Abstract
The release of cellular factors by means of extracellular vesicles (EVs) is conserved in archaea, bacteria and eukaryotes. EVs are released by growing bacteria as part of their interaction with their environment and, for pathogenic bacteria, constitute an important component of their interactions with the host. While EVs released by gram-negative bacteria have been extensively studied, the vesicles released by thick cell wall microorganisms like mycobacteria were recognized only recently and are less well understood. Nonetheless, studies of mycobacterial EVs have already suggested roles in pathogenesis, opening exciting new avenues of research aimed at understanding their biogenesis and potential use in antitubercular strategies. In this minireview we discuss the discovery of mycobacterial vesicles, the current understanding of their nature, content, regulation, and possible functions, as well as their potential therapeutic applications.
Keywords: mycobacterium, vesicles, siderophores, iron, lipoproteins, vaccines
Introduction
Mycobacterium tuberculosis (Mtb), the bacterium that causes tuberculosis, has co-evolved with humans, who are its only known reservoir. As a result of this co-evolution Mtb engages in multiple interactions with the host via cell surface components and by secretion of a variety of molecules into the extracellular environment. Recently, another means by which pathogenic mycobacteria interact with the host was discovered: it was shown that mycobacteria can concentrate and pack various types of macromolecules into membrane vesicles, which are released into the environment. Although the study of mycobacterial microvesicles is in its early stages and much still needs to be learned about their biogenesis and functions, it is clear that microvesicle formation is a regulated process by which Mtb can manipulate host responses and promote bacterial adaptation. This review summarizes what is currently known about mycobacterial microvesicles and discusses possible ways to utilize microvesicles to modulate pathogen-host interactions in ways that lead to positive outcomes for the host.
The discovery of mycobacterial extracellular vesicles
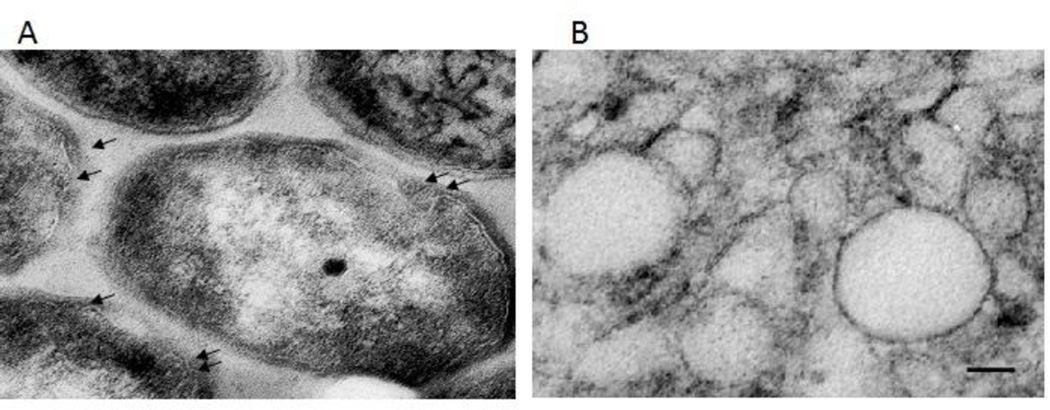
Mycobacterial extracellular vesicles (MEVs) were first discovered embedded in the extracellular matrix of Mycobacterium ulcerans biofilms (Marsollier et al., 2007). Subsequently, they were recovered – by differential sedimentation – from the culture supernatants of several mycobacterium species including M. tuberculosis, M. bovis BCG, M. kansasii, M. avium, M. smegmatis and M. phlei (Prados-Rosales et al., 2011). The observation that fast and slow growers, virulent and non-virulent mycobacteria all produce vesicles suggests that this is a conserved phenomenon in the mycobacterium genus. MEVs were observed by transmission electronmicrographic analysis (TEM) in the pellets recovered from culture supernatants and were also associated with only live, metabolically active bacteria (Prados-Rosales et al., 2011) (Figure 1). Furthermore, M. tuberculosis (Mtb) and BCG associated microvesicles were also observed during intracellular infection of macrophages in culture and in mice, confirming that MEVs production happens not only in vitro but also in vivo (Prados-Rosales et al., 2011).
Fig 1. Microvesicles produced by Mycobacterium tuberculosis.
Transmission electron micrograph showing vesicles budding from the surface of the bacteria (arrows) (A) and isolated membrane vesicles (Scale Bar: 50 nm) (B).
Copyright © 2014, American Society for Microbiology. Journal of Bacteriology. 196(6). 1250–1256. doi:10.1128/JB.01090-13
The biogenesis of MEVs
MEVs vary in size from 60 to 300 nm in diameter, which is similar to the size range of EVs produced by gram-negative bacteria. MEVs are composed of lipids and lipoproteins present in the mycobacterial plasma membrane, indicating their plasma membrane origin (Prados-Rosales et al., 2011). However, while in gram-negative bacteria the vesicles emerge from the outer membrane and are released without any obstruction, mycobacteria have a complex cell wall that surrounds the plasma membrane. This cell wall is composed of peptidoglycan covalently attached to arabinogalactan, which in turn is decorated with mycolic acids and intercalating free lipids, forming the mycomembrane (Daffe, 2015). Furthermore, a capsule composed of polysaccharides, proteins and lipids surrounds the cell wall. Thus, one of the most important unanswered questions is how MEVs traverse the cell wall. This question is also relevant to other thick cell wall microorganisms, such as gram-positive bacteria and fungi. The presence of cell wall remodeling enzymes in fungal EVs and peptidoglycan degrading enzymes in Staphylococcus aureus EVs suggests that remodeling of the cell wall may facilitate EV transit across the cell wall (Lee et al., 2009). This and other non-mutually exclusive hypotheses have been proposed to explain the mechanism of vesicle release in mycobacteria; however, there is as yet no definitive information and this remains an area of intense investigation.
The regulation of MEV production by iron limitation
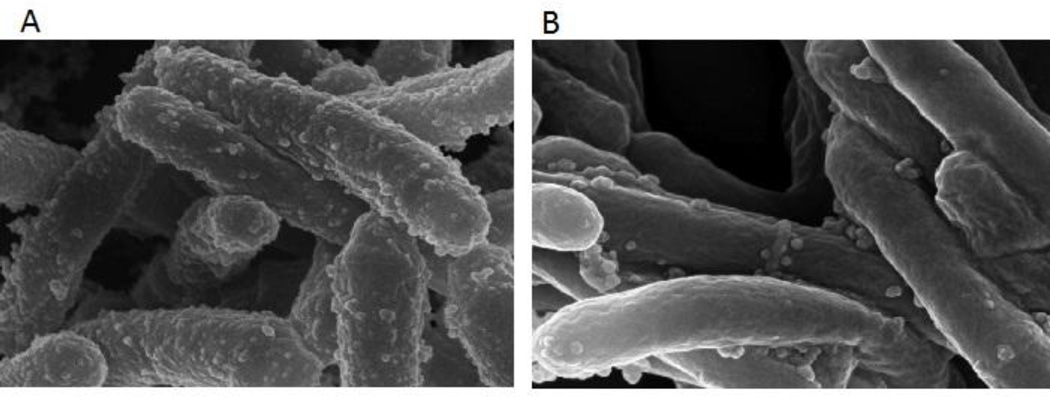
In response to iron limitation Mtb enhances EV production (Figure 2) (Prados-Rosales et al., 2014b). This behavior and the increased vesiculation observed in the Mtb strain lacking the vesiculogenesis and immune response regulator, VirR, indicates that MEV production is genetically regulated (Rath et al., 2013). VirR is a cytoplasmic protein that interacts with the plasma membrane and at least one lipoprotein that is included in MEVs. It has been suggested that VirR is part of a high-order protein complex that controls vesicle formation and cargo selection (Rath et al., 2013). However, the precise mechanism by which VirR influences vesiculogenesis remains to be elucidated. Similarly, how iron limitation signals to promote MEV generation and the molecular mechanisms involved in this response are still unknown. For instance, while virR expression is downregulated in response to iron deficiency (M. Rodriguez, unpublished), it is still not clear whether reduced virR expression is responsible for Mtb's hypervesiculation under iron limitation. Since iron restriction is a natural host response to infection (Weinberg, 1984) that frequently signals bacteria to produce virulence factors (Litwin & Calderwood, 1993), it is likely that MEVs released in response to iron limitation in vivo are involved in pathogenesis. Characterization of MEVs produced under iron restriction and other conditions that resemble the host environment will aid understanding the function of MEVs in Mtb interactions with the host (see below).
Fig 2. Microvesicle production is regulated by iron.
Scanning electron micrograph of Mtb cultured under iron limiting (A) and iron sufficient conditions (B). The number of spherical vesicles associated to the mycobacterial surface is higher in iron deficient cultures.
Copyright © 2014, American Society for Microbiology. Journal of Bacteriology. 196(6). 1250–1256. doi:10.1128/JB.01090-13
MEV content
In E. coli, 0.2%–0.5% of outer membrane (OM) and periplasmic proteins are packed in vesicles (Hoekstra et al., 1976, Kesty & Kuehn, 2004), suggesting that vesicle formation is an energy demanding process and thus is likely to serve an important cellular function. In addition to proteins, a large variety of other molecules, including phospholipids, nucleic acids, lipopolysacharide and periplasmic components, can be found encapsulated in outer membrane vesicles (OMVs) in gram-negative bacteria (Kuehn & Kesty, 2005). Because of their potential implications in host-pathogen interactions, vesicles produced by pathogenic bacteria have been most extensively studied and have been shown to deliver a variety of virulence factors like adhesins, toxins, and immunomodulatory effectors (Kuehn & Kesty, 2005). In addition, OMVs have been associated with inter-bacterial transfer of material that contributes to survival and genetic diversity, including antibiotic-resistance enzymes and chromosomal, plasmid and phage DNA (Ciofu et al., 2000, Kolling & Matthews, 1999). Analogous to gram-negative bacteria, gram-positive pathogens like S. aureus use EVs to deliver cytotoxic material to host cells (Gurung et al., 2011). Similarly, M. ulcerans EVs contain the main virulence factor produced by this mycobacterium, the cytotoxin mycolactone (Marsollier et al., 2007). Importantly, mycolactone enclosed in EVs is more potent than isolated toxin, supporting a role of EVs in pathogenicity of M. ulcerans (Marsollier et al., 2007).
While it is hypothesized that the quantity, stability and sub-cellular localization of a protein might influence its availability for inclusion as vesicle cargo, little is known regarding the determinants of EV cargo inclusion or exclusion. Global protein composition of EVs produced by Mtb, BCG and M. smegmatis (Msmg) cultured in a defined minimal medium, identified 48, 66, and 64 vesicular proteins, respectively (Prados-Rosales et al., 2011). BCG and Mtb EVs were similarly enriched in lipoproteins and proteins belonging to the functional categories of cell wall, membrane function, as well as intermediate metabolism and respiration. BCG EVs contained more proteins classified in the category of lipid metabolism than Mtb and Msmg EVs. The poor representation of lipoproteins in Msmg EVs, despite their similar abundance in the cell, suggests that different species regulate incorporation of EV cargo differently. The lipid content of EVs released by Mtb cultured under conditions of iron sufficiency or deficiency, analyzed by mass spectrometry, showed predominately polar lipids, phosphatidylinositol (PI), acylated phosphatidylinositol dimmannosides (PIM2), cardiolipin (CL) and phosphatidylethanolamine (PE) in both conditions, consistent with the membrane origin of the vesicles (Prados-Rosales et al., 2011). However, EVs from iron deficient Mtb were also enriched in acylated glicerides, and PE, while acyl trehalose, an important mycobacterial cell wall component, was more abundant in iron sufficient Mtb EVs. Lipoarabinomanan, an important immunologically active glycolipid released by bacilli replicating within macrophage phagosomes, was also found associated with pathogenic mycobacteria produced EVs. A strong difference between low and high iron EVs was detected in the levels of the lipidic siderophore mycobactin, which was enriched in EVs released by iron limited Mtb (Prados-Rosales et al., 2014b). The finding of mycobactin in Mtb EVs and the stimulation of EV production in response to iron limitation have important implications for pathogenesis as discussed in the next section. DNA was also detected in purified and intact MEVs (R. Prados-Rosales, unpublished) but the implications of this finding are not yet known.
MEVs and host-pathogen interactions
Protein and lipid analysis of MEVs has revealed enrichment of lipoproteins and immunologically active glycolipids known to be ligands of the pattern recognition Toll-like receptor 2 (TLR-2), suggesting that MEVs participate in immune stimulation. Consistent with this idea, macrophage exposure to MEVs in vitro and administration of isolated MEVs to mice induced a TLR-2 dependent pro-inflammatory response distinct and more intense than that elicited by MEVs from non-virulent Msmg. Notably, naive and BCG vaccinated mice challenged with Mtb aerosols showed acute inflammation and a higher lung bacillary load when they were also injected intratracheally with BCG MEVs, indicating an overall impairment in control of infection and suggesting a role of MEVs in pathogenesis (Prados-Rosales et al., 2014a). These studies demonstrated that one function of MEVs is to deliver factors that modify the response of host cells to infection in a way that benefits the pathogen.
Iron availability is a critical factor that affects all bacteria living within a host. Because basic cellular metabolic activities require iron, this metal is absolutely essential for growth. However, due to its poor solubility and potential toxicity under aerobic conditions, free iron is not available in the host. Successful pathogens must be able to obtain iron and to adapt their metabolic activity according to iron availability. To obtain iron, Mtb synthesizes and secretes siderophores named mycobactins, which are essential for virulence (De Voss et al., 2000, Reddy et al., 2013). Two forms of mycobactins are produced: carboxymycobactin, an amphiphilic molecule that is secreted into the medium, and mycobactin, a cell surface associated lipophilic molecule (Ratledge & Dover, 2000, Snow, 1970, Snow & White, 1969, Gobin et al., 1995). Carboxymycobactin effectively sequesters ferric iron from the environment and transfers it to mycobactin (Gobin & Horwitz, 1996) or brings it into the cell via the iron-regulated transporter IrtAB {Rodriguez, 2006 #1580 (Ryndak et al., 2010)}. The fate of iron bound by mycobactin and its overall contribution to iron uptake are unclear. Mature mycobactin is included in MEVs released by Mtb experiencing iron limitation, whereas mycobactin synthesis intermediates are abundant in iron sufficient Mtb MEVs (Prados-Rosales et al., 2014b). Also, MEVs produced during iron limitation can deliver iron and support proliferation of iron-deficient bacteria only if they contain mycobactin (Prados-Rosales et al., 2014b). Although the molecular mechanism of MEV-associated mycobactin-mediated iron delivery remains to be characterized, these studies indicate a role for MEVs in pathogenesis.
MEV-mediated iron capture may be critical for Mtb survival during infection, especially in the context of intense iron deprivation in the granuloma (Basaraba et al., 2008). Immune cells forming the granuloma release siderocalin, which binds carboxymycobactin (Holmes et al., 2005), thereby compromising its role in iron acquisition. However, it is possible that vesicular mycobactin is not accessible to siderocalin. If that is the case, by “disguising” mycobactin in the EVs, Mtb may be able to overcome the interference of siderocalin with its iron acquisition. In addition, iron homeostasis and the regulation of macrophages’ antimicrobial response are tightly interconnected (Cairo et al., 2011). It is not impossible that MEV-associated mycobactin interferes with macrophage iron homeostasis and thereby perturbs macrophage immune function. Support for this idea comes from the observation of exogenously added mycobactin-containing liposomes in association with phagosomal vacuoles in infected macrophages (Luo Minkui et al., 2005). The inclusion of mycobactin in MEVs may also be an example of collaborative Mtb interactions since mycobactin-contained in MEVs can benefit both the bacterium producing the MEVs and its neighbours.
MEV translational applications
Although MEV biogenesis is yet to be fully understood, the accumulation of knowledge, especially in gram-negative bacteria, has opened several avenues for therapeutic MEV applications. For example, vesicles derived from bacterial pathogens have been extensively used in the development of immunogenic vaccine candidates, and vesicles from gram-negative microorganisms have been recently developed into therapeutic vaccines (Acevedo et al., 2014). The vaccine potential of naturally produced MEVs isolated from BCG and Mtb was tested in a mouse model (Prados-Rosales et al., 2014a). Only immunization with Mtb-derived MEVs showed a comparable capacity to control bacterial replication in the lung similar to that of standard BCG vaccination. Analysis of immunogenicity showed a mixed antibody and cellular response directed to lipoproteins and bacterial cell surface components. Although mycobacterial MEVs may represent a promising alternative vaccine, there are still some practical aspects of their production that need to be tailored. For instance, inconsistent protection efficacy of three different preparations of Mtb MEVs produced under the same conditions was observed and composition analysis of multiple preparations showed variable protein cargo (R. Prados-Rosales, unpublished). These observations suggest that naturally produced EVs might not represent an ideal vaccine candidate. Furthermore, although the immune response to Mtb MEVs is directed against lipoproteins and bacterial cell surface components, the MEV-associated components that explain their protection efficacy remain to be elucidated. When this information is in hand, artificial Mtb microvesicles incorporating recombinantly expressed MEV-associated proteins and lipids could be developed and tested as an alternative vaccine.
Because MEVs represent a bacterial compartment with a unique protein composition, they may be useful as an alternative biomarker for tuberculosis. Recently, the human antibody response to naturally produced BCG and Mtb EVs was evaluated to look for novel biomarkers in a small cohort of patients, including smear-positive, smear-negative HIV uninfected pulmonary tuberculosis (TB), and BCG-vaccinated with and without latent TB (Ziegenbalg et al., 2013). A combination of three MEV-associated antigens was clearly recognized by sera from TB patients and not by the control group, encouraging further study of these structures as diagnostic tools. Similarly to the vaccine applications discussed above, MEVs’ natural variable composition may cause inconsistencies in the serology performance. In this scenario, artificially produced microvesicles with uniform content may represent an ideal source of experimental and therapeutic material.
Conclusions
Production of EVs in mycobacteria has now been clearly demonstrated. These vesicles originate from the plasma membrane and are associated with virulence factors and immunomodulators, indicating that they play a role in pathogenesis. MEVs from virulent mycobacteria elicit an immune response that can be protective. These findings suggest that the immunogenic potential of mycobacterial vesicles can be harnessed for vaccine development. Although the study of MEVs has intensified, the mechanisms of vesicle production and release and how these processes are regulated remain poorly understood. Also important to understand are the factors that contribute to cargo selection or exclusion and how environmental signals impact vesicle production and content. Future molecular and immunological studies will likely reveal novel roles of vesicles in mycobacterial physiology and pathogenesis and will stimulate the search of ways to exploit MEVs for antitubercular applications.
Acknowledgments
RP's work discussed here was supported by a grant from the Bill and Melinda Gates Foundation. GMR's work discussed here was supported by the National Institutes of Health grant AI044856. The authors thank Erika Shor for help in manuscript preparation.
Footnotes
Ethical Approval: All procedures with Mtb-infected animals conducted by the authors and mentioned in this review were approved by the Albert Einstein College of Medicine animal care and use committee.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Acevedo R, Fernandez S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, Perez JL. Bacterial outer membrane vesicles and vaccine applications. Frontiers in immunology. 2014;5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaraba RJ, Bielefeldt-Ohmann H, Eschelbach EK, Reisenhauer C, Tolnay AE, Taraba LC, Shanley CA, Smith EA, Bedwell CL, Chlipala EA, Orme IM. Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis (Edinb) 2008;88:69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends in immunology. 2011;32:241–247. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;45:9–13. doi: 10.1093/jac/45.1.9. [DOI] [PubMed] [Google Scholar]
- Daffe M. The cell envelope of tubercle bacilli. Tuberculosis (Edinb) 2015;95(Suppl 1):S155–S158. doi: 10.1016/j.tube.2015.02.024. [DOI] [PubMed] [Google Scholar]
- De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, BCE The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin J, Horwitz M. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell wall. J. Exp. Med. 1996;183:1527–1532. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin J, Moore CH, Reeve JR, Jr, Wong DK, Gibson BW, Horwitz MA. Iron acquisition by Mycobacterium tuberculosis: Isolation and characterization of a family of iron-binding exochelins. Proc. Natl. Acad. Sci. USA. 1995;92:5189–5193. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim SI, Lee JC. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One. 2011;6:e27958. doi: 10.1371/journal.pone.0027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, van der Laan JW, de Leij L, Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976;455:889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279:2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol. 1999;65:1843–1848. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Kesty NC. Bacterial outer mebrane vesicles and the host-pathogen interactions. Genes and Development. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- Litwin CM, Calderwood SB. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkui Luo, Fadeev Evgeny A, Groves JT. Mycobactin-mediated iron acquisition within macrophages. Nature Chemical Biology. 2005;1:149–153. doi: 10.1038/nchembio717. [DOI] [PubMed] [Google Scholar]
- Marsollier L, Brodin P, Jackson M, Kordulakova J, Tafelmeyer P, Carbonnelle E, Aubry J, Milon G, Legras P, Andre JP, Leroy C, Cottin J, Guillou ML, Reysset G, Cole ST. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 2007;3:e62. doi: 10.1371/journal.ppat.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Jr, Porcelli SA, Casadevall A. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121:1471–1483. doi: 10.1172/JCI44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Carreno LJ, Batista-Gonzalez A, Baena A, Venkataswamy MM, Xu J, Yu X, Wallstrom G, Magee DM, LaBaer J, Achkar JM, Jacobs WR, Jr, Chan J, Porcelli SA, Casadevall A. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. MBio. 2014a;5 doi: 10.1128/mBio.01921-14. e01921-01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R, Weinrick B, Piqué D, Jacobs WR, Jr, Casadevall A, Rodriguez GM. Role for Mycobacterium tuberculosis membrane vesicles in iron acquistion. J Bacteriol. 2014b;196:1250–1256. doi: 10.1128/JB.01090-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath P, Huang C, Wang T, Wang T, Li H, Prados-Rosales R, Elemento O, Casadevall A, Nathan CF. Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2013;110:E4790–E4797. doi: 10.1073/pnas.1320118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- Reddy PV, Puri RV, Chauhan P, Kar R, Rohilla A, Khera A, Tyagi AK. Disruption of Mycobactin Biosynthesis Leads to Attenuation of Mycobacterium tuberculosis for Growth and Virulence. J Infect Dis. 2013 doi: 10.1093/infdis/jit250. [DOI] [PubMed] [Google Scholar]
- Ryndak M, Wang S, I S, Rodriguez GM. The Mycobacterium tuberculosis high-affinity iron importer, IrtA, contains an FAD-binding domain. J. Bacteriology. 2010;192:861–869. doi: 10.1128/JB.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow GA. Mycobactins: iron chelating growth factors from mycobacteria. Bacteriol. Rev. 1970;34:99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow GA, White AJ. Chemical and biological properties of mycobactins isolated from various mycobacteria. Biochem. J. 1969;115:1031–1045. doi: 10.1042/bj1151031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Iron withholding: A defense against infection and neoplasia. Physiological reviews. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Ziegenbalg A, Prados-Rosales R, Jenny-Avital ER, Kim RS, Casadevall A, Achkar JM. Immunogenicity of mycobacterial vesicles in humans: identification of a new tuberculosis antibody biomarker. Tuberculosis (Edinb) 2013;93:448–455. doi: 10.1016/j.tube.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]