Abstract
Background:
Gradual weight reduction has been shown to be associated with improvements in liver enzymes. However, some evidence demonstrated that liver enzymes may transiently increase immediately after a diet-induced weight loss.
Objectives:
This study was designed to assess the effects of a hypocaloric, almond-enriched diet (AED) compared with a hypocaloric nut-free diet (NFD) on liver function tests in the context of a three-month weight reduction program in overweight/obese women.
Patients and Methods:
This randomized controlled clinical trial was registered at Iranian Registry of Clinical Trials with ID number of IRCT2013062313751N1. Overweight and obese Iranian women [n = 108; age = 42.7 y, body mass index = 29.6 kg/m2] were randomly assigned to consume an AED or NFD. The carefully planned hypocaloric diets were identical for both groups except for the AED group who consumed 50 grams of almonds daily for three months. Anthropometric measurements and laboratory measurements including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and γ-glutamyltransferase (GGT) were assessed before and immediately after the intervention.
Results:
Of 108 participants, 50 women in AED group and 50 women in NFD group completed the protocol of the study (response rate: 92.6 %). The AED led to a median weight loss of 3.79 kg (interquartile range: 4.4 kg). Significant decreases within AED and NFD were observed in ALT (-16.6 ± 16.3 and -11.7 ± 16.8, P < 0.001, respectively). Similar significant decreases were observed in AST (-13.6 ± 15.7 and -7.7 ± 16.1; P < 0.001, respectively). The decrease in GGT was also significant in both groups (-11.4 ± 21.6 and -6.2 ± 19.8; P < 0.001 respectively). ALT, AST and GGT decreased significantly in the AED group compared to the NFD group (P < 0.001).
Conclusions:
AED improved liver enzymes in obese women. However, mild, transient increases in ALT and AST values can be observed immediately after an NFD in women.
Keywords: Nuts, Liver Function Tests, Caloric Restriction, Overweight, Obesity, Women
1. Background
Obesity and overweight are common public health concerns worldwide (1) and carry serious health consequences, including liver disease such as nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) (2). Therefore, there is a demand for an effective and safe balanced hypocaloric diet that can produce and maintain weight loss and improve comorbidities (3). Overweight and in particular obesity are major risk factors in the pathogenesis of NAFLD; it is expected that weight loss should be therapeutic (3). Serum aminotransferases such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), commonly referred as “liver enzymes”, are by far the most accessible and cheapest paraclinical examination as an indicator of liver diseases (4). Gradual weight reduction has been shown to be associated with improvements in liver enzymes (5-7). However, some evidence demonstrated that liver enzymes may transiently increase immediately after a diet-induced weight loss (8, 9). In addition, alkaline phosphatase (ALP), γ- glutamyltransferase (GGT), bilirubin, total protein and albumin are commonly measured sensitive markers of liver function (10).
A few randomized trials have demonstrated the effect of low-calorie diet (LCD) enriched with almonds (11), LCD enriched with pistachios or pretzels (12) and LCD enriched with peanuts (13) in a weight-loss program. This literature review aimed to report contradictory findings about the effects of nut consumption on the weight loss. A recent study designed to examine the effects of an almond-enriched, hypocaloric diet in the treatment of obesity showed clinically significant and comparable weight loss (14). However, the effect of nuts as a component of healthful, hypocaloric diet on liver enzymes of obese people is unclear. Nuts are highly nutritious foods rich in unsaturated fatty acids, fiber, vitamins, minerals and some bioactive substances, such as phenolic antioxidants and phytosterols (15) and due to these wholesome benefits, individuals living with liver disease are usually advised to include nuts in their diet (16). In spite of the nut benefits, many obese individuals trying to lose weight may consciously avoid consuming nuts because of their high fat and energy density (14).
So far, the effect of almond-enriched, hypocaloric diet on liver enzymes remains unclear. Therefore, the current trial was undertaken to determine the effects of a hypocaloric, almond-enriched diet (AED) compared with a hypocaloric nut-free diet (NFD) on liver function tests in a three-month weight reduction program in overweight/obese women.
2. Objectives
This study was designed to assess the effects of a hypocaloric, almond-enriched diet (AED) compared with a hypocaloric nut-free diet (NFD) on liver function tests in a three-month weight reduction program in overweight/obese women.
3. Patients and Methods
3.1. Study Design and Participants
The current study was a randomized controlled trial conducted between March and September 2013 in Shiraz, Iran. Participants were recruited through public advertisements. Overweight/obese volunteer women were asked to visit Valiasr charity clinic, Shiraz, Iran. The project used a convenience sample of 152 women volunteered to participate in this study. Finally, 108 women fulfilling the inclusion criteria were selected.
A sample of 80 based on BMI was calculated to detect the predicted difference between the means of two groups with a power level of 80% and the significance level set as 0.05 if the standard deviation (SD) was 12.7 and 12.8 (11). The calculated required sample size was 40 in each group. A sample size of 108 subjects was selected to account for the assumed attrition rate (54 in each group).
Scope and methodology were explained and a written informed consent was obtained from each woman before the enrolment. A total of 108 women had inclusion criteria. Participants were eligible for enrollment if they were premenopausal women, aged 20 to 55 years, had a body mass index (BMI; in kg/m2) ≥ 25 and had light physical activity (doing household tasks, riding in a car and light activity while siting). The exclusion criteria in both groups were having a chronic diseases (e.g., cancer, renal failure, cardiovascular disease, diabetes, liver and lung failure), uncontrolled hypertension (defined as a blood pressure ≥ 180/100 mmHg), taking lipid-lowering medications or vitamin/mineral supplements, an inflammatory condition (e.g., lupus), working night shifts, pregnancy or lactation, smoking, alcohol consumption or any known allergy or sensitivity to nuts. Participants on weight control diets or any specific diets at the time and using medications known to affect body weight or a weight-loss of ≥ 5 kg in the preceding 6 months were not included. In addition, participants who did not comply with their diet, did not do their recommended walking or did not intend to continue the study were excluded.
The study protocol was in accordance with the ethical standards of the University and approved by the ethics committee of human experimentation of Shiraz university of medical sciences. This trial was registered in Iranian Registry of Clinical Trials with ID number of IRCT2013062313751N1.
3.2. Treatment Groups
3.2.1. Common Protocol
Participants were assigned using the balanced block randomization method (block size of four) to follow either the AED or the NFD for three months as described below.
At the beginning of treatment, all participants were instructed to follow a balanced low calorie diet, which was nutritionally adequate except for energy providing 50% - 55% carbohydrate, 15% - 25% protein and ≤ 30% fat (17). Energy requirement was calculated by the Harris Benedict equation and then reduced to 1000 kcal/day. In the both groups, the same percentage of energy from macronutrients was used to design the diets (approximately 54% carbohydrates, 16% proteins and 30% fats). Participants in the both groups were counseled regarding their assigned therapeutic diet, healthy nutrition, self-monitoring and stimulus control separately and advised to maintain their usual activities and encouraged to walk with medium speed 30 minutes every day.
All participants were given a request to comply with their diets for 3 months. The diet compliance and lifestyle intervention were assessed every 15 days by phone contact. In addition, suggestions to enhance compliance were provided. Moreover, three 24-hour dietary recalls (two weekdays and one for weekend day) were collected from all participants at baseline, the end of each month and the end of study by a trained dietitian to assess participants’ compliance.
3.2.2. Almond-enriched, Low-calorie Diet
Fifty-four participants were assigned to receive AED. Participants were provided 50 grams raw almond (~ 25 grams raw almond in each snack) to consume daily throughout the study. These participants were instructed to abstain from alternative nut consumption. Supplies of prepackaged almonds (each package contains 50 grams of almond) were distributed at their group clinic meetings monthly. The primary behavioral targets were adherence to the total energy intake goal and consumption of 50 grams almonds/d.
3.2.3. Nut-free, Low-calorie Diet
Fifty-four participants were assigned to receive NFD. This group was instructed to abstain from the consumption of nuts (e.g., peanuts, peanut butter, cashews, macadamia nuts, walnuts and pistachios). Instead of almond, compensatory serving from meat and fat exchange lists (like sunflower oil and corn oil) were consumed by NFD group participants.
3.3. Outcomes
To assess the effects of an AED relative to an NFD, biochemical and anthropometric variables were collected at baseline and after 3 months of the end of study. The AED and NFD women attended separate treatment groups to promote adherence to the intervention.
3.3.1. Anthropometric Measurements
Body weight was measured on a Seca electronic calibrated scale to the nearest 0.1 kg using the standard protocols, while the participants were wearing light clothes and without shoes at baseline and the end of study. Height was measured to the nearest 0.1 cm according to the standard protocols with a stadiometer at baseline only. Weight (kg) was then divided by the square root of height (m2) for calculation of BMI. Waist and hip circumferences were measured using non-stretch tape without pressure to body surfaces and recorded to the nearest 0.1 cm. Waist circumference (WC) was measured midway between the last rib and the ileac crest. Waist to hip ratio (WHR) was calculated by dividing waist circumference to the hip circumference.
3.3.2. Laboratory Investigations
The participants of each group were recruited for a blood sample collection on separate days. A volume of 10 mL venous blood samples were taken after 12 hours of overnight fasting at baseline and after the intervention period. To eliminate day-to-day laboratory variances, all samples from a given individual were labeled by code and analyzed in the same batch. The details of plasma separation were described previously (18). Blood sample analysis included ALT, AST, GGT, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and fasting blood sugar (FBS). All lipid profiles and FBS were measured by a photometric method (Autoanalyzer BT 1500, Blotecnica Instruments, Italy). AST, ALT and GGT levels were determined based on enzyme-linked immunosorbent assay method by auto-analyzer. ALP activity was determined with ALP assay kit (Parsazmun, Tehran, Iran) using p-nitrophenyl phosphate as substrate and alkaline phosphatase provided in the kit as standard. Moreover, to eliminate the effect of freeze-thawing of samples that may lower enzyme activity values, ALT, AST, ALP and GGT testing was conducted on samples immediately transferred to the laboratory. Total protein, albumin and bilirubin blood tests were performed by routine laboratory methods.
3.4. Statistical Analyses
Analysis was performed using IBM SPSS statistical software (version 19, IBM Company, Armonk, NY, USA). Numerical variables were expressed as mean ± standard deviation (SD). Kolmogorov-Smirnov test was used to test the normality of variable distribution. The variables with non-normal distribution were transformed to natural logarithm (Ln) values. The between-group comparisons for baseline characteristics and their changes after three months of intervention were performed by independent sample t-test. Within-group comparisons of the measurements before and after the study in each group were performed with paired t-test. All reported P values were two tailed and those < 0.05 were considered statistically significant in all statistical tests.
4. Results
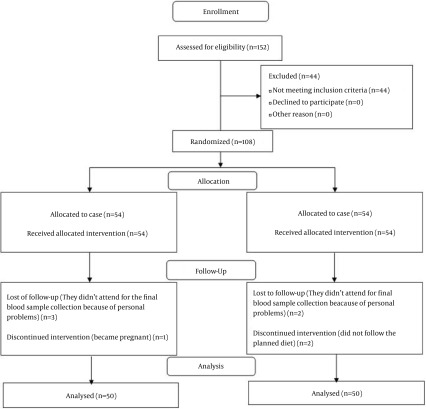
A total of 100 participants completed the trial. Eight participants were lost to follow-up (four women from each group). During the study, in the AED group, one woman due to pregnancy and three participants for personal reasons did not continue the study. Two women in the NFD group did not follow the planned diet and excluded. However, two women due to their personal reasons did not continue the study. Finally, 50 participants in each group completed the intervention. Equal numbers of lost to follow-up in the both groups made them comparable regarding this variable. No adverse effects were reported by participants. Patient screening, enrollment and retention by treatment groups are shown in Figure 1.
Figure 1. Flowchart of Design and Protocol of the Study.
Participants’ baseline characteristics are summarized in Table 1. Mean ± SD of age at randomization were 42.36 ± 4.30 and 42.94 ± 6.82 for AED and NFD groups, respectively; there was no significant difference between age of the groups (P = 0.686). At baseline, there were no significant differences in anthropometric factors, demographic characteristics and nutritional status between the AED and NFD groups. At baseline, there were no significant differences in all the measured variables between the two groups except for FBS.
Table 1. Baseline Characteristics of the Study Participantsa.
| Characteristics | Treatment Groups | P Valueb | |
|---|---|---|---|
| AED | NFD | ||
| Anthropometric characteristics | |||
| Height, m | 1.59 ± 0.47 | 1.60 ± 0.41 | .463 |
| Weight, Kg | 76.39 ± 2.69 | 75.58 ± 2.42 | .118 |
| BMI, Kg/m2 | 29.91 ± 1.20 | 29.37 ± 1.73 | .075 |
| WC, cm | 107.78 ± 6.28 | 106.20 ± 6.16 | .207 |
| HC, cm | 108.64 ± 4.98 | 107.37 ± 4.81 | .201 |
| WHR | 0.99 ± 0.05 | 0.98 ± 0.05 | .777 |
| Liver function tests | |||
| ALT, U/L | 50.08 ± 16.01 | 55.58 ± 15.72 | .874 |
| AST, U/L | 46.08 ± 12.25 | 45.03 ± 12.00 | .667 |
| GGT, U/L | 36.7 ± 22.5 | 38.1 ± 20.9 | .348 |
| ALP, U/L | 67.91 ± 17.3 | 69.33 ± 16.7 | .267 |
| Total bilirubin | 1.09 ± 0.38 | 1.07 ± 0.42 | .489 |
| Albumin | 3.70 ± 0.33 | 3.66 ± 0.32 | .256 |
| Total protein, g/dL | 7.48 ± 0.30 | 7.37 ± 0.29 | .369 |
| Laboratory characteristics | |||
| FBSc | 102.70 ± 4.72 | 100.32 ± 5.26 | .019 |
| TGc | 270.46 ± 64.86 | 262.31 ± 62.94 | .525 |
| TCc | 261.40 ± 34.83 | 254.56 ± 33.63 | .320 |
| LDL-Cc | 134.86 ± 9.22 | 131.86 ± 9.18 | .106 |
| HDL-Cc | 42.72 ± 4.23 | 41.26 ± 4.69 | .106 |
Abbreviations: AED, almond-enriched diet; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FBS, fasting blood sugar; GGT, γ-glutamyltransferase; HC, hip circumference; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; NFD, nut-free diet; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WHR; waist hip ratio.
aAll data are presented as mean ± SD and n = 50.
bP values were resulted from independent sample t-test.
cUnit of data is mg/dL.
AED was associated with greater reduction in BMI (4.9 vs. 1.7%, P = 0.018), WC (11.6 vs. 3.5%, P < 0.001) and WHR (11.1 vs. 1.0%, P = 0.018) in comparison with NFD.
Liver function tests of participants at baseline and the end of study are shown in Table 2. At baseline, there were no significant differences in all the measured liver function variables between the two groups. After intervention, ALT, AST, GGT, ALP, albumin and total protein, decreased significantly in the both groups. In the current study, total bilirubin levels were not altered by both hypocaloric diets.
Table 2. Comparison of Liver Function Tests Before and After the Study in the Both Groupsa.
| AED | P Valueb | NFD | P Valueb | |
|---|---|---|---|---|
| ALT, U/L | < 0.001 | < 0.001 | ||
| Before | 50.1 ± 16.0 | 55.6 ± 15.7 | ||
| After | 34.5 ± 6.7 | 47.9 ± 6.7 | ||
| AST, U/L | < 0.001 | < 0.001 | ||
| Before | 46.1 ± 14.3 | 45.0 ± 14.0 | ||
| After | 32.5 ± 16.9 | 39.2 ± 18.3 | ||
| GGT, U/L | < 0.001 | 0.009 | ||
| Before | 36.7 ± 22.5 | 38.1 ± 20.9 | ||
| After | 25.3 ± 20.6 | 31.9 ± 19.6 | ||
| ALP, U/L | < 0.001 | < 0.001 | ||
| Before | 67.9 ± 17.3 | 69.3 ± 16.7 | ||
| After | 51.6 ± 14.1 | 55.3 ± 15.3 | ||
| Total bilirubin, mg/dL | 0.074 | 0.09 | ||
| Before | 1.09 ± 0.38 | 1.07 ± 0.42 | ||
| After | 1.05 ± 0.62 | 1.05 ± 0.45 | ||
| Albumin, g/dL | 0.007 | 0.009 | ||
| Before | 3.70 ± 0.33 | 3.66 ± 0.32 | ||
| After | 3.49 ± 0.41 | 3.47 ± 0.38 | ||
| Total protein, g/dL | < 0.001 | < 0.001 | ||
| Before | 7.48 ± 0.30 | 7.37 ± 0.29 | ||
| After | 7.09 ± 0.39 | 7.03 ± 0.31 |
Abbreviations: AED, almond-enriched diet; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; NFD, nut-free diet.
aAll data are presented as mean ± SD and n = 50.
bP values were resulted from paired t-test.
Table 3 provides the effects of two hypocaloric diet on liver function tests. In the AED group, greater significant reduction in ALT, AST and GGT were found than the NFD group after 3 months (P < 0.001). However, ALP, total protein, albumin and bilirubin decreased in both groups over time and insignificant differences were found between the two groups after 3 months.
Table 3. Comparison of Changes in Liver Function Tests Between the Both Groups (U/L)a.
| AED | NFD | P Valueb | |
|---|---|---|---|
| ALT, (U/L) | -16.6 ± 16.3 | -11.7 ± 16.8 | < 0.001 |
| AST, (U/L) | -13.6 ± 15.7 | -7.7 ± 16.1 | < 0.001 |
| GGT, (U/L) | -11.4 ± 21.6 | -6.2 ± 19.8 | < 0.001 |
| ALP, (U/L) | -16.3 ± 15.9 | -14.0 ± 16.6 | 0.074 |
| Total bilirubin | -0.05 ± 0.55 | -0.02 ± 0.51 | 0.063 |
| Albumin | -0.21 ± 0.38 | -0.19 ± 0.39 | 0.125 |
| Total protein | -0.39 ± 0.34 | -0.34 ± 0.30 | 0.092 |
Abbreviations: AED, almond-enriched diet; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; NFD, nut-free diet.
aAll data are presented as mean ± SD and n = 50.
bP values were resulted from independent sample t-test.
5. Discussion
This study aimed to determine the effects of hypocaloric AED compared to hypocaloric NFD on liver function tests. To date no reports exist in the literature about the effect of hypocaloric AED on liver function tests in the context of a three-month weight reduction program in overweight/obese women. This study showed that hypocaloric AED significantly decreased ALT, AST and GGT in overweight/obese women.
Although some researches showed the impact of weight loss on improving liver enzymes (6), some studies reported no such effects (8, 9, 19, 20). Therefore, findings regarding the effects of weight loss on liver enzymes are inconsistent. In the current study, significant declines in weight, BMI, WC, ALT, AST and GGT were observed in the AED group compared with the NFD group. However, no difference in ALP, total protein, albumin and total bilirubin was found between the study groups.
The present findings seem to be consistent with previous researches, which found that a hypocaloric, almond-enriched diet led to greater reduction in weight, BMI and WC (11, 21). This study results were in accordance with the findings of most previous investigations in this field, supporting that weight loss through lifestyle modification, medication or bariatric surgery is associated with a decrease in liver enzymes (6, 7, 22-26). In contrast, a study by Gasteyger et al. examined the effects of an 8-week dietary induced weight loss on liver enzymes in obese subjects. In women, liver enzymes increased significantly, although mildly; however, this increase was transient (3). A study by Muller et al. outside the context of weight reduction showed hepatoprotective activity of aqueous extract of shells of pecan nut against ethanol-induced liver damage (27).
It is strongly recommended to use a well-balanced diet with nutritious foods to manage body weight at all times. There is a qualified health claim stating that regular nut intakes are associated with many health benefits in adults. Besides the low risk of toxicity, nuts are highly nutritious, being a good source of protein, high in antioxidants and full of healthful, unsaturated fatty acids. One of the advisable factors for weight management program is increasing the frequency of nuts consumption. The results of our previous study support the notion that consumption of 50 g/d almonds as a component of hypocaloric diet can acutely beneficially lead to greater weight loss and better improvements in cardiovascular risk factors for three months (18). Mechanisms underlying the effects of nut consumption on weight loss are unclear, but they are likely related to altered resting energy expenditure, inefficient absorption of energy from nuts or increased satiety due to high content of fiber, protein, unsaturated fats, various phytonutrients and low glycemic index; all of them are dietary factors associated with satiety responses (28-31). Almonds contain 21 grams protein per 100 grams, a significant amount of macronutrient associated with increased satiation (32). Almonds are a significant source of fiber, a component with documented satiating properties (33) and the crunchy textural property of almonds may also promote satiety (32). During the AED period, intake of omega-3 fatty acids increased, which may be adversely related to liver function (34, 35). Almonds contain magnesium, vitamin E and selenium. The effects of magnesium (36, 37), vitamin E and selenium (38) supplementation on the liver function were shown in previous studies.
5.1. Strength and Limitations of Study
The present study had several strengths. In previous studies the effect of hypocaloric AED was shown on weight reduction, but the current study performed to assess the effect of hypocaloric AED on liver function tests in overweight/obese women. The high participation rate of individuals in our research (more than 90%) was another advantage of this study.
The current study had several limitations. The first limitation was that liver function was only assessed by measuring liver enzymes and computed tomography scans, magnetic resonance scanning and liver biopsies were not performed. Further studies, considering these measurements are needed. Second, physical activity was not assessed during the intervention. Therefore, changes in physical activity level could have influenced liver enzymes during the dietary-induced weight loss. Third, the duration of this study was short, so it is not clear whether changes in body weight and liver enzyme levels are sustained for long time. Fourth, participants were females; therefore, the findings may not be generalized to males. Finally, only participants who completed hypocaloric AED and NFD were included in the analysis, which may have introduced a potential selection bias.
5.2. Conclusions
In conclusion, results of this randomized clinical trial showed that the balanced hypocaloric AED in comparison to the balanced hypocaloric NFD may decrease ALT and AST. During a dietary-induced weight loss i.e. NFD, a modest decrease in ALT and AST can be observed. These results provide further support for the hypothesis that a low-calorie, nut-containing diet could have beneficial effects on liver enzymes in overweight/obese women. These findings could be used by dietitian to design a well-balanced hypocaloric diet.
Acknowledgments
The authors are thankful to all participants and the research assistants who participated in this project for their effort.
Footnotes
Authors’ Contribution:Study concept and design: Mousa Salehi, Zohreh Abazarfard; analysis and interpretation of data: Ghazaleh Eslamian; drafting of the manuscript: Ghazaleh Eslamian, Zohreh Abazarfard; critical revision of the manuscript for important intellectual content: Mousa Salehi, Sareh Keshavarzi; statistical analysis: Sareh Keshavarzi, Ghazaleh Eslamian, Zohreh Abazarfard; Administrative, technical and material support: Sareh Keshavarzi; study supervision: Mousa Salehi.
Funding/Support:The present manuscript was extracted from the thesis written by Zohreh Abazarfard and financially supported by Shiraz university of medical sciences with grant No. 92-6498.
References
- 1.Katagiri S, Nitta H, Nagasawa T, Izumi Y, Kanazawa M, Matsuo A, et al. High prevalence of periodontitis in non-elderly obese Japanese adults. Obes Res Clin Pract. 2010;4(4):e247–342. doi: 10.1016/j.orcp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330–7. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87(5):1141–7. doi: 10.1093/ajcn/87.5.1141. [DOI] [PubMed] [Google Scholar]
- 4.Koike T, Miyamoto M, Oshida Y. Alanine aminotransferase and gamma-glutamyltransferase as markers for elevated insulin resistance-associated metabolic abnormalities in obese Japanese men younger than 30 years of age. Obes Res Clin Pract. 2010;4(1):e1–e82. doi: 10.1016/j.orcp.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53(3):413–9. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang RT, Koretz RL, Yee HF. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115(7):554–9. doi: 10.1016/s0002-9343(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 7.Straznicky NE, Lambert EA, Grima MT, Eikelis N, Nestel PJ, Dawood T. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabet Obes Metabol. 2012;14(2):139–48. doi: 10.1111/j.1463-1326.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoy MK, Heshka S, Allison DB, Grasset E, Blank R, Abiri M, et al. Reduced risk of liver-function-test abnormalities and new gallstone formation with weight loss on 3350-kJ (800-kcal) formula diets. Am J Clin Nutr. 1994;60(2):249–54. doi: 10.1093/ajcn/60.2.249. [DOI] [PubMed] [Google Scholar]
- 9.Friis R, Vaziri ND, Akbarpour F, Afrasiabi A. Effect of rapid weight loss with supplemented fasting on liver tests. J Clin Gastroenterol. 1987;9(2):204–7. doi: 10.1097/00004836-198704000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Purkins L, Love ER, Eve MD, Wooldridge CL, Cowan C, Smart TS, et al. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br J Clin Pharmacol. 2004;57(2):199–208. doi: 10.1046/j.1365-2125.2003.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wien MA, Sabate JM, Ikle DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord. 2003;27(11):1365–72. doi: 10.1038/sj.ijo.0802411. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr. 2010;29(3):198–203. doi: 10.1080/07315724.2010.10719834. [DOI] [PubMed] [Google Scholar]
- 13.Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, Kris-Etherton PM. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79(2):204–12. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 14.Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr. 2012;96(2):249–54. doi: 10.3945/ajcn.112.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001–11. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han JM, Jo AN, Lee SM, Bae HS, Jun DW, Cho YK, et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2014;29(6):1265–72. doi: 10.1111/jgh.12520. [DOI] [PubMed] [Google Scholar]
- 17.Mahan LK, Escott-Stump S, Raymond JL, Krause MV. Nutrition in weight management. 13th ed. St. Louis,: Elsevier/Saunders; 2012. [Google Scholar]
- 18.Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: A randomized controlled clinical trial. J Res Med Sci. 2014;19(5):457–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Kreitzman SN, Pedersen M, Budell W, Nichols D, Krissman P, Clements M. Safety and effectiveness of weight reduction using a very-low-calorie formulated food. Arch Intern Med. 1984;144(4):747–50. [PubMed] [Google Scholar]
- 20.Luyckx FH, Desaive C, Thiry A, Dewe W, Scheen AJ, Gielen JE, et al. Liver abnormalities in severely obese subjects: Effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22(3):222–6. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 21.McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord. 2001;25(10):1503–11. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- 22.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S39–43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 23.Phillips ML, Boase S, Wahlroos S, Dugar M, Kow L, Stahl J, et al. Associates of change in liver fat content in the morbidly obese after laparoscopic gastric banding surgery. Diabetes Obes Metab. 2008;10(8):661–7. doi: 10.1111/j.1463-1326.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 24.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100(5):1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 25.Dixon JB, Bhathal PS, O'Brien PE. Weight loss and non-alcoholic fatty liver disease: Falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16(10):1278–86. doi: 10.1381/096089206778663805. [DOI] [PubMed] [Google Scholar]
- 26.de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R. Effect of two different hypocaloric diets in transaminases and insulin resistance in nonalcoholic fatty liver disease and obese patients. Nutr Hosp. 2010;25(5):730–5. [PubMed] [Google Scholar]
- 27.Muller LG, Pase CS, Reckziegel P, Barcelos RC, Boufleur N, Prado AC, et al. Hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Exp Toxicol Pathol. 2013;65(1-2):165–71. doi: 10.1016/j.etp.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Jaceldo-Siegl K, Sabate J, Rajaram S, Fraser GE. Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. Br J Nutr. 2004;92(3):533–40. doi: 10.1079/bjn20041223. [DOI] [PubMed] [Google Scholar]
- 29.Sabate J. Nut consumption and body weight. Am J Clin Nutr. 2003;78(3 Suppl):647S–50S. doi: 10.1093/ajcn/78.3.647S. [DOI] [PubMed] [Google Scholar]
- 30.Jackson CL, Hu FB. Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutr. 2014;100 Suppl 1:408S–11S. doi: 10.3945/ajcn.113.071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadivel V, Kunyanga CN, Biesalski HK. Health benefits of nut consumption with special reference to body weight control. Nutrition. 2012;28(11-12):1089–97. doi: 10.1016/j.nut.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98(3):651–6. doi: 10.1017/S0007114507734608. [DOI] [PubMed] [Google Scholar]
- 33.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21(3):411–8. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Oosthuizen W, van Graan A, Kruger A, Vorster HH. Polyunsaturated fatty acid intake is adversely related to liver function in HIV-infected subjects: the THUSA study. Am J Clin Nutr. 2006;83(5):1193–8. doi: 10.1093/ajcn/83.5.1193. [DOI] [PubMed] [Google Scholar]
- 35.Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231–48. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- 36.Poikolainen K, Alho H. Magnesium treatment in alcoholics: A randomized clinical trial. Subst Abuse Treat Prev Policy. 2008;3:1. doi: 10.1186/1747-597X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gullestad L, Dolva LO, Soyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16(5):986–90. doi: 10.1111/j.1530-0277.1992.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 38.Sodhi S, Sharma A, Brar RS. A protective effect of vitamin E and selenium in ameliorating the immunotoxicity of malathion in chicks. Vet Res Commun. 2006;30(8):935–42. doi: 10.1007/s11259-006-2503-5. [DOI] [PubMed] [Google Scholar]