Abstract
BACKGROUND
The use of combination antiretroviral therapy (cART) has significantly decreased the morbidity and mortality associated with HIV infection. Lipid disorders, including lipodystrophy, hypertriglyceridemia, hypercholesterolemia, and dyslipidemia, remain the most commonly reported metabolic disorders among those treated with long-term cART. Mounting evidence suggests an association between drug abuse and poor glycemic control and diabetes complications. Substance related disorders (SRD) may increase the risk of metabolic syndrome.
MATERIALS AND METHODS
The aim of this retrospective cohort study was to examine the relationship between SRD, cART, and lipid-lowering agent use in an HIV infected population. A total of 276 subjects with HIV infection were included, 90 (33%) received lipid-lowering agents, and 31 (34%) had SRD. Patients received efavirenz or protease inhibitor-based cART for at least 6 months. Prescription information was retrieved from the medical records. The primary outcome was the use of lipid-lowering agents including statins, fibrates and fish oil. The impact of SRD and cART was assessed on the lipid-lowering agent use.
RESULTS
Smoking was prevalent among subjects with SRD (84% vs. 15%, p<0.001). Statins were the mainstay for the management of dyslipidemia (66%), followed by the fibrates (24%), omega-3 fatty acids (5%), nicotinic acid (3%) and the cholesterol absorption inhibitors (3%). Use of statins or fibrates was significantly higher among subjects without SRD than those with (40% vs. 23%, p=0.005). The type of cART, including efavirenz and protease inhibitors, appeared to have no significant impact on the use pattern of lipid-lowering agents. Lopinavir/r was mostly prescribed for subjects with SRD (25% vs. 8%, p=0.02).
CONCLUSIONS
Among HIV-infected patients, statins remain the mainstay for the management of dyslipidemia in routine clinical care, followed by fibrates. A significant high risk of metabolic disorders among patients with SRD is implicated by heavy tobacco use and prevalent lopinavir/r-based treatment. Significantly low rate of lipid-lowering agent use in this population underscores the importance of lipid disorder scrutiny and cART treatment optimization for HIV-infected patients with SRD.
Keywords: substance-related disorders, HIV, metabolic disorder, dyslipidemia, lipid-lowering therapy, statins, fibrates
Introduction
Although the use of combination antiretroviral therapy (cART) targeting the human immunodeficiency virus (HIV) has significantly decreased the morbidity and mortality associated with the HIV infection, issues such as lipodystrophy, hypertriglyceridemia, hypercholesterolemia and diabetes still plague those treated with protease inhibitors (PIs). While dyslipidemia has been associated with the use of non-nucleoside-reverse transcriptase inhibitors, PI-based regimens appear to have significantly higher prevalence, ranging from 28–80% (1–5). The mechanism of PI-induced dyslipidemia is not fully understood, but might involve PI binding to cytoplasmic retinoic acid-binding protein type 1 (CRABP-1) which is structurally similar to the catalytic region of HIV-1 protease (6). Notably, there is conflicting results about the risk of myocardial infarction association with the use of PIs (7–9).
Statins are considered as first line treatment for primary hypercholesterolemia. Fluvastatin, pitavastatin and pravastatin (except for pravastatin with darunavir/r) have the least potential for drug-drug interactions, according to the guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (10). Simvastatin and lovastatin are contraindicated with PIs due to an increased risk of myopathy and rhabdomyolysis as a result of drug interactions (10). Fibrates, such as gemfibrozil and fenofibrate, are also commonly used in hypercholesterolemia, hypertriglyceridemia and mixed dyslipidemia. No clinically relevant interaction has been noted between PIs and fibrates (11).
The global HIV epidemic continues to grow, and is associated with substance-related disorders (SRD) (12–16). This underscores the need to develop individualized treatment programs that provide clinical care for both HIV and SRD in an integrated setting (17–26). Although it is well appreciated that cART adherence is important to achieve sustained viral suppression, many clinicians often indicate that this goal is a challenge in patients with SRD due to poor adherence (27–31). In addition, patients with SRD are often perceived to have comorbidities that preclude successful antiretroviral adherence. Disease complexity factors such as co-infection with tuberculosis, malaria, hepatitis B and C, or behavioral health disorders and cancer may influence antiretroviral exposure (32–35). There is evidence that suggests those with a history of drug abuse are more likely to have poor glycemic control and diabetes complications (36, 37). SRD per se may increase the risk of metabolic syndrome (38). This retrospective analysis aims to examine the relationship between SRD, antiretroviral therapy and lipid lowering agent use.
Methods
Patient Population
This was a retrospective cohort study that examined 276 subjects from four HIV treatment and research centers including Bronx, New York; Rochester, New York; Miami, Florida and Cleveland, Ohio. Subjects were eligible for the study if they had a confirmed HIV-1 infection, were >18 years of age, and on either an efavirenz- or PI-based combination ART regimen with dual nucleoside analogs for >6 months. Data was obtained from a therapeutic drug monitoring registry maintained by the University at Buffalo. This was a secure, interactive website (www.tdm.buffalo.edu) that was developed by the pharmacology laboratory for centralized data entry. The data of interest was from a period spanning from May 2003 to May 2007. The data entry process included adherence verification for antiretrovirals and concurrent medications according to the prescribed dosing schedule. Information was extracted from this registry, including baseline demographics, presence or absence of active SRD, plasma HIV-1 RNA viral load, CD4 cell count, co-infection status (HCV or HBV), ART utilization, and concomitant medication profile.
Antiretroviral therapy, Lipid Lowering Agents and Substance-Related Disorders
Antiretroviral therapy was categorized into efavirenz (EFV), atazanavir (ATV), lopinavir/ritonavir (LPV/r), or other PI-based regimens. Lipid lowering agents were divided into the following classes: statins, fibrates, omega-3 fatty acids, nicotinic acids and cholesterol absorption inhibitors. A complete list of medication in each class is listed in appendix-1. The number of prescriptions a subject received toward each drug class was used to quantify medication utilization.
Subjects with active substance use were identified between 2003 and 2007 according to the National Institute on Drug Abuse (http://www.drugabuse.gov/) criteria (see (39) for additional information). Subjects were categorized as SRD positive (SRD+, n=130) or negative (SRD−, n=146) based on individual reporting on the use of the following substances within 30 days before their entry visit: opiates or benzodiazepines not prescribed by a physician, anabolic steroids, cigarettes, alcohol, cocaine, barbiturates, “club” drugs, phencyclidine, amphetamines, inhalants, marijuana, or treatment for opioid addition, such as with methadone, levo-alpha-acetylmethadol, naltrexone, naloxone or buprenorphine. SRD− patients might have a history of smoking, alcohol or marijuana use. The determination of SRD− was at the discretion of the clinician.
The difference in utilization of lipid lowering agents and ART was examined between SRD+ and SRD− groups. Additional analyses was performed to explore specific lipid lowering agent(s) used by SRD+ and SRD− subjects, and by those receiving PI-based regimens (ATV, LPV/r, other PI-based) or NNRTI-based regimens (EFV).
Statistical Analysis
Differences between patients with and without active SRD were compared by the χ2 and Fisher’s exact tests for categorical variables, while continuous variables were compared by the Student’s t-test. The Yates continuity correction was used for the 2x2 contingency tables. Statistical analysis was performed using Minitab, and p < 0.05 (two-sided) was considered significant.
Results
Data from 90 subjects, 33% of 276 total enrollment in the study, between 2003 and 2007 was retrospectively evaluated from 4 different HIV treatment and research centers for use of lipid-lowering agent prescriptions. Overall, 34% (n=31) of the subjects were SRD+. Approximately 25% of the subject population were female, and the majority of subjects were African Americans (32%), Caucasians (32%) and Hispanics (32%). A significant difference was found in smoking status between the SRD+ and SRD− groups (84% vs. 15%, respectively, p<0.001). The demographic characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of study subjects
| Characteristics | SRD+ n (%) | SRD− n (%) |
|---|---|---|
| No. of Subjects | 31 (34) | 59 (66) |
|
| ||
| Age (y) | 44 (8) | 48 (8) |
| Mean (SD) | ||
|
| ||
| BMI | 27.7 (5.1) | 22.5 (5.9) |
| Mean (SD) | ||
|
| ||
| Male | 24 (77) | 41 (70) |
|
| ||
| Ethnicity | ||
| African American | 13 (42) | 16 (27) |
| Caucasian | 11 (36) | 18 (31) |
| Hispanic | 6 (19) | 23 (39) |
| Others | 1 (3) | 2 (3) |
|
| ||
| Glucose (mg/dL) | 94 (43) | 118 (78) |
| Mean (SD) | ||
|
| ||
| Hepatitis C | 6 (19) | 12 (20) |
|
| ||
| CD4 count (cells/mm3) | 645 (343) | 554 (277) |
| Mean (SD) | ||
|
| ||
| HIV viral load (c/ml) | 3690 (13,181) | 4183 (15,645) |
| Mean (SD) | ||
|
| ||
| Substance-related disorders | ||
| Tobacco* | 26 (84) | 9 (15) |
| Alcohol | 12 (39) | 17 (29) |
| Cocaine | 2 (6) | |
| Methadone or buprenorphine | 2 (6) | |
| Marijuana | 4 (13) | 10 (17) |
|
| ||
| Protease inhibitor use | 21 (68) | 39 (66) |
p<0.001.
SRD: substance-related disorders. Age, BMI, glucose, CD4 count, and HIV viral load are expressed as mean (standard deviation, SD).
The difference in use of lipid-lowering agents in the SRD+ and SRD− groups is listed in Table 2. Overall, 23% (30/130) of subjects with SRD received statins or fibrates treatment, in comparison to 40% (58/146) without SRD (p=0.005). Statins and fibrates had comparable use within the SRD+ and SRD− groups (statins: 68% vs. 65%, p=0.87, fibrates: 21% vs. 26%, p=0.76). Omega-3 fatty acids, nicotinic acid and cholesterol absorption inhibitor were the least utilized agents and did not show a statistical difference between the two groups. Twenty one percent (21%) of subjects (19/90) used combination lipid-lowering agents.
Table 2.
Use of lipid-lowering agents in HIV-infected subjects with or without substance-related disorders –n (%)
| Prescriptions | SRD+ | SRD− | Total | p |
|---|---|---|---|---|
| Statins | 26 (68) | 48 (65) | 74 (66) | 0.87 |
| Fibrates | 8 (21) | 19 (26) | 27 (24) | 0.76 |
| Omega-3 fatty acids | 2 (5) | 4 (5) | 6 (5) | 1.00* |
| Nicotinic acids | 1 (3) | 1 (1) | 2 (2) | 1.00* |
| Cholesterol absorption inhibitor | 1 (3) | 2 (3) | 3 (3) | 1.00* |
| Total | 38 (34) | 74 (66) | 112 (100) |
Fisher’s exact test. SRD: substance-related disorders.
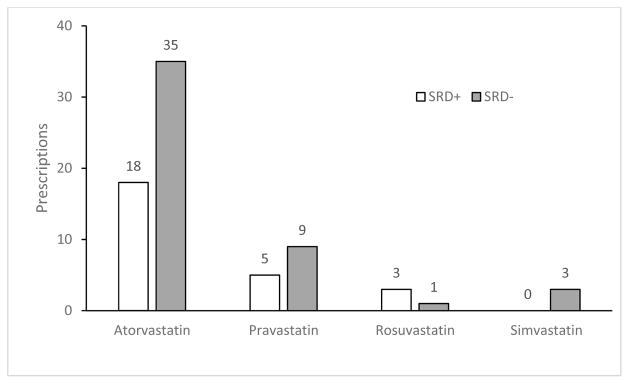
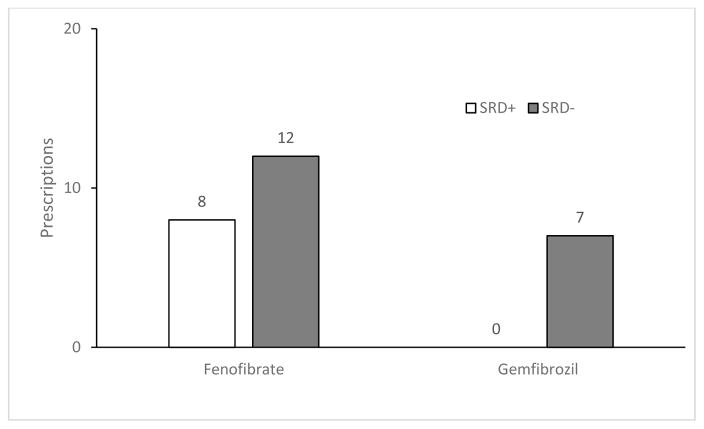
Statins were the mainstay of dyslipidemia treatment for HIV-infected patients (66%), followed by fibrates (24%), omega-3 fatty acids (5%), nicotinic acid (3%) and the cholesterol absorption inhibitor (3%). Atorvastatin was the most frequently prescribed statin in both the SRD+ and SRD− groups, followed by pravastatin (Figure 1); with fenofibrate being the most commonly prescribed fibrate (Figure 2). When looking at interactions between utilization patterns of antiretrovirals and lipid lowering agents within the SRD+ group, there was a comparable trend of statin use in the ATV, LPV/r, and EFV groups and comparable fibrate use in all four groups (Table 3). While 30% of patients (58/193) on PI-based antiretroviral regimen received statins or fibrates, 36% on EFV (26/73) required either statin or fibrate containing lipid lowering treatment (p=0.141). In the SRD− group, LPV/r group had the greatest number of statins users, followed by EFV, other-protease inhibitors, and ATV (Table 3). No difference was noted between PI-based and efavirenz-based regimens in the use of statins and fibrates (89% vs. 91%, respectively, p=1.00). Fibrates were mostly used in the ATV group, with comparable use between the remaining groups. Comparing the use of antiretrovirals between the SRD+ and SRD− groups, a significant difference was noted in the use of LPV/r between the SRD+ and SRD− groups (25 vs. 8%, p=0.02) (Table 4).
Figure 1.
Prescriptions of HMG CoA reductase inhibitors among HIV-infected subjects with and without SRD
SRD: substance-related disorders
Figure 2.
Prescriptions of fibrates among HIV-infected subjects with and without SRD
SRD: substance-related disorders
Table 3.
Use of lipid lowering agents per antiretroviral regimen in HIV-infected patients with and without substance-related disorders – n (%)
| Efavirenz | Atazanavir | Lopinavir/r | Other PIs | |||||
|---|---|---|---|---|---|---|---|---|
| SRD+ | SRD− | SRD+ | SRD− | SRD+ | SRD− | SRD+ | SRD− | |
| Statins | 7 (78) | 16 (70) | 8 (67) | 13 (50) | 7 (70) | 5 (83) | 5 (56) | 15 (71) |
| Fibrates derivatives | 1 (11) | 5 (22) | 3 (25) | 9 (35) | 3 (30) | 1 (17) | 2 (22) | 4 (19) |
| Omega-3 fatty acids | 1 (11) | 2 (8) | 1 (8) | 3 (12) | 1 (5) | |||
| Nicotinic acid | 1 (11) | 1 (5) | ||||||
| Cholesterol absorption inhibitor | 1 (3) | 1 (11) | ||||||
| Efavirenz | Protease inhibitors (including atazanavir, lopinavir/r and others) | |||||||
| SRD+ | SRD− | SRD+ | SRD− | |||||
| Statins or fibrates | 8 (89) | 21 (92) | 28 (90) | 47 (89) | ||||
PIs: protease inhibitors; SRD: substance-related disorders.
Table 4.
Use of cART regimens with or without SRD
| Prescription –n (%) | SRD+ | SRD− | Total | p |
|---|---|---|---|---|
| Atazanavir | 12 (30) | 26 (34) | 38 (33) | 0.80 |
| Efavirenz | 9 (23) | 23 (30) | 32 (28) | 0.50 |
| Lopinavir/r | 10 (25) | 6 (8) | 16 (14) | 0.02 |
| Other protease inhibitors | 9 (23) | 21 (28) | 30 (26) | 0.71 |
cART: combination antiretroviral therapy, SRD: substance-related disorders
The lipid parameters between SRD+ and SRD− groups were summarized in Table 5. While total cholesterol and LDL appeared to be largely under control with borderline high (~200 mg/dL) and near optimal (~110 mg/dL) levels, respectively, for subjects receiving lipid-lowering agents regardless their SRD status, the average triglyceride levels remained high for both groups (>250 mg/dL), particularly among those with SRD (329 mg/dL), suggesting suboptimal triglycerides management with lipid-lowering therapy.
Table 5.
Lipid parameters in HIV-infected patients with or without SRD
| SRD+ | SRD− | p | |
|---|---|---|---|
| Triglyceride (mg/dL) | 329 (178) | 257 (164) | 0.062 |
| Total cholesterol (mg/dL) | 211 (40) | 204 (44) | 0.399 |
| HDL (mg/dL) | 43 (10) | 47 (16) | 0.165 |
| LDL (mg/dL) | 112 (27) | 114 (38) | 0.930 |
Mean (SD), SRD: substance-related disorders, LDL, low-density lipoprotein cholesterol, HDL, high-density lipoprotein cholesterol
Discussion
This retrospective analysis of drug utilization data from a multicenter study explored the effect of SRD on lipid lowering medication use in an HIV-infected population. We identified a statistically higher rate of lipid-lowering medication use in those without SRD. The parent study was not powered to distinguish between the increased risk in those already on cART, cART-induced dyslipidemia, or effects of SRD on lipid metabolism. The 90 patients included in this analysis undergoing lipid-lowering treatment, accounted for a significant portion of 275 subjects (33%) enrolled in the parent study, indicating the clinical relevance of lipid management as well as cardiovascular complications in HIV-infected patient population.
Statins (66%) were the most commonly prescribed medication for dyslipidemia in this analysis, followed by fibrates (24%), omega-3 fatty acids (5%), cholesterol absorption inhibitors (3%) and nicotinic acid (2%), regardless of SRD status. These prescribing patterns were consistent with the clinical outcomes associated with statins and other lipid-lowering agents when used for treatment of dyslipidemia and primary prevention of cardiovascular disease. Atorvastatin was the most prescribed (72%), followed by pravastatin (19%), rosuvastatin (5%) and simvastatin (4%) suggesting efficacy remained the first priority in the lipid management. Although the CURVE study demonstrated atorvastatin 10, 20 and 40 mg produced greater reduction in low-density lipoprotein (LDL) cholesterol at equivalent doses of simvastatin, pravastatin, lovastatin and fluvastatin; cautions should be exercised with atorvastatin and most PI co-administration, except tipranavir, which is contraindicated with atorvastatin use. For co-administration, atorvastatin should be started with a low dose and gradually titrated to higher doses (10). Despite of a favorable drug interaction profile, pravastatin only accounted for 19% prescription likely due to its low efficacy. Pitavastatin has the least potential for drug-drug interactions with PIs, but was not available during the study period (10, 40, 41). Simvastatin is contraindicated with all PIs, but safe with concurrent use of efavirenz.
While the association between LPV/r and dyslipidemia was implicated in a number of studies, non-nucleoside reverese transcriptase inbhibitors, particularly nevirapine, have demonstrated a more lipid-friendly profile than LPV/r with an HDL increase and a remarkable reduction in total cholesterol/HDL ratio (42). In the present study, there was no significant difference in statin or fibrate use between the protease inhibitor and efavirenz groups (30% vs. 36%, p=0.141), consistent with previous findings from the 2NN study and ACTG A5142 study that efavirenz and ritonavir-enhanced PIs had similar and significant potential of inducing dyslipidemia (43, 44).
Substance-related disorders, particularly high cigarett smoking rate, are well estabolished cardiovascular risk factors commonly found in patients with HIV infection. In the present study, the SRD+ group had a remarkably higher percentage of subjects smoking compared to that in the SRD− group (84% vs. 15%, p<0.001). Evidence has revealed that cigarette smoking promoted systemic atherosclerosis by damaging the arterial endothelium, suggesting that smoking might contribute to the pathogenesis of dyslipidemia that requires lipid-lowering medication use (45). As well, a recent national survey has estimated a significantly higher rate of smoking in the SRD positive population than that in the general population (46). Current IDSA guideline recommends smoking cessation regardless of cardiovascular risk due to its multiple adverse effects on metabolism, drug interactions, bone health and cardovascular system (47). Signifantly more prescriptions of statins and fibrates among subjects without SRD noted in the present study (40% vs. 23%, p=0.005) confirmed the high prevalence of dyslipidemia among HIV-infected patient population and reinforced the importance of proper management using statins and fibrates regardless SRD status. High triglyceride levels were noted in this study, particularly among patients with SRD indicating an urgent need for triglyceride management with more potent lipid-lowering regimens. The analysis of interactions between lipid-lowering agent use, SRD status and cART indicated no significant relationship likely due to the small sample size; thus, in order to distinguish the contribution of these factors to the increased risk of dyslipidemia and metabolic disorders, a large clinical study would be warranted.
Additional analysis of cART use and SRD revealed that statistically more SRD+ subjects received LPV/r-based regimen compared to those in the SRD− group (25% vs. 8%, p=0.02). This finding might reflect more severe HIV disease and suboptimal treatment outcomes among HIV-infected subject with SRD since LPV/r is typically reserved as the second-line treatment. A number of previous studies demonstrated that LPV/r-based regimens have the highest risk of dyslipidemia and metabolic syndrome among cART (1, 4), representing a unique challenge for management of dyslipidemia among HIV-infected subjects with SRD undergoing LPV/r-based treatment.
Several limitations might interfere the validity of findings from this study, including a small sample size, the retrospective cross-sectional design, misclassification of medications, and selection bias. The data might not be representative of the HIV-infected population although they were collected from four different institutions. Misclassification of medications could occur, e.g., benzodiazepines might be used for anticonvulsant purposes and as sleeping aid, while methadone could be used for substance-related disorders and pain control (48). Lastly, selection bias was also possible since the subjects were enrolled from four different research centers and each center, and clinician might have different opinions for defining active SRD. Nevertheless, the study assessed the relationship between lipid lowering agents, SRD status, and cART and identified a high prevalence of dyslipidemia among HIV-infected patients requiring lipid lowering medications regardless their SRD status.
Conclusions
The study demonstrated a high prevalence of lipid lowering medication use among HIV-infected patients regardless their SRD status. Statins were the mainstay for the lipid management, followed by fibrates. Notably, the majority of SRD+ patients receiving lipid-lowering agents were smokers and on LPV/r-based regimens. This combination could substantially increase the risk of atherosclerosis and cardiovascular complications. The limitations of this study might have precluded statistical significance, however, future studies with a large sample size are warranted to investigate the impact of SRD and cART on development of dyslipidemia as well as lipid management.
Supplementary Material
Acknowledgments
The contributions of Raju Sadal, Julie Sarlo, Carol Greisberger, Leslie Thompson, Norma Storer and the clinical research staff at the Montefiore Medical Center AIDS Center, the University of Rochester HIV Program, the University of Miami AIDS Research Unit, and the University Hospitals Case Medical Center HIV Program and the patients at each of the participating HIV treatment centers is appreciated. The dedication of the research staff of the UB Pharmacotherapy Research Center, Core Analytical Laboratory is also appreciated. The study was supported by R01DA015024 from the National Institute on Drug Abuse. Dr. Zingman was supported in part by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI51519). Dr. Ma was supported in part by the Clinical Scientist Research Career Development Award from the National Institute of Mental Health (K08MH098794).
References
- 1.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998 May 7;12(7):F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999 Jun 19;353(9170):2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 3.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992 May;74(5):1045–52. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000 Jan 1;23(1):35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Saves M, Raffi F, Capeau J, Rozenbaum W, Ragnaud JM, Perronne C, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002 May 15;34(10):1396–405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 6.Calza L, Manfredi R, Chiodo F. Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. J Antimicrob Chemother. 2004 Jan;53(1):10–4. doi: 10.1093/jac/dkh013. [DOI] [PubMed] [Google Scholar]
- 7.Group DADS, Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 8.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002 Aug 15;30(5):471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rickerts V, Brodt H, Staszewski S, Stille W. Incidence of myocardial infarctions in HIV-infected patients between 1983 and 1998: the Frankfurt HIV-cohort study. Eur J Med Res. 2000 Aug 18;5(8):329–33. [PubMed] [Google Scholar]
- 10.Myerson M, Malvestutto C, Aberg JA. Management of lipid disorders in patients living with HIV. J Clin Pharmacol. 2015 Sep;55(9):957–74. doi: 10.1002/jcph.473. [DOI] [PubMed] [Google Scholar]
- 11.Calza L, Manfredi R, Chiodo F. Use of fibrates in the management of hyperlipidemia in HIV-infected patients receiving HAART. Infection. 2002 Jan;30(1):26–31. doi: 10.1007/s15010-001-2052-3. [DOI] [PubMed] [Google Scholar]
- 12.Beyrer C. HIV epidemiology update and transmission factors: risks and risk contexts--16th International AIDS Conference epidemiology plenary. Clin Infect Dis. 2007 Apr 1;44(7):981–7. doi: 10.1086/512371. [DOI] [PubMed] [Google Scholar]
- 13.Chawarski MC, Mazlan M, Schottenfeld RS. Heroin dependence and HIV infection in Malaysia. Drug Alcohol Depend. 2006 Apr;82(Suppl 1):S39–42. doi: 10.1016/s0376-8716(06)80007-4. [DOI] [PubMed] [Google Scholar]
- 14.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005 Oct 1;41(7):1027–34. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005 Feb;100(2):150–8. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 16.Surratt HL, Inciardi JA, Weaver JC, Falu VM. Emerging linkages between substance abuse and HIV infection in St. Croix, US Virgin Islands. AIDS Care. 2005 Jun;17(Suppl 1):S26–35. doi: 10.1080/09540120500121151. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006 Mar 1;42(5):716–21. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- 18.Brown LS, Jr, Kritz SA, Goldsmith RJ, Bini EJ, Rotrosen J, Baker S, et al. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2006 Jun;30(4):315–21. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce RD. Medical interventions for addictions in the primary care setting. Top HIV Med. 2010 Feb-Mar;18(1):8–12. [PubMed] [Google Scholar]
- 20.Bruce RD. Methadone as HIV prevention: high volume methadone sites to decrease HIV incidence rates in resource limited settings. Int J Drug Policy. 2010 Mar;21(2):122–4. doi: 10.1016/j.drugpo.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007 Mar;21(1):149–79. ix. doi: 10.1016/j.idc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy. 2007 Aug;18(4):326–8. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce RD, Kresina TF, McCance-Katz EF. Medication-assisted treatment and HIV/AIDS: aspects in treating HIV-infected drug users. AIDS. 2010 Jan 28;24(3):331–40. doi: 10.1097/QAD.0b013e32833407d3. [DOI] [PubMed] [Google Scholar]
- 24.Kresina TF, Eldred L, Bruce RD, Francis H. Integration of pharmacotherapy for opioid addiction into HIV primary care for HIV/hepatitis C virus-co–infected patients. AIDS. 2005 Oct;19(Suppl 3):S221–6. doi: 10.1097/01.aids.0000192093.46506.e5. [DOI] [PubMed] [Google Scholar]
- 25.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007 Aug;18(4):306–12. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner BJ, Laine C, Lin YT, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch Intern Med. 2005 Aug 8–22;165(15):1769–76. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- 27.MacMaster SA. Experiences with and perceptions of, barriers to substance abuse and HIV services among African American women who use crack cocaine. J Ethn Subst Abuse. 2005;4(1):53–75. doi: 10.1300/J233v04n01_05. [DOI] [PubMed] [Google Scholar]
- 28.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004 Mar;99(3):361–8. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004 Apr;29(2):117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 30.Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Health Psychol. 2004 Jul;23(4):363–70. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- 31.Ware NC, Wyatt MA, Tugenberg T. Adherence, stereotyping and unequal HIV treatment for active users of illegal drugs. Soc Sci Med. 2005 Aug;61(3):565–76. doi: 10.1016/j.socscimed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004 Jan 1;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004 May;16(4):471–84. doi: 10.1080/09540120410001683402. [DOI] [PubMed] [Google Scholar]
- 34.Palmer NB, Salcedo J, Miller AL, Winiarski M, Arno P. Psychiatric and social barriers to HIV medication adherence in a triply diagnosed methadone population. AIDS Patient Care STDS. 2003 Dec;17(12):635–44. doi: 10.1089/108729103771928690. [DOI] [PubMed] [Google Scholar]
- 35.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003 May;114(7):573–80. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 36.Ng RS, Darko DA, Hillson RM. Street drug use among young patients with Type 1 diabetes in the UK. Diabet Med. 2004 Mar;21(3):295–6. doi: 10.1046/j.1464-5491.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 37.Virmani A, Binienda Z, Ali S, Gaetani F. Links between nutrition, drug abuse, and the metabolic syndrome. Ann N Y Acad Sci. 2006 Aug;1074:303–14. doi: 10.1196/annals.1369.027. [DOI] [PubMed] [Google Scholar]
- 38.Virmani A, Binienda ZK, Ali SF, Gaetani F. Metabolic syndrome in drug abuse. Ann N Y Acad Sci. 2007 Dec;1122:50–68. doi: 10.1196/annals.1403.004. [DOI] [PubMed] [Google Scholar]
- 39.Slish J, Ma Q, Zingman BS, Reichman RC, Fischl MA, Gripshover B, et al. Assessing the impact of substance use and hepatitis coinfection on atazanavir and lopinavir trough concentrations in HIV-infected patients during therapeutic drug monitoring. Ther Drug Monit. 2007 Oct;29(5):560–5. doi: 10.1097/FTD.0b013e31806db8ae. [DOI] [PubMed] [Google Scholar]
- 40.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study) Am J Cardiol. 1998 Mar 1;81(5):582–7. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 41.Malvestutto CD, Ma Q, Morse GD, Underberg JA, Aberg JA. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. J Acquir Immune Defic Syndr. 2014 Dec 1;67(4):390–6. doi: 10.1097/QAI.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estrada V, Portilla J. Dyslipidemia related to antiretroviral therapy. AIDS Rev. 2011 Jan-Mar;13(1):49–56. [PubMed] [Google Scholar]
- 43.Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009 Jun 1;23(9):1109–18. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004 Oct;1(1):e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010 May 4;55(18):1988–95. doi: 10.1016/j.jacc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. [PubMed] [Google Scholar]
- 47.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jan;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 48.Neumann AM, Blondell RD, Jaanimagi U, Giambrone AK, Homish GG, Lozano JR, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis. 2013;32(1):68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.