9 days (range, 5 to 15 days). One postsurgical death occurred in a patient diagnosed with an intraoperative diverticular abscess requiring resection of the sigmoid colon (Table 5). In four patients, hospital discharge was delayed beyond 10 days (two patients for ileus, one patient for an abdominal abscess, and one patient for a peritoneal fluid drain).
Table 5.
Surgery-Related Toxicity in the 18 Neoadjuvant Patients
| Postsurgical Toxicity | No. of Patients | % |
|---|---|---|
| Hospital stay, days | ||
| Median | 9 | |
| Range | 5-17 | |
| Postsurgical death* | 1 | 6 |
| Delayed discharge (> 10 days) | 4 | 22 |
| Ileus | 2 | 11 |
| Abdominal abscess | 1 | 6 |
| Peritoneal fluid drain | 1 | 6 |
| Rhabdomyolysis | 1 | 6 |
| Intraoperative hemorrhage | 1 | 6 |
| None | 12 | 67 |
A diverticular abscess was found intraoperatively, requiring emergency sigmoid colon resection.
DISCUSSION
There are no standard treatment recommendations for SCUC. Current strategies, which encompass surgery, radiation, chemotherapy, or any combination of two of these strategies, have been necessarily based on anecdotal reports and small retrospective series. Long-term survivors have been described with all of these approaches. However, the small patient numbers represented by these studies raise the potential for selective reporting bias. Thus, the need for prospective data collected in a systematic fashion becomes exceedingly important, especially for physicians who face the dilemma in providing treatment for patients with rare malignancies.
The benefits of incorporating neoadjuvant chemotherapy can be multifold. Whereas it may take time to schedule an operation or for patients in this age group to complete preoperative clearance, systemic chemotherapy can be initiated quickly, providing timely control of this rapidly growing chemotherapy-sensitive tumor. SCUC can frequently be downstaged, resulting in a surgery that is more likely to achieve negative margins.6 The pathologic stage after preoperative chemotherapy may also provide valuable prognostic information, with one retrospective series suggesting no disease-related deaths in patients downstaged to ≤ pT2N0M0.6
Two retrospective series suggest an improvement in patient outcomes with neoadjuvant therapy. Walther12 observed that five of seven patients were alive and cancer free at 36 months, most as a result of preoperative chemotherapy. A larger series observed a 5-year disease-specific survival rate of approximately 78% in 21 patients treated by preoperative chemotherapy, compared with a 5-year disease-specific survival rate of 36% in 25 patients treated with initial cystectomy, regardless of whether they received adjuvant chemotherapy.6 Although many different chemotherapy combinations were reported, there was a suggestion that neuroendocrine regimens, such as EP or IA, were more likely to eradicate the small-cell component compared with regimens more typically used in transitional cell tumors.6
Although it is impossible to definitively state that preoperative chemotherapy is the optimal strategy without a randomized trial, this clinical trial substantiates the concept that long-term disease control can occur with neoadjuvant chemotherapy. Pathologic downstaging was quite frequent, with an improved survival in those downstaged to ≤ pT2N0M0. Most of the deaths from recurrent SCUC occurred within 2 years of therapy.
The biggest impact on survival seemed to be in patients with muscle-invasive bladder cancer. However, patients with cT3b tumors did not fare as well. Although four cycles of preoperative chemotherapy may be adequate in the setting of muscle-invasive tumors, it is possible that this is simply not enough chemotherapy when faced with a larger tumor of the bladder. Supporting this finding was the presence of small cell in the pathologic specimen in three of four patients with cT3b cancer. In comparison, none of the patients with muscle-invasive tumor (cT2) had small cell remaining at cystectomy. However, one might also argue that more aggressive tumor biology could account for this finding.
Interestingly, a recent retrospective report from the Mayo Clinic advocates surgery alone in patients with surgically resectable SCUC, especially for those with muscle-invasive tumors (pT2N0M0).7 Although it is not clear how many patients were upstaged at surgery, of 12 patients with pT2N0M0 SCUC, the 3-year OS rate was 63.6%; however, half of these patients experienced relapse. In the present study, the 2- and 5-year OS rates were 87% and 77%, respectively, for patients downstaged to ≤ pT2N0M0, with only one of 16 downstaged patients experiencing relapse with SCUC. The Mayo Clinic results are also in marked contrast to another retrospective study that suggests an approximately 25% 3-year survival rate in 30 patients with organ-confined disease (≤ pT2N0M0).3 This latter report also indicates no apparent benefit with multimodality therapy with chemotherapy or radiation, although it is not clear which patients received combined-modality treatment.
The University of Southern California Norris Cancer Center experience also suggests poor outcomes with initial surgery, with 76% of patients having lymph node involvement at the time of cystectomy.2 Like other studies, they report a few more long-term survivors with adjuvant chemotherapy.8–10 Similarly, poor survival outcomes and a high risk of pathologic upstaging in seven of 11 patients with cT2N0M0 tumors treated with initial surgery (regardless of receiving adjuvant chemotherapy) at the M. D. Anderson Cancer Center6 raise a valid concern about initial surgery inadequately treating a substantial portion of these patients. Without a large randomized trial, it is impossible to confirm the benefits of neoadjuvant chemotherapy; however, the improved clinical outcomes and high likelihood of pathologic downstaging seen in the current clinical trial support the utility of neoadjuvant chemotherapy.
In keeping with the small-cell lung cancer experience, some investigators have reported long-term survivors when using radiation therapy rather than cystectomy as the local consolidative measure of choice.13,14 However, many patients treated with radiation had recurrent tumor requiring additional treatment and/or cystectomy.13 A reason for this high relapse rate may be the mixed histology seen with SCUC arising in combination with transitional cell carcinoma2,4,6,7,18 or with carcinoma in situ,2 which is a contraindication to radiation therapy. In this clinical trial, carcinoma in situ was observed in three patients preoperatively and seven patients postoperatively. Although these findings are based on small patient cohort, we have favored surgery as the consolidative measure of choice. However, when a patient cannot undergo a cystectomy or refuses surgery, consolidation with radiation therapy is a reasonable option.
Results from systemic chemotherapy in metastatic SCUC are similar to the experience with small-cell tumors of other organ sites. Although initial response rates remain quite high, the vast majority of patients eventually relapse, with few long-term survivors. Surgical consolidation was performed in three of five patients with initial nodal metastases; however, only one of these patients has not relapsed with SCUC.
There have been no prior studies on the incidence of brain metastases in SCUC. In this clinical trial, brain metastases developed in 50% of patients with bulky tumors; none were observed in organ-confined SCUC. Given the potential impact of PCI on small-cell tumors of the lung, we have now begun offering PCI to patients with bulky tumors at initial diagnosis.
In the absence of a large comparative trial, we cannot draw definitive conclusions regarding the best multimodality strategy for the treatment of SCUC. Currently available literature suggests that surgery alone is not optimal and that integrating chemotherapy can improve long-term disease control. The results of this clinical trial suggest that neoadjuvant chemotherapy followed by localized therapy to the pelvis may be the optimal strategy. Whether radiation or cystectomy provides optimal local consolidation is not currently known. Given the presence of carcinoma in situ that has been observed in these patients and the risk for recurrent cancer when the bladder remains intact, we have advocated for cystectomy as the localized therapy of choice.
Once metastatic disease occurs, the prognosis remains poor. The association between brain metastases and higher stage SCUC may help define the cohort of patients for whom the benefits of PCI outweigh the risks. Multi-institutional trials are necessary to help better define the impact of combined-modality therapy in SCUC and to confirm the findings of this small, single-institution trial.
Footnotes
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Ashish M. Kamat, TetraLogic Pharmaceuticals, Halozyme Therapeutics, Indevus Pharmaceuticals Research Funding: Ashish M. Kamat, Abbott Molecular, Bioniche Life Sciences, Bayer Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Randall E. Millikan
Administrative support: Dallas L. Williams
Provision of study materials or patients: Arlene O. Siefker-Radtke, Ashish M. Kamat, H. Barton Grossman, Dallas L. Williams, Colin P. Dinney, Randall E. Millikan
Collection and assembly of data: Arlene O. Siefker-Radtke, Dallas L. Williams, Randall E. Millikan
Data analysis and interpretation: Arlene O. Siefker-Radtke, Wei Qiao, Peter F. Thall
Manuscript writing: Arlene O. Siefker-Radtke
Final approval of manuscript: Arlene O. Siefker-Radtke, Ashish M. Kamat, H. Barton Grossman, Dallas L. Williams, Wei Qiao, Peter F. Thall, Colin P. Dinney, Randall E. Millikan
REFERENCES
- 1.Sternberg CN, Swanson DA. Non-transitional cell bladder cancer. In: Raghavan D, Scher HI, Leiber SA, et al., editors. Principles and Practice of Genitourinary Oncology. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 322–323. [Google Scholar]
- 2.Quek ML, Nichols PW, Yamzon J, et al. Radical cystectomy for primary neuroendocrine tumors of the bladder: The University of Southern California experience. J Urol. 2005;174:93–96. doi: 10.1097/01.ju.0000162085.20043.1f. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Pan CX, Yang XJ, et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer. 2004;101:957–962. doi: 10.1002/cncr.20456. [DOI] [PubMed] [Google Scholar]
- 4.Blomjous CE, Vos W, De Voogt HJ, et al. Small cell carcinoma of the urinary bladder: A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989;64:1347–1357. doi: 10.1002/1097-0142(19890915)64:6<1347::aid-cncr2820640629>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Holmäng S, Borghede G, Johansson SL, et al. Primary small cell carcinoma of the bladder: A report of 25 cases. J Urol. 1995;153:1820–1822. [PubMed] [Google Scholar]
- 6.Siefker-Radtke AO, Dinney CP, Abrahams NA, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: A retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481–484. doi: 10.1097/01.ju.0000132413.85866.fc. [DOI] [PubMed] [Google Scholar]
- 7.Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder: The Mayo Clinic experience. Cancer. 2005;103:1172–1178. doi: 10.1002/cncr.20903. [DOI] [PubMed] [Google Scholar]
- 8.Grignon DJ, Ro JY, Ayala AG, et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 22 cases. Cancer. 1992;69:527–536. doi: 10.1002/1097-0142(19920115)69:2<527::aid-cncr2820690241>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Abbas F, Civantos F, Benedetto P, et al. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–630. doi: 10.1016/S0090-4295(99)80290-8. [DOI] [PubMed] [Google Scholar]
- 10.Oesterling JE, Brendler CB, Burgers JK, et al. Advanced small cell carcinoma of the bladder: Successful treatment with combined radical cystoprostatectomy and adjuvant methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1990;65:1928–1936. doi: 10.1002/1097-0142(19900501)65:9<1928::aid-cncr2820650910>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Sved P, Gomez P, Manoharan M, et al. Small cell carcinoma of the bladder. BJU Int. 2004;94:12–17. doi: 10.1111/j.1464-410X.2003.04893.x. [DOI] [PubMed] [Google Scholar]
- 12.Walther PJ. Adjuvant/neo-adjuvant etoposide/cisplatin and cystectomy for management of invasive small cell carcinoma of the bladder. J Urol. 2002;167:285. [Google Scholar]
- 13.Lohrisch C, Murray N, Pickles T, et al. Small cell carcinoma of the bladder: Long term outcome with integrated chemoradiation. Cancer. 1999;86:2346–2352. doi: 10.1002/(sici)1097-0142(19991201)86:11<2346::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Bastús R, Caballero JM, Gonzâalez G, et al. Small cell carcinoma of the urinary bladder treated with chemotherapy and radiotherapy: Results in five cases. Eur Urol. 1999;35:323–326. doi: 10.1159/000019870. [DOI] [PubMed] [Google Scholar]
- 15.Snedecor GW, Cochran WG. Statistical Methods. ed 7. Ames, IA: Iowa State University Press; 1980. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Therneau TM, Grambsch PM. Modeling Survival Data. New York, NY: Springer; 2000. [Google Scholar]
- 18.Abrahams NA, Moran C, Reyes AO, et al. Small cell carcinoma of the bladder: A contemporary clinicopathological study of 51 cases. Histopathology. 2005;46:57–63. doi: 10.1111/j.1365-2559.2004.01980.x. [DOI] [PubMed] [Google Scholar]