Abstract
Inorganic polyphosphate (polyP) accumulates in acidocalcisomes, acidic calcium stores that have been found from bacteria to human cells. Proton pumps, such as the vacuolar proton pyrophosphatase (V-H+-PPase, or VP1), the vacuolar proton ATPase (V-H+-ATPase), or both, maintain their acidity. A vacuolar transporter chaperone complex (VTC) is involved in the synthesis and translocation of polyP to these organelles in several eukaryotes, such as yeast, trypanosomatids, Apicomplexan, and algae. Studies in trypanosomatids have revealed the role of polyP and acidocalcisomes in osmoregulation and calcium signaling.
Introduction
The organelles known as acidocalcisomes were first identified in trypanosomatids [1, 2], although they were known before with other names such as metachromatic [3] or volutin [4] granules. However, these granules, first described in bacteria, were though to lack a delimiting membrane [5] until two species of bacteria were found in which a surrounding membrane is present [6, 7]. When Wiame reported the presence of polyphosphate (polyP) in the yeast vacuole [8] they were also named as polyP granules or bodies. Recent work is mammalian cells have discovered the presence of polyP in organelles which were then recognized as acidocalcisomes, such as the human platelet dense granules [9], and mast cell and basophil granules [10]. These studies established that acidocalcisomes are membrane-bounded organelles that have been conserved from bacteria to human cells, and are defined by their common properties: the abundant presence of polyP, calcium and other cations, and their acidity.
It has been proposed that acidocalcisomes occur in all domains of life, including archaea, and may date back as far as to the last universal common ancestor or unancestor [11]. Some acidocalcisomes, like those of trypanosomes, share their biogenesis mechanism with organelles known as lysosome-related organelles (LROs) [10, 12]. Adaptor protein 3 (AP-3), a protein complex involved in transport of membrane proteins to LROs of mammalian cells [13] is involved in the biogenesis of acidocalcisomes of Leishmania major [14] and Trypanosoma brucei [15]. These results suggest that similar membrane-bounded polyP-containing acidic calcium stores could have appeared either autogenously or by convergent evolution.
Acidocalcisomes have been well studied in bacteria [6, 7], Dictyostelium discoideum [16], Chlamydomonas reinhardtii [17, 18], trypanosomatids [1, 2], Apicomplexan parasites [19, 20], human cells [9, 10] and eggs of different origin [21, 22]. PolyP was found to accumulate in other acidic organelles that could also be considered acidocalcisomes, such as the small granules found in the Gram-negative sporulating bacterium Acetonema longum [23], the acidocalcisome-like vacuoles of the arbuscular mycorrhizal fungus Rhizophagus sp. [24], or the spherites found in the midgut of the caterpillar Anticarsia gemmatalis [25].
Transporters and channels in acidocalcisomes
The acidity of acidocalcisomes is maintained by two proton pumps, a vacuolar H+-pyrophosphatase (V-H+-PPase), and a vacuolar H+-ATPase (V-H+-ATPase), or by both [26]. Few organelles are known to have these two proton pumps together in addition to acidocalcisomes [26–28], such as the plant vacuole [29], the malaria vacuole [30], the T. cruzi [31], C. reinhardtii [17], and Dictyostelium discoideum [16] contractile vacuoles, and the T. gondii plant-like vacuole [32]. A Ca2+-ATPase involved in Ca2+ uptake was characterized in acidocalcisomes of different species [33] and a recent proteomic analysis of acidocalcisome of Trypanosoma brucei revealed the presence of other transporters involved in transport of Pi, Zn2+, Fe2+, and polyamines [26], in addition to Ca2+/H+ and Na+/H+ exchangers [20, 34]. At least two channels have been described in acidocalcisomes, an aquaporin or water channel in T. cruzi [35], and an inositol 1,4,5-trisphosphate receptor or calcium channel in T. brucei [36]. The presence of mechanisms for uptake and efflux of Ca2+ suggests an important role for acidocalcisomes in Ca2+ signaling. Fig. 1 shows a scheme of the pumps, channels, and exchangers present in procyclic stages of T. brucei.
Figure 1. Pumps, channels and exchangers in acidocalcisomes of procyclic stages of T. brucei.
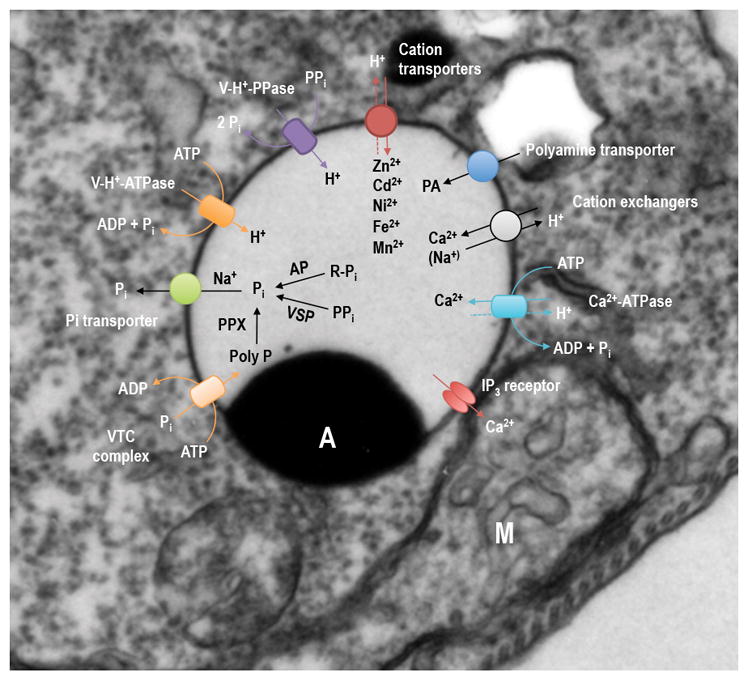
Electron micrograph of a procyclic trypomastigote of T. brucei showing an acidocalcisome (A, recognized by the presence of an electron-dense inclusion of electron dense material) in close contact with the mitochondrion (M). Ca2+ is taken up by a H+-countertransporting Ca2+-ATPase and released by the inositol 1,4,5, trisphosphate receptor (IP3 receptor). H+ are pumped in electrogenically by either the vacuolar H+-PPase (V-H+-PPase) or the multisubunit vacuolar H+-ATPase (V-H+-ATPase). Na+/H+ or Ca2+/H+ exchangers are used for Na+ uptake in exchange for H+ or Ca2+ release in exchange of H+. Cations transporters are used for either Zn2+, Cd2+, Ni2+, Fe2 or Mn2+ uptake. There is also a polyamine (PA) transporter. A vacuolar transporter chaperone complex (VTC) with at least two subunits (Vtc1 and Vtc4) synthesizes polyP using ATP and translocates it into the organelle. A Na+/Pi symporter releases Na+ and Pi form acidocalcisomes. Within acidocalcisomes there is a vacuolar soluble PPase (VSP), an exopolyphosphatase (PPX) and an acid phosphatase (AP).
Polyphosphate synthesis and degradation, and acidocalcisomes
Early work in T. cruzi demonstrated the presence of large amounts of polyP (Fig. 2) and polyP kinase and exopolyphosphatase activities in isolated acidocalcisomes [37]. Work in D. discoideum localized a polyP kinase 1 (DdPPK1) to small vesicles that could correspond to acidocalcisomes [38]. A second PPK, named DdPPK2, which share characteristics and sequence identity with actin-related proteins, was also proposed to be in acidocalcisomes [39]. More recent work in Sacharomyces cerevisiae found that a vacuolar transporter chaperone 4 (ScVtc4p) is a polyP polymerase that uses ATP to generate polyP [40]. A previous report already revealed that null mutants of several Vtc proteins resulted in less polyP synthesis [41]. Vtc4p is the catalytic subunit of a complex of four proteins (Vtc1-4p) that form heterotrimeric complexes that couple synthesis and translocation of polyP to the acidocalcisome-like vacuole preventing its toxicity when in the cytosol and using an electrochemical gradient as a driving force [42]. A similar role was found for the products of the VTC4 genes present in acidocalcisomes of T. brucei [43] and T. cruzi [44]. These enzymes (TbVtc4 and TcVtc4) synthesize predominantly short chain polyP (~100–300 Pi residues). TbVtc4 [43] and TbVtc1 [45] are essential in T. brucei. Vtc2 and Vtc4 homologs were also found in Toxoplasma gondii, where they are also involved in polyP synthesis although it is not clear whether they are in acidocalcisomes because Vtc2 does not co-localize with the V-H+-PPase [46]. A Vtc1 homolog necessary for acidocalcisome formation was also found in C. reinhardtii, which also possesses a Vtc4 homolog [47]. Mutants deficient in CrVtc1 contain less polyP and acidocalcisomes [47]. Proteomic studies of acidocalcisomes of the red alga Cyanidioschyzon merolae (Vtc1) [28] and of T. brucei (Vtc1, Vtc4) [26] revealed the presence of Vtc proteins in these organelles.
Figure 2. Short chain polyphosphate from T. brucei (Tb) and T. cruzi (Tc).
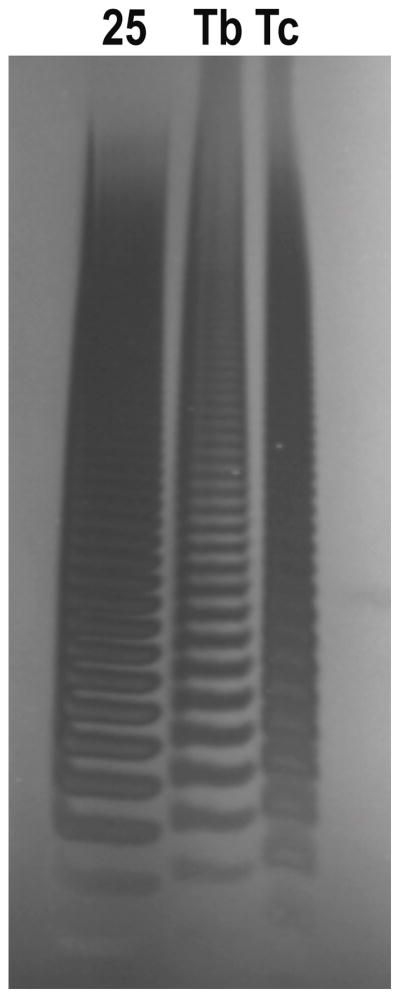
PolyP was extracted with titanium dioxide beads (62) and resolved by 35% polyacrylamide gel electrophoresis. 25 corresponds to short chain polyphosphate of maximum length of 25 Pi units. Gels were stained with 4′,6-diamidino-2-phenylindole (DAPI).
A vacuolar soluble pyrophosphatase (VSP) localizes to acidocalcisomes and the cytosol of different trypanosomatids [48–50]. This enzyme has two distinct domains, an N-terminal EF-hand-like domain and a C-terminal catalytic domain and can hydrolize either PPi or polyP. Its overexpression in T. cruzi results in significant decrease in cytosolic PPi, and short and long chain polyP levels, accompanied by a growth defect, less responsiveness to hyperosmotic stress and reduced persistence of the parasite in tissues of mice, suggesting a role of PPi and polyP under stressful conditions in the host and in maintaining a persistent infection [50]. Table 1 summarizes the acidocalcisome enzymes involved in polyP metabolism that have been identified in eukaryotes
Table 1.
Enzymes involved in polyP metabolism studied in eukaryotes.
| Enzyme abbreviation | Enzyme name | Protein Function | Organism | NCBI Reference number | Ref. |
|---|---|---|---|---|---|
| DdPPK1 | PolyP kinase 1 | PolyP synthesis | D. discoideum | AAD53165.1 | 38 |
| DdPPK2 | PolyP kinase 2 | Reversible polyP synthesis | D. discoideum | XP_636500.1, XP_645275.1, XP_636191.1 | 39 |
| Vtc1p | Vacuolar Transporter Chaperone 1 | PolyP synthesis (subunit of VTC complex) | S. cerevisiae | NP_010995.1 | 41, 42 |
| Vtc2p | Vacuolar Transporter Chaperone 2 | PolyP synthesis (subunit of VTC complex) | S. cerevisiae | NP_116651.1 | 41, 42 |
| Vtc3p | Vacuolar Transporter Chaperone 3 | PolyP synthesis (subunit of VTC complex) | S. cerevisiae | NP_015306.1 | 41, 42 |
| Vtc4p | Vacuolar Transporter Chaperone 4 | PolyP synthesis (catalytic subunit of VTC complex) | S. cerevisiae | NP_012522.2 | 40–42 |
| CrVtc1 | Vacuolar Transporter Chaperone 1 | PolyP synthesis (subunit of VTC complex) | C. reinhardtii | XP_001690865.1 | 47 |
| CmVtc1 | Vacuolar Transporter Chaperone 1 | PolyP synthesis (subunit of VTC complex) | C. merolae | XP_005537755.1 | 28 |
| TbVtc1 | Vacuolar Transporter Chaperone 1 | PolyP synthesis (subunit of VTC complex) | T. brucei | XP_846013.1 | 26, 45 |
| TbVtc4 | Vacuolar Transporter Chaperone 4 | PolyP synthesis (catalytic subunit of VTC complex) | T. brucei | XP_829284.1 | 26, 43, 44 |
| TcVtc4 | Vacuolar Transporter Chaperone 4 | PolyP synthesis (catalytic subunit of VTC complex) | T. cruzi | XP_821342.1 | 44 |
| TgVtc2 | Vacuolar Transporter Chaperone 2 | PolyP synthesis (subunit of VTC complex) | T. gondii | XP_002372055.1 | 46 |
| ScPPX1 | Exopoly phosphatase 1 | PolyP hydrolysis | S. cerevisiae | AAA65933.1 | 58 |
| ScPPN1 | Endopoly phosphatase 1 | PolyP hydrolysis | S. cerevisiae | NP_010740.3 | 59 |
| TcPPX | Exopoly phosphatase | PolyP hydrolysis | T. cruzi | AAQ11880.1 | 60 |
| LmPPX | Exopoly phosphatase | PolyP hydrolysis | L. major | CBZ11834.1 | 61 |
| TbPPX | Exopoly phosphatase | PolyP hydrolysis | T. brucei | AAP74699.1 | 62 |
| TbVSP | Vacuolar Soluble Pyrophosphate | PolyP and PPi hydrolysis | T. brucei | AAP74702.1 | 48 |
| LaVSP | Vacuolar Soluble Pyrophosphate | PolyP and PPi hydrolysis | L. amazonensis | AAP74700.1 | 49 |
| TcVSP | Vacuolar Soluble Pyrophosphate | PolyP and PPi hydrolysis | T. cruzi | XP_807207.1 | 50 |
Function of acidocalcisome polyphosphate in osmoregulation
The role of acidocalcisome polyphosphate in osmoregulation was better studied in T. cruzi. When epimastigote stages are exposed to hyposmotic stress there is a microtubule- and cyclic AMP-mediated fusion of acidocalcisomes to the contractile vacuole that results in translocation of a water channel or aquaporin (TcAQP1) to this organelle [35]. A model has been proposed according to which this fusion of acidocalcisomes with the contractile vacuole together with a rise in ammonia and its accumulation in acidocalcisomes as NH4+ would result in activation of acidocalcisome polyP hydrolysis and the resulting transfer of osmolytes (amino acids, cations and Pi) and water (through the AQP) to the contractile vacuole facilitating its swelling. After water release to the extracellular medium amino acids, cations and Pi would return to the cytosol helping the regulatory volume decrease (RVD). Evidence in favor of this model is the presence of a phosphodiesterase C (PDEC), which would terminate cyclic AMP stimulation, in the spongiome of the contractile vacuole [51], the inhibition of RVD by PDEC inhibitors [52], microscopic evidence of fusion of these organelles [53], and the presence in the contractile vacuole of a sodium-phosphate symporter that could be involved in recycling of Pi produced by the hydrolysis of polyP during RVD [54]. In addition, proteins involved in organellar fusion were detected in the contractile vacuole (SNAREs, TcRab32), and in acidocalcisomes (TcVAMP7) [31, 53].
In contrast to what occurs under hyposmotic stress, hyperosmotic stress results in increased synthesis of acidocalcisome polyP, which by complexing cations results in decreased cytosolic ions that, together with water release by the contractile vacuole through TcAQP1, results in shrinking of the cells. In support of this model treatment of epimastigotes with HgCl2, a known inhibitor of T. cruzi aquaporin 1 (TcAQP1), or knockdown of TcAQP1 expression reduces the intensity of shrinking after hyperosmotic stress while overexpression of TcAQP1 increased shrinking, suggesting that the contractile vacuole mediates water efflux during hyperosmotic challenge. Shrinking is also favored by cation elimination through a cation channel (TcCAT) that is translocated to the plasma membrane of epimastigotes submitted to hyperosmotic stress [55]. Inhibitors of TcCAT (BaCl2, 4-aminopyridine) inhibit shrinking of trypomastigotes under hyperosmotic stress [55]. Synthesis of polyP and inorganic ions sequestration in acidocalcisomes prevents the deleterious effects of a cellular increase in ionic strength. Amino acids are the compatible osmolytes that replace the inorganic ions sequestered in acidocalcisomes, and they initially accumulate by a reduction in their catabolism, and later on by protein degradation and by uptake through induced amino acid transporters [56, 57].
Osmoregulation defects are observed when expression of acidocalcisome enzymes involved in polyP synthesis [43] or degradation [50] are altered, evidencing the involvement of acidocalcisomes in this process.
Acknowledgments
Funding
This work was supported in part by the United States National Institutes of Health [grant numbers AI-077538 and AI-108222] and from the São Paulo Research Foundation (FAPESP), Brazil [grant number 2013/50624-0]. N.L. is a postdoctoral fellow of FAPESP [grant number 2014/08995-4].
References
- 1.Vercesi AE, Moreno SN, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docampo R, Scott DA, Vercesi AE, Moreno SN. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem J. 1995;310:1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babes V. Beobachtungen über die metachromatischen köperchen, sporenbidung, verzwiegung, kolben- und kasel-bildung pathogener bakterien. Zentralbl Bakeriol Parasitenkd Infektionskr Hyg. 1895;20:412–420. [Google Scholar]
- 4.Meyer A. Orientierende untersuchungen über verbreitung. Morphologie, und chemie des volutins. Bot Zeit. 1904;62:113–152. [Google Scholar]
- 5.Shively JM. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28:167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- 6.Seufferheld M, Lea CR, Vieira M, Oldfield E, Docampo R. The H(+)-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–51202. doi: 10.1074/jbc.M406099200. [DOI] [PubMed] [Google Scholar]
- 7.Seufferheld M, Vieira MC, Ruiz FA, Rodrigues CO, Moreno SN, Docampo R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–29978. doi: 10.1074/jbc.M304548200. [DOI] [PubMed] [Google Scholar]
- 8.Wiame JM. Etude d’une substance polypshophoré, basophile et métachromatique chez les levures. Biochim Biophys Acta. 1947;1:234–255. [Google Scholar]
- 9.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seufferheld MJ, Kim KM, Whitfield J, Valerio A, Caetano-Anolles G. Evolution of vacuolar proton pyrophosphatase domains and volutin granules: clues into the early evolutionary origin of the acidocalcisome. Biol Direct. 2011;6:50. doi: 10.1186/1745-6150-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docampo R, Ulrich P, Moreno SN. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Phil Trans Royal Soc London Series B, Biol Sci. 2010;365:775–784. doi: 10.1098/rstb.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121:561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Fang J, Sant’Anna C, Li ZH, Wellems DL, Rohloff P, Docampo R. Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei. J Biol Chem. 2011;286:36619–36630. doi: 10.1074/jbc.M111.284661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchesini N, Ruiz FA, Vieira M, Docampo R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. J Biol Chem. 2002;277:8146–8153. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz FA, Marchesini N, Seufferheld M, Govindjee, Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J Biol Chem. 2001;276:46196–46203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- 18.Hong-Hermesdorf A, Miethke M, Gallaher SD, Kropat J, Dodani SC, Chan J, Barupala D, Domaille DW, Shirasaki DI, Loo JA, Weber PK, Pett-Ridge J, Stemmler TL, Chang CJ, Merchant SS. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat Chem Biol. 2014;10:1034–1042. doi: 10.1038/nchembio.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz FA, Luo S, Moreno SN, Docampo R. Polyphosphate content and fine structure of acidocalcisomes of Plasmodium falciparum. Microsc Microanal. 2004;10:563–567. doi: 10.1017/S1431927604040875. [DOI] [PubMed] [Google Scholar]
- 20.Rohloff P, Miranda K, Rodrigues JC, Fang J, Galizzi M, Plattner H, Hentschel J, Moreno SN. Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii. PLoS One. 2011;6:e18390. doi: 10.1371/journal.pone.0018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos I, Gomes F, Koeller CM, Saito K, Heise N, Masuda H, Docampo R, de Souza W, Machado EA, Miranda K. Acidocalcisomes as calcium- and polyphosphate-storage compartments during embryogenesis of the insect Rhodnius prolixus Stahl. PLoS One. 2011;6:e27276. doi: 10.1371/journal.pone.0027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos IB, Miranda K, Ulrich P, Ingram P, LeFurgey A, Machado EA, de Souza W, Docampo R. Calcium- and polyphosphate-containing acidocalcisomes in chicken egg yolk. Biol Cell. 2010;102:421–434. doi: 10.1042/BC20100011. [DOI] [PubMed] [Google Scholar]
- 23.Tocheva EI, Dekas AE, McGlynn SE, Morris D, Orphan VJ, Jensen GJ. Polyphosphate storage during sporulation in the gram-negative bacterium Acetonema longum. J Bacteriol. 2013;195:3940–3946. doi: 10.1128/JB.00712-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikuchi Y, Hijikata N, Yokoyama K, Ohtomo R, Handa Y, Kawaguchi M, Saito K, Ezawa T. Polyphosphate accumulation is driven by transcriptome alterations that lead to near-synchronous and near-equivalent uptake of inorganic cations in an arbuscular mycorrhizal fungus. New Phytol. 2014;204:638–649. doi: 10.1111/nph.12937. [DOI] [PubMed] [Google Scholar]
- 25.Gomes FM, Carvalho DB, Peron AC, Saito K, Miranda K, Machado EA. Inorganic polyphosphates are stored in spherites within the midgut of Anticarsia gemmatalis and play a role in copper detoxification. J Insect Physiol. 2012;58:211–219. doi: 10.1016/j.jinsphys.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, Moreno SN, Orlando R, Docampo R. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014;10:e1004555. doi: 10.1371/journal.ppat.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott DA, Docampo R. Characterization of isolated acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 2000;275:24215–24221. doi: 10.1074/jbc.M002454200. [DOI] [PubMed] [Google Scholar]
- 28.Yagisawa F, Nishida K, Yoshida M, Ohnuma M, Shimada T, Fujiwara T, Yoshida Y, Misumi O, Kuroiwa H, Kuroiwa T. Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae. Plant J. 2009;60:882–893. doi: 10.1111/j.1365-313X.2009.04008.x. [DOI] [PubMed] [Google Scholar]
- 29.Maeshima M. Vacuolar H+-pyrophosphatase. Biochim Biophys Acta. 2000;1465:37–51. doi: 10.1016/s0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 30.Saliba KJ, Allen RJ, Zissis S, Bray PG, Ward SA, Kirk K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J Biol Chem. 2003;278:5605–5612. doi: 10.1074/jbc.M208648200. [DOI] [PubMed] [Google Scholar]
- 31.Ulrich PN, Jimenez V, Park M, Martins VP, Atwood J, 3rd, Moles K, Collins D, Rohloff P, Tarleton R, Moreno SN, Orlando R, Docampo R. Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS One. 2011;6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, Coppens I, Sibley LD, Moreno SN. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vercesi AE, Docampo R. Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei. Biochem J. 1996;315:265–270. doi: 10.1042/bj3150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- 36.Huang G, Bartlett PJ, Thomas AP, Moreno SN, Docampo R. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc Natl Acad Sci USA. 2013;110:1887–1892. doi: 10.1073/pnas.1216955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem. 2001;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Gomez-Garcia MR, Shi X, Rao NN, Kornberg A. Polyphosphate kinase 1, a conserved bacterial enzyme, in a eukaryote, Dictyostelium discoideum, with a role in cytokinesis. Proc Natl Acad Sci USA. 2007;104:16486–16491. doi: 10.1073/pnas.0706847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Garcia MR, Kornberg A. Formation of an actin-like filament concurrent with the enzymatic synthesis of inorganic polyphosphate. Proc Natl Acad Sci USA. 2004;101:15876–15880. doi: 10.1073/pnas.0406923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324:513–516. doi: 10.1126/science.1168120. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerasimaite R, Sharma S, Desfougeres Y, Schmidt A, Mayer A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J Cell Sci. 2014;127:5093–5104. doi: 10.1242/jcs.159772. [DOI] [PubMed] [Google Scholar]
- 43.Lander N, Ulrich PN, Docampo R. Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J Biol Chem. 2013;288:34205–34216. doi: 10.1074/jbc.M113.518993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulrich PN, Lander N, Kurup SP, Reiss L, Brewer J, Soares Medeiros LC, Miranda K, Docampo R. The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi. J Eukaryot Microbiol. 2014;61:155–165. doi: 10.1111/jeu.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Rohloff P, Miranda K, Docampo R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007;407:161–170. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rooney PJ, Ayong L, Tobin CM, Moreno SN, Knoll LJ. TgVTC2 is involved in polyphosphate accumulation in Toxoplasma gondii. Mol Biochem Parasitol. 2011;176:121–126. doi: 10.1016/j.molbiopara.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aksoy M, Pootakham W, Grossman AR. Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation. Plant Cell. 2014;26:4214–4229. doi: 10.1105/tpc.114.129270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, Docampo R, Bakalara N. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–3425. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- 49.Espiau B, Lemercier G, Ambit A, Bringaud F, Merlin G, Baltz T, Bakalara N. A soluble pyrophosphatase, a key enzyme for polyphosphate metabolism in Leishmania. J Biol Chem. 2006;281:1516–1523. doi: 10.1074/jbc.M506947200. [DOI] [PubMed] [Google Scholar]
- 50.Galizzi M, Bustamante JM, Fang J, Miranda K, Soares Medeiros LC, Tarleton RL, Docampo R. Evidence for the role of vacuolar soluble pyrophosphatase and inorganic polyphosphate in Trypanosoma cruzi persistence. Mol Microbiol. 2013;90:699–715. doi: 10.1111/mmi.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoijet AC, Miranda K, Medeiros LC, de Souza W, Flawia MM, Torres HN, Pignataro OP, Docampo R, Alonso GD. Defining the role of a FYVE domain in the localization and activity of a cAMP phosphodiesterase implicated in osmoregulation in Trypanosoma cruzi. Mol Microbiol. 2011;79:50–62. doi: 10.1111/j.1365-2958.2010.07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King-Keller S, Li M, Smith A, Zheng S, Kaur G, Yang X, Wang B, Docampo R. Chemical validation of phosphodiesterase C as a chemotherapeutic target in Trypanosoma cruzi, the etiological agent of Chagas’ disease. Antimicrob Agents Chemother. 2010;54:3738–3745. doi: 10.1128/AAC.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niyogi S, Jimenez V, Girard-Dias W, de Souza W, Miranda K, Docampo R. Rab32 is essential for maintaining functional acidocalcisomes and for growth and infectivity of Trypanosoma cruzi. J Cell Sci. 2015;128:2363–2373. doi: 10.1242/jcs.169466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez V, Docampo R. TcPho91 is a contractile vacuole phosphate sodium symporter that regulates phosphate and polyphosphate metabolism in Trypanosoma cruzi. Mol Microbiol. 2015 doi: 10.1111/mmi.13075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimenez V, Docampo R. Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi. PLoS Pathog. 2012;8:e1002750. doi: 10.1371/journal.ppat.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li ZH, Alvarez VE, De Gaudenzi JG, Sant’Anna C, Frasch AC, Cazzulo JJ, Docampo R. Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi. J Biol Chem. 2011;286:43959–43971. doi: 10.1074/jbc.M111.311530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int Rev Cell Mol Biol. 2013;305:69–113. doi: 10.1016/B978-0-12-407695-2.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wurst H, Shiba T, Kornberg A. The gene for a major exopolyphosphatase from Saccharomyces cerevisiae. J Bacteriol. 1995;177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sethuraman A, Rao NN, Kornberg A. The endopolyphosphatase gene: essential in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2001;98:8542–8547. doi: 10.1073/pnas.151269398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang J, Ruiz FA, Docampo M, Luo S, Rodrigues JL, Motta LS, Rohloff P, Docampo R. Overexpression of a Zn2+-sensitive soluble exopolyphosphatase from Trypanosoma cruzi depletes polyphosphate and affects osmoregulation. J Biol Chem. 2007;282:32501–32510. doi: 10.1074/jbc.M704841200. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues CO, Ruiz FA, Vieira M, Hill JE, Docampo R. An acidocalcisomal exopolyphosphatase from Leishmania major with high affinity for short chain polyphosphate. J Biol Chem. 2002;277:50899–50906. doi: 10.1074/jbc.M208940200. [DOI] [PubMed] [Google Scholar]
- 62.Luginbuehl E, Kunz S, Wentzinger L, Freimoser F, Seebeck T. The exopolyphosphatase of Trypanosoma brucei. BMC Microbiol. 2011;11:4. doi: 10.1186/1471-2180-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson MS, Bulley SJ, Pisani F, Irvine RF, Saiardi A. A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present inn human plasma or uring. Open Biol. 2015;5:150014. doi: 10.1098/rsob.150014. [DOI] [PMC free article] [PubMed] [Google Scholar]


