Abstract
There is little knowledge about how emotion regulation difficulties interplay with psychopathology in terms of smoking cessation. Participants (n = 250; 53.2 % female, Mage = 39.5, SD = 13.85) were community-recruited daily smokers (≥8 cigarettes per day) who self-reported motivation to quit smoking; 38.8 % of the sample met criteria for a current (past 12-month) psychological disorder. Emotion regulation deficits were assessed pre-quit using the Difficulties with Emotion Regulation Scale (DERS; Gratz and Roemer in J Psychopathol Behav Assess 26(1):41–54, 2004) and smoking behavior in the 28 days post-quit was assessed using the Timeline Follow-Back (TLFB; Sobell and Sobell in Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press, Totowa, 1992). A Cox proportional-hazard regression analysis was used to model the effects of past-year psychopathology, DERS (total score), and their interaction, in terms of time to lapse post-quit day. After adjusting for the effects of gender, age, pre-quit level of nicotine dependence, and treatment condition, the model revealed a non-significant effect of past-year psychopathology (OR = 1.14, CI95 % = 0.82–1.61) and difficulties with emotion regulation (OR = 1.01, CI95 % = 1.00–1.01) on likelihood of lapse rate. However, the interactive effect of psychopathology status and difficulties with emotion regulation was significant (OR = 0.98, CI95 % = 0.97–0.99). Specifically, there was a significant conditional effect of psychopathology status on lapse rate likelihood at low, but not high, levels of emotion regulation difficulties. Plots of the cumulative survival functions indicated that for smokers without a past-year psychological disorder, those with lower DERS scores relative to elevated DERS scores had significantly lower likelihood of early smoking lapse, whereas for smokers with past-year psychopathology, DERS scores did not differentially impact lapse rate likelihood. Smokers with emotion regulation difficulties may have challenges quitting, and not having such difficulties, especially without psychopathology, decreases the potential likelihood of early lapse.
Keywords: Tobacco, Emotion regulation, Lapse, Nicotine, Psychopathology
Introduction
Smokers with psychopathology represent a vulnerable subpopulation of the tobacco-using population [National Institute of Health (NIH) 2006]. The prevalence of smoking among individuals with any psychological disorder is significantly higher relative to the general population (36.1 vs. 21.4 %, respectively) and smokers with psychopathology smoke at higher rates relative to their non-disordered counterparts [Center for Disease Control and Prevention (CDCP) 2013]. Cigarette smoking also contributes to the onset and maintenance of psychopathology and can interfere with mental health treatment (Richards et al. 2013, Ziedonis et al. 2008). Epidemiological and clinical trial data suggest that smokers with psychopathology, relative to those without, are less likely to quit smoking successfully (e.g., CDCP 2013; Ferguson et al. 2003; Lasser et al. 2000; Piper et al. 2010). In fact, psychopathology appears to be a key factor in the stagnation of smoking cessation rates in the United States over recent decades (Goodwin et al. 2012, 2014). Specifically, poorer cessation outcomes have been documented among smokers across various forms of psychopathology (e.g., depressive disorders, posttraumatic stress disorder, anxiety disorders, co-morbid substance use disorders; Beckham et al. 2013; Hitsman et al. 2013; Leventhal et al. 2014; Piper et al. 2010; Zvolensky et al. 2008a).
In the context of smoking cessation, early smoking lapse, typically defined as latency to the first instance of smoking (even a puff), is important because it is associated with an increased likelihood of return to regular smoking (i.e., relapse; Brown et al. 2005, 2009). Smokers with psychopathology may be particularly at risk for early smoking re-initiation given smoking lapse is typically preceded by heightened levels of negative affect (e.g., Shiffman and Waters 2004; Shiffman 2005). For example, some work suggests anxiety psychopathology is associated with early lapse (Zvolensky et al. 2008a). However, limited empirical work has examined factors that may moderate the relationship between psychopathology and early lapse. Such research has the potential to identify factors that may exacerbate early lapse likelihood, which could be potentially targeted when preparing smokers with psychopathology to quit.
One possible individual difference factor that may be involved in early smoking lapse is emotion regulation difficulties. Emotion regulation is often broadly understood as adaptive responding to emotional distress (i.e., that makes it easier for individuals to behave in socio-emotionally appropriate ways; John and Eng 2014) versus efforts to control/suppress emotional arousal (Gratz and Roemer 2004). Specifically, emotion regulation has been described on four dimensions: (a) flexible use of adaptive strategies to modulate (vs. eliminate) emotional intensity/ duration; (b) maintaining behavioral control when distressed; (c) emotional awareness, clarity and acceptance; and (d) willingness to experience emotional distress to pursue meaningful activities. Difficulties or deficits in any one domain are indicative of emotion “dysregulation”—or difficulties in emotion regulation (Gratz and Tull 2010) and may demarcate deficits in one’s ability to use situationally appropriate regulation strategies flexibility to modulate emotional responses (Gratz and Roemer 2004). As a result of having such difficulties with emotion regulation, (a) the experience of negative affective states may persist longer and be more severe and (b) attempts may be made to inflexibily escape or modulate distress states; both may promote or maintain internalizing and externalizing disorders (e.g., Mennin et al., 2007; Tice et al., 2001). Indeed, difficulties with emotion regulation are implicated in various forms of psychopathology, including anxiety disorders (Baker et al. 2004a; McLaughlin et al. 2007; Mennin et al. 2009; Tull and Roemer 2007), post-traumatic stress disorder (Ehring and Quack 2010; Weiss et al. 2012), mood disorders (Johnson 2005; Joormann and Siemer 2014; Nolen-Hoeksema 2000), substance use disorders (Fox et al. 2008; Kun and Demetrovics 2010; Riley and Schutte 2003), and eating disorders (Gianini et al. 2013; Harrison et al. 2010).
Notably, available work suggests difficulties with emotion regulation are related to smoking recency (Adams et al. 2012), craving and attentional bias to smoking cues (Fucito et al. 2010; Szasz et al. 2012), affect-regulatory smoking expectancies (Johnson et al. 2008), and perceived barriers for quitting and certain reasons (motives) for smoking (e.g., stimulation, habitual, and sensorimotor reasons; Gonzalez et al. 2008). Yet, it is unclear how difficulties in emotion regulation relate to smoking lapse in general, or in the context of psychopathology. It is possible that smokers with psychopathology who also experience greater difficulties with emotion regulation may struggle to maintain abstinence during a cessation attempt. For example, these smokers may lack adaptive strategies to effectively/adaptively ‘down-regulate’ negative emotional states (i.e., thus rely on smoking as a strategy for managing negative emotional states). This is consistent with affect-regulatory theories of addiction that posit that negative reinforcement learning (i.e., self-administration of drugs followed by reduction in aversive stimulus) can maintain addiction, especially because affective distress can inhibit cognitive control resources (McCarthy et al. 2010). Additionally, smokers (especially those with psychological disorders) also may have difficulties with recognizing and understanding emotional states, and may have difficulties with maintaining goal-directed behavior in the context of distressing emotional states, which may promote early smoking lapse. In contrast, smokers without psychopathology and without emotion regulation deficits may be most successful in maintaining sustained abstinence due to better adaptive strategies for managing distress or emotional competence (Kun and Demetrovics 2010).
Together, the present study sought to test whether difficulties with emotion regulation may interplay with psychopathology in the prediction of smoking lapse among smokers. Specifically, the current study examined the interactive effects of psychopathology and difficulties in emotion regulation in terms of time to lapse post-cessation attempt among smokers enrolled in a randomized controlled smoking cessation treatment trial. It was hypothesized that psychopathology status and difficulties with emotion regulation would synergistically interplay, such that smokers with psychopathology in the context of greater difficulties with emotion regulation would have the earliest lapse profile, relative to those with psychopathology but lower difficulties with emotion regulation or smokers without past-year psychopathology (regardless of emotion regulation difficulties). In contrast, smokers with the combination of no past-year psychopathology and lower emotional regulation difficulties would have a profile indicative of longest latency to lapse, relative to other groups.
Methods
Participants
Participants (N = 250) were adult treatment-seeking daily smokers (Mage = 39.5, SD = 13.85; 53.2 % female) who primarily identified as White (86.4 %). Participants were generally well educated (78.8 % reported completing at least part of college) and the majority reported marital status as never married (38.0 %) or married/cohabitating (37.6 %). The average daily cigarettes per day in the sample was 16.0 (SD = 8.84) and on average, moderate levels of nicotine dependence were reported (Fagerström Test for Nicotine Dependence [FTND]: M = 5.1, SD = 2.26). On average, participants reported starting smoking at age 14.9 (SD = 3.59), regular daily smoking at age 17.8 (SD = 4.18), and during their heaviest period of smoking, were smoking an average of 24.9 (SD = 12.54) cigarettes per day. Participants reported an average of 3.5 (SD = 2.32) previous ‘serious’ quit attempts.
The presence of current (past 12-month) psychological disorders were assessed using the Structural Clinical Interview of DSM-IV Disorders (SCID-I/NP; First et al. 2007)—38.8 % met criteria for a diagnosis. Of those with a past-year disorder, the average number of diagnoses was 1.6 (SD = 0.90; range = 1–5), and specific diagnoses included: social anxiety disorder (32.0 %), specific phobia (21.6 %), generalized anxiety disorder (20.6 %), alcohol use disorder (19.6 %), major depressive disorder (13.4 %), dysthymia (12.4 %), posttraumatic stress disorder (11.3 %), cannabis use disorder (9.3 %), obsessive–compulsive disorder (4.1 %), other depressive disorders (3.1 %), anxiety disorder not otherwise specified (NOS; 3.1 %), other substance use disorder (2.1 %), or other psychological disorders (7.2 %).
Measures
A demographics form was used to collect gender, age, race/ethnicity and educational background.
Past-Year Psychopathology
The Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-I/NP; First et al. 2007) was administered to assess the presence of past 12-month (current) psychological disorders. This version of the SCID is commonly used in research with community participants. Interviews were administered by trained research assistants or doctoral-level staff and supervised by independent doctoral-level professionals. A random sample (12.5 %) of cases were sampled and checked by two independent raters for diagnostic accuracy (no discrepancies were noted). A dichotomous variable was created to indicate those who met criteria for any psychological disorder (=1) versus those who did not (=0). The presence of historical diagnoses (disorders that were successfully treated or remitted) was not included in the coding of this dichotomous variable. Examining the role of current psychopathology in terms of risk for smoking cessation lapse is conceptually the most relevant.
Emotion Regulation Difficulties
The Difficulties with Emotion Regulation Scale (DERS; Gratz and Roemer 2004) is a 36-item self-report measure that assesses, on a five-point Likert-type scale (1 = Almost never to 5 = Almost always), the degree to which respondents experience greater difficulties in regulating emotional states. Items can be summed to produce a total score, with higher scores reflecting greater difficulties with emotion regulation (possible range 36–180). The psychometric properties of the DERS have been documented, including internal consistency, test–retest reliability, and predictive validity (Gratz and Roemer 2004). Internal consistency in the current sample was α = 0.95.
Smoking-Relevant Measurement
Nicotine Dependence
The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991) is a 6-item self-report scale that was used to assess gradations in tobacco dependence. Scores range from 0 to 10, with higher scores reflecting higher levels of physiological dependence on nicotine. Internal consistency in the current sample was α = 0.66.
Smoking History
The Smoking History Questionnaire (SHQ; Brown et al. 2005, 2009) is a self-report questionnaire used to assess smoking history (e.g., onset of regular daily smoking), pattern (e.g., number of cigarettes consumed per day), and quit history. In the present study, the SHQ was employed to describe the sample on smoking history and patterns of use (e.g., smoking rate, years as a regular smoker).
Lapse Behavior
The Timeline Follow-Back Interview (TLFB; Sobell and Sobell 1992) is a calendar-based assessment that was originally developed to assess alcohol use, but has been adapted for other substances including tobacco (Robinson et al. 2014). Data on the quantity and frequency of cigarette use are collected using clinician-guided retrospective recall. Participants are encouraged to use notable events (e.g., birthdays, holidays, special events) and patterns of use (e.g., weekends vs. week days, locations, time of day) to complete the calendar. The TLFB was conducted at each follow-up visit post-quit day. Only the first 28 days post-quit were utilized in the present study given the high rate of smoking lapse that occurs shortly after quitting. Computation of outcome variables from the TLFB is described in detail in Data Analytic Plan, below. This form of data collection has been found to have very strong psychometric properties up to 90-days, including excellent inter-rater reliability, test–retest reliability, and strong convergent validity based on collateral interviews (Robinson et al. 2014). Internal consistency in the current sample was α = 0.98.
Procedure
Participants were recruited for potential inclusion in a randomized controlled trial examining the efficacy of two smoking cessation interventions in terms of smoking cessation outcomes and prevention of panic disorder (clinicaltrials.gov #NCT01753141). Participants were recruited from the community via flyers, radio advertisements, free online postings, and word-of-mouth referrals. The current study is based on secondary analyses of data for a sub-set of the sample. Inclusion criteria for the parent study included daily cigarette use (average ≥8 cigarettes per day for at least 1 year), between ages 18–65, and reported motivation to quit smoking of at least 5 on a 10-point scale. Exclusion criteria included: inability to give informed consent, current use of smoking cessation products or treatment, past-month suicidality, history of panic psychopathology (per aims of parent study; i.e., panic prevention), and history of psychotic-spectrum disorders. Individuals responding to study advertisements were scheduled for an in-person, baseline evaluation. All participants provided informed consent prior to participation. All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Boards where the data were collected, and with the 1964 Helsinki Declaration and its later amendments. After providing written informed consent, participants completed a computerized battery of baseline (pre-treatment) self-report questionnaires, including the DERS, FTND, and SHQ, and completed an interview-based assessment of psychopathology (per the SCID-I/NP).
Eligible participants were randomly assigned to one of two 4-session smoking cessation treatment programs: (1) Standard Cessation Program (n = 136, 54.4 %; Fiore et al. 2008), which included standard cognitive-behavioral strategies for smoking cessation, or (2) Panic- Smoking Prevention Program (n = 114, 45.6 %; Zvolensky et al. 2008b), which included the standard strategies for smoking cessation with the addition of specific strategies for addressing anxiety. The intervention is described in detail elsewhere (Farris et al. 2015). Self-report TLFB data were collected at scheduled follow-up assessments which occurred at weeks 1, 2, and 4 post-quit day. Cases for the current study were selected from a larger dataset, which included all participants who were screened for the parent study not necessarily enrolled in the treatment phase of the protocol. Data were retained for the current secondary analysis on the basis of having available data for all study variables including the TLFB for the 28-day following the cessation attempt. Participants were compensated for completion of the baseline appointment ($12.50) and all follow-up appointments ($20).
Data Analytic Plan
Data were analyzed using SPSS Version 22.0. All data were checked for inconsistences, outliers, and data entry errors. Descriptive differences between smokers with and without a past-year psychological disorders were examined in addition to bivariate associations between study variables. Smoking lapses were determined based on self-reported TLFB. It was assumed that the presence of missing smoking status data indicated the occurrence of the outcome event (i.e., cigarette use) when the closest available data point was a smoking day (required for only five cases). From these TFLB data, a dichotomously-coded variable was created based whether the occurrence of “any lapse” occurred in the 28-period post-quit day (i.e., indicating a self-report smoking lapse). Then, a variable was coded based on the number of days elapsed since quit day before the first lapse. Next, a multivariate Cox proportional-hazard regression analysis was used to examine the predictive value of past-year psychopathology status (0 = No past-year psychological disorder; 1 = Past-year psychological disorder), difficulties with emotion regulation (DERS-Total score), and their interaction, in terms of lapse rate. This analytic approach estimates and models the distribution of “survival” time it takes before an event (lapse) occurs (Cox 1972). This strategy was selected, in part, for its robustness to censored data. Baseline demographic factors (gender, age) and level of nicotine dependence were included as model covariates based on the well-documented role of these variables in smoking maintenance and cessation (e.g., Baker et al. 2007; Jarvis et al. 2013). Additionally, because participants were randomized to two different treatment conditions (as part of the parent trial), treatment condition (0 = Standard Cessation Program; 1 = Panic-Smoking Prevention Program) was included as a covariate to adjust for any treatment-specific variance accounted for in lapse outcomes. Specifically, the covariates were entered in Step 1 of the model, the main effects of psychopathology status and DERS-Total score were entered in Step 2, and the interaction term was entered in Step 3.
Results
Smokers with and without past year psychological disorders did not differ in terms of age (M = 39.8, SD = 13.6 vs. M = 39.3, SD = 14.1, respectively), race (85.6 vs. 86.9 % white), educational attainment (73.2 vs. 82.4 % completed of at least part college). Smokers with a history of past-year psychopathology were more frequently female relative to smokers without a history of past-year psychological disorders (see Table 1). In terms of smoking history, those with and without a history of psychological disorders did not differ in terms of age of initiating regular daily smoking (M = 17.1, SD = 3.7 vs. M = 18.2, SD = 4.4), number of years as a smoker (M = 21.3, SD = 14.1 vs. M = 20.3, SD = 13.9, respectively), or average number of cigarette smoked per day in the week prior to baseline appointment (M = 16.0, SD = 8.5 vs. M = 15.9, SD = 8.5). As indicated in Table 1, groups did not differ in terms of number of prior quit attempts or level of nicotine dependence at baseline. Regarding bivariate associations, past-year psychopathology status was significantly correlated with DERS total scores, although medium in size (r = .35, p <.001). For smokers with past-year psychopathology, female gender was significantly correlated with higher DERS scores. For smokers without a past-year psychological disorder, younger age was associated with higher DERS scores.
Table 1.
Descriptive characteristics and bivariate correlations among smokers with and without a past-year psychopathology
| Variable | No disorder (n = 153) |
Past-year disorder (n = 97) |
t or x2 | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|---|---|---|
| 1. Gender (% female) | 73 (47.7 %) | 60 (61.9 %) | 4.769* | – | .11 | −.09 | .01 | .10 | −.14 |
| 2. Age | 39.3 (14.1) | 39.8 (13.6) | −0.269 | .17 | – | .32** | .28** | .37** | −.32** |
| 3. Cigarettes/day | 15.9 (8.5) | 16.0 (8.5) | −0.072 | −.19 | .31** | – | .20* | .60** | −.12 |
| 4. # prior quit attempts | 3.6 (2.4) | 3.2 (2.1) | 1.596 | .12 | .17 | −.08 | – | .20* | −.12 |
| 5. FTND | 4.9 (2.3) | 5.4 (2.2) | −1.63 | −.04 | .50** | .61** | .03 | – | −.12 |
| 6. DERS | 67.7 (17.4) | 82.6 (22.9) | −5.491** | .29* | −.19 | −.11 | −.04 | −.03 | – |
Above diagonal are correlations for sample without a past-year psychological disorder; below the diagonal are correlations for sample with a past-year psychological disorder. Gender (coded 0 = Male, 1 = Female); Cigarettes/day (average number of cigarettes smoked per day in the past week); # prior quit attempts (number of “serious” lifetime quit attempts)
FTND Fagerström Test of Nicotine Dependence, DERS Difficulties With Emotion Regulation Scale * p < .05; ** p < .01
Predicting Time to Lapse
Descriptive Overview
Overall, 70.8 % of the participants self-reported a smoking lapse during the 28-day post-quit period. The median time to lapse was 6.8 days, with 27.0 % reporting smoking lapse on the first day following the quit attempt. Among smokers with a past-year psychological disorder, 77.3 % reported a smoking lapse during the quit period (median time to lapse was 5.1 days). For those smokers without a past-year psychological disorder, 66.7 % reported a smoking lapse (median time to lapse was 9.2 days).
Survival Analysis
Next, a step-wise multivariate Cox-proportional hazard regression model was constructed to test the effect of psychopathology status, DERS scores, and their interaction in terms of predicting time to lapse after the quit attempt, adjusting for gender, age, level of nicotine dependence, and treatment condition (entered in Step 1 of the model). Results indicated that the overall model was significant (x2(7) = 15.50, p = .030). As presented in Table 2, results indicated that older age was a significantly predictive of likelihood of lower lapse rate, whereas the effects of gender, nicotine dependence, and treatment condition were non-significant predictors of time to smoking lapse (step 1: (x2(4) = 6.09, p = .193). The main effects of past-year psychopathology status and DERS score were also non-significant (x2(6) = 10.34, p = .111). The addition of the interaction term accounted for a significant Chi square change (x2(1) = 5.30, p = .021) and this effect was a significant predictor of likelihood of lower lapse rate.
Table 2.
Prediction of rate of smoking lapse as a function of past-year psychopathology, difficulties with emotion regulation, and their interaction
| Step | Predictor | OR | Sig. | CI 95 % (L) | CI 95 % (U) |
|---|---|---|---|---|---|
| 1 | Gender | 0.95 | .729 | 0.70 | 1.28 |
| Age | 0.99 | .022 | 0.97 | 0.98 | |
| FTND | 1.05 | .194 | 0.98 | 1.13 | |
| TX condition | 0.88 | .398 | 0.65 | 1.19 | |
| 2 | Psychological Dx | 1.14 | .435 | 0.82 | 1.61 |
| DERS-total | 1.01 | .138 | 1.00 | 1.01 | |
| 3 | Interaction | 0.98 | .021 | 0.97 | 0.99 |
Note: Gender (Male = 0, Female = 1); Age = at baseline appointment; FTND = Fagerström Test of Nicotine Dependence; TX Condition (Control = 0, Active = 1); Psychological DX = any past-year psychopathology disorder per the SCID-I/NP (No disorder = 0, Past-Year disorder = 1); DERS-total = Difficulties with Emotion Regulation Scale (Total score); Interaction (Psychological DX * DERS-Total)
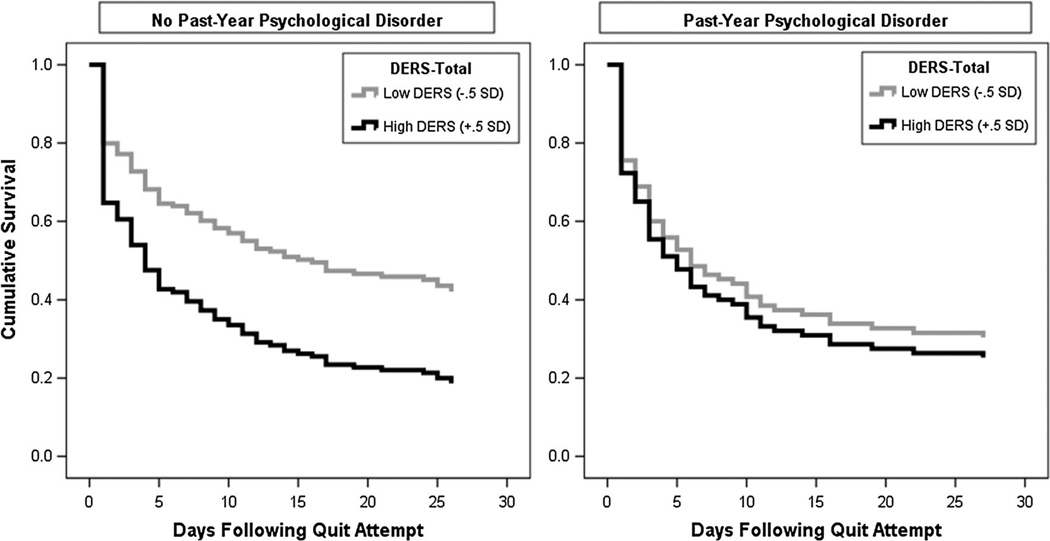
To specifically test the nature of the interaction, tests of simple slopes (entered with ±0.5 SD DERS scores) were conducted. Results indicated that the interplay between psychopathology status was not differentially predictive of likelihood of lapse rate when scores were high on the DERS (OR = 0.96, CI95 % = 0.70–1.40) but was significant when scores were low on the DERS (OR = 1.70, CI95 % = 1.06–2.72). Figure 1 presents plots of the adjusted cumulative survival functions for smokers without and with a past-year psychological disorder, as impacted by DERS scores. As illustrated, the impact of difficulties with emotion regulation is specifically seen in smokers with no past-year psychological disorder (Fig. 1; panel a) relative to those with a past-year psychological disorder (Fig. 1; panel b).
Fig. 1.
Visualization of interaction of psychopathology and DERS-Total score in terms of time to first smoking lapse. Cases with no past-year psychological disorder (n = 153); Cases with a past-year psychological disorder (n = 97)
Discussion
Consistent with expectation, the combined effect of psychopathology status and difficulties with emotion regulation was statistically significant. Yet, the form of the interactive effect was only partially in support with the a priori hypothesis. Specifically, as expected, at lower levels of difficulties with emotion regulation, smokers without past-year psychopathology were at a significantly lower risk for early lapse rate relative to those smokers a past-year psychological disorder. In contrast, in the context of high levels of emotion regulation difficulties, likelihood of early lapse did not differ between smokers with and without past-year psychological disorders. Thus, while data do not support an ‘amplifying’ effect of emotion regulation difficulties in the context of past-year psychopathology per se, findings indicate that even among smokers without a history of past-year psychopathology, those smokers who struggle to regulate emotional states may be ‘high-risk’ for early cessation failure. Interestingly, the non-significant main effect of emotion regulatory difficulties suggests that this vulnerability may only confer risk for smokers during a cessation attempt, if considered in the context of psychopathology (presence of psychopathology or not), but not incrementally after adjusting for other covariates including psychopathology status.
Findings also indicated that there was a non-significant main effect of psychopathology status, after adjusting for relevant covariates (gender, age, nicotine dependence, and treatment condition). This non-significant finding is somewhat surprising given other work that has documented increased lapse likelihood among smokers with psychopathology relative to non-disordered smokers (e.g., at 6-months follow-up; Ferguson et al. 2003). It is possible that smokers in the ‘no psychopathology’ group in the current sample had a past history of psychopathology (i.e., successfully treated/remitted disorders). Indeed, lifetime psychopathology, relative to no history psychopathology, has been found to be associated with decreased likelihood of short-term smoking abstinence, particularly with a history of comorbid psychopathology (not only one lifetime disorder; Cougle et al. 2010; Piper et al. 2010). Additionally, it is possible that certain disorder-specific effects were undetectable in the current study based on the grouping of all past-year psychological disorders into one group. For example, in terms of short-term cessation outcomes (i.e., early lapse), one large study found that, relative to smokers with no psychiatric history, smokers with a past-year mood or anxiety disorder (but not substance use disorder) had lower abstinence rates after 8-week after quit attempt (Piper et al. 2010). Thus, while not explicitly testing latency to lapse, Piper and colleagues suggest that specific disorder groups may differentially be associated with smoking outcomes. For instance, based on the literature documenting the strong associations between difficulties with emotion regulation and certain anxiety/mood disorders (e.g., generalized anxiety disorder, depression; McLaughlin et al. 2007; Mennin et al. 2005; Nolen-Hoeksema et al. 2008), differential predictive and interactive effects may emerge for these disorders relative to other psychological disorders. Differentiation between the predictive effects of specific psychological disorders in the context of emotion regulation difficulties was beyond the scope of the current study, but is worthy for further explication.
It is worth noting that, while difficulties with emotion regulation was modeled here as a global construct, some work suggests specific difficulties with emotion regulation may differentially relate to aspects of psychopathology and substance use. For example, non-acceptance of emotional states (i.e., the tendency to judge or negatively evaluate one’s emotions), specifically, has been linked to depression/ smoking associations (Adams et al. 2012) and marijuana coping motives among daily cannabis users (Bonn-Miller et al. 2008). Additionally, a meta-analysis found that identification of emotions and regulation of emotions are specifically linked to substance use disorders (Kun and Demetrovics 2010). Moreover, while 8 items on the DERS measure assess perceived access to effective emotion regulation strategies, the current study did not explicitly measure/examine emotion regulation strategies, which may differ across psychological disorders (e.g., Aldao et al. 2010; Vine and Aldao 2014). Further work is recommended to examine the nature of specific emotion regulation strategies among cigarettes smokers with and without psychopathology (e.g., Fucito et al. 2010), and the extent to which certain aspects of emotion regulation difficulties are associated with lapse likelihood and smoking behavior.
There are several limitations of the current investigation. First, self-report data were exclusively used to index latency to smoking lapse (Shiffman et al. 1997). Thus, is it possible that reporting biases or retrospective recall may have impacted the findings. Second, past (lifetime) psychopathology (successfully treated or remitted) was not included in the categorization of current (past-year/12-month) psychopathology. It is possible that naturally remitting psychopathology might function differently (Piper et al. 2010), especially in the context of high negative emotional states or situational stressors (like quitting smoking). Additionally, based on exclusion criteria of the larger parent study, the current sample did not include individuals with panic disorder or psychotic-spectrum psychopathology, so it important to consider the extent to which these findings generalize to all mental health disorders. Third, the patterning of results should be examined in more racially/ethnically and educationally diverse samples of smokers given the current sample comprised of relatively well-educated, white smokers.
Fourth, we examined the combination of psychopathology and difficulties with emotion regulation in the context of smoking lapse behavior. It would be important to examine other contextually-relevant mechanistic processes, like severity of nicotine withdrawal. It is also possible that emotion regulation deficits may explain (mediate) the associations between psychopathology and lapse behavior and this matter could be usefully explored in future work. Fifth, we did not model latency to relapse in the current study. Although early lapse is associated with increased risk for relapse (Brown et al. 2005, 2009), the lack of sample variability and relatively short follow-up period post quit-attempt (28-day post-quit), did not permit computation of relapse status. Sixth, we employed the DERS as primary assessment of difficulties with emotion regulation. Although the DERS is a psychometrically valid and reliable measure, it is not specific to smoking behavior. Therefore, it is unclear how the emotion regulatory processes tapped by the DERS relate to smoking-specific emotion regulation. Seventh, antecedents and situational factors involved in the first lapse episodes were unknown in these data (i.e., lack of ecological assessment data, or experimental context; Kahler et al. 2010; Shiffman et al. 1996, 2007). Although it may be inferred that a lapse episode for a smoker with difficulties with emotion regulation involved a failure in the smokers’ ability to adaptively cope with a stressor (whether psychologically-relevant, smoking-specific, or otherwise), the true context of the lapse episodes were unknown. Lastly, the current study modeled difficulties with emotion regulation as a time-invarying process, although certainly, it is possible that smoking cessation treatment or achieving abstinence could impact emotion regulation abilities, which in turn may dynamically impact lapse likelihood.
Overall, smokers without past-year psychopathology and lower self-reported difficulties with emotion regulation were at lowest risk for early smoking lapse. Relative to others, this sub-set of smokers appears to represent a psychologically ‘healthier’ group that is likely more apt to endure early distress experienced while quitting. In contrast, the findings suggest that smokers with either past-month history of psychopathology (in the context of low emotion regulation difficulties), difficulties with emotion regulation (without psychopathology), or the combination of the two (past-year psychopathology and difficulties with emotion regulation), represent relatively comparable groups of ‘psychologically vulnerable’ smokers. Based on this finding, first, difficulties with emotion regulation may be best described as having a unifying function across diverse symptoms/maladaptive behaviors (Gross and Munoz 1995), rather than having an amplifying effect. Second, these findings are broadly consistent with the negative reinforcement models of drug addiction (Baker et al. 2004b), and to a lesser extent, “self-medication” theories of substance use (Khantzian 1985) which generally posit that negative affective states motivate use of substance use, and attenuation in distress (whether perceived or actual) following substance use can reinforce subsequent drug administration. Indeed, negative affective states are documented antecedents to smoking craving, which commonly motivate smoking re-initiation to aid in regulation (amelioration) of craving (Shiffman et al. 1996, 2013). This may promote early smoking lapse behavior. Third, smokers who do not have current psychopathology, but do have difficulties with emotion regulation, may represent a vulnerable group in terms of early smoking lapse (Adams et al. 2014) and in terms of increased risk for the development of psychopathology (e.g., Hofmann et al. 2012). Thus, these findings have both prevention and treatment implications. For smokers with psychopathology and those without (with difficulties in emotion regulation), it may be useful to employ targeted intervention strategies to address ‘psychological vulnerability processes’. It also may be important to provide smokers with information about the function of both cigarette smoking in maintaining (and amplifying risk for) clinically-significant levels of negative emotional states, as is done in certain integrated treatments for smoking and affective vulnerability (Zvolensky et al. 2014). It is recommended that further work in this arena explore the nature of the interplay between deficits in emotion regulation, and specific forms of psychopathology, to further explicate the way in which difficulties with emotion regulation is related to smoking lapse.
Acknowledgments
This work was funded by a National Institute of Mental Health grant awarded to Drs. Michael J. Zvolensky and Norman B. Schmidt (R01-MH076629-01A1). Ms. Farris acknowledges support from a pre-doctoral National Research Service Award (F31-DA035564).
Footnotes
Compliance with Ethical Standards
Animal Rights No animal studies were carried out by the authors for this paper.
Conflict of Interest Ms. Farris, and Drs. Zvolensky and Schmidt declare that they have no conflict of interest.
Informed Consent Informed consent was obtained from all individual subjects participating in the study.
Ethical standard All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Boards where the data were collected (University of Vermont, Florida State University) and analyzed (University of Houston), and with the 1964 Helsinki Declaration and its later amendments.
References
- Adams CE, Heppner WL, Houchins S, Stewart DW, Vidrine JI, Wetter DW. Mindfulness meditation and addictive behaviors. In: Singh NN, editor. Psychology of Meditation. Hauppauge, NY: Nova Science Publishers; 2014. pp. 311–343. [Google Scholar]
- Adams CE, Tull MT, Gratz KL. The role of emotional nonacceptance in the relation between depression and recent cigarette smoking. The American Journal on Addictions. 2012;21(4):293–301. doi: 10.1111/j.1521-0391.2012.00238.x. doi: 10.1111/j.1521-0391.2012.00238.x. [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Baker R, Holloway J, Thomas PW, Thomas S, Owens M. Emotional processing and panic. Behaviour Research and Therapy. 2004a;42(11):1271–1287. doi: 10.1016/j.brat.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(S.4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004b;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine & Tobacco Research. 2013;15(6):1122–1129. doi: 10.1093/ntr/nts252. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Vujanovic AA, Zvolensky MJ. Emotional dysregulation: Association with coping-oriented marijuana use motives among current marijuana users. Substance Use and Misuse. 2008;43(11):1656–1668. doi: 10.1080/10826080802241292. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25(6):713–733. doi: 10.1016/j.cpr.2005.05.003. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11:493–502. doi: 10.1093/ntr/ntp041. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥ 18 years with mental illness—United States, 2009–2011. Morbidity and Mortality Weekly Report. 2013;62(5):81–87. [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Zvolensky MJ, Fitch KE, Sachs-Ericsson N. The role of comorbidity in explaining the associations between anxiety disorders and smoking. Nicotine & Tobacco Research. 2010;12:355–364. doi: 10.1093/ntr/ntq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society: Series B. 1972;34:187–220. [Google Scholar]
- Ehring T, Quack D. Emotion regulation difficulties in trauma survivors: The role of trauma type and PTSD symptom severity. Behavior Therapy. 2010;41(4):587–598. doi: 10.1016/j.beth.2010.04.004. doi: 10.1016/j.beth.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, DiBello AM, Schmidt NB. Validation of the Avoidance and Inflexibility Scale (AIS) among treatment-seeking smokers. Psychological Assessment. 2015;27(2):467–477. doi: 10.1037/pas0000059. doi: 10.1037/pas0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JA, Patten CA, Schroeder DR, Offord KP, Eberman KM, Hurt RD. Predictors of 6-month tobacco abstinence among 1224 cigarette smokers treated for nicotine dependence. Addictive Behaviors. 2003;28(7):1203–1218. doi: 10.1016/s0306-4603(02)00260-5. doi: 10.1016/S0306-4603(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCIDI/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors. 2008;33(2):388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Juliano LM, Toll BA. Cognitive reappraisal and expressive suppression emotion regulation strategies in cigarette smokers. Nicotine & Tobacco Research. 2010;12(11):1156–1161. doi: 10.1093/ntr/ntq146. doi: 10.1093/ntr/ntq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianini LM, White MA, Masheb RM. Eating pathology, emotion regulation, and emotional overeating in obese adults with Binge Eating Disorder. Eating Behavior. 2013;14(3):309–313. doi: 10.1016/j.eatbeh.2013.05.008. doi: 10.1016/j.eatbeh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zvolensky MJ, Vujanovic AA, Leyro TM, Marshall EC. An evaluation of anxiety sensitivity, emotional dysregulation, and negative affectivity among daily cigarette smokers: Relation to smoking motives and barriers to quitting. Journal of Psychiatric Research. 2008;43(2):138–147. doi: 10.1016/j.jpsychires.2008.03.002. doi: 10.1016/j.jpsychires.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Wall MM, Choo T, Galea S, Horowitz J, Zvolensky MJ, Hasin D. Changes in mood and anxiety disorders among male and female current smokers from 1990 to 2001. Annals of Epidemiology. 2014;24:493–497. doi: 10.1016/j.annepidem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Zvolensky MJ, Keyes KM, Hasin DS. Mental disorders and cigarette use among adults in the United States. The American Journal on Addictions. 2012;21(5):416–423. doi: 10.1111/j.1521-0391.2012.00263.x. doi: 10.1111/j.1521-0391.2012.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- Gratz KL, Tull MT. The relationship between emotion dysregulation and deliberate self-harm among inpatients with substance use disorders. Cognitive Therapy and Research. 2010;34(6):544–553. doi: 10.1007/s10608-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychological Medicine. 2010;40(11):1887–1897. doi: 10.1017/S0033291710000036. doi: 10.1017/S0033291710000036. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K. The fagerström test for nicotine dependence: A revision of the fagerström tolerance questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, Demott A, Herrera MJ, Spring B, et al. Past major depression and smoking cessation outcome: A systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. doi: 10.1111/add.12009. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety. 2012;29(5):409–416. doi: 10.1002/da.21888. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA. Dispelling myths about gender differences in smoking cessation: Population data from the USA, Canada and Britain. Tobacco Control: An International Journal. 2013;22(5):356–360. doi: 10.1136/tobaccocontrol-2011-050279. doi: 10.1136/tobaccocontrol-2011-050279. [DOI] [PubMed] [Google Scholar]
- John OP, Eng J. Three approaches to individual differences in affect regulation: Conceptualizations, measures, and findings. In: Gross JJ, editor. Handbook of emotion regulation. 2nd ed. New York, NY: Guilford Press; 2014. pp. 321–345. [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25(2):241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Zvolensky M, Marshall EC, Gonzalez A, Abrams K, Vujanovicv AA. Linkages between cigarette smoking outcome expectancies and negative emotional vulnerability. Addictive Behaviors. 2008;33(11):1416–1424. doi: 10.1016/j.addbeh.2008.05.001. doi: 10.1016/j.addbeh.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Siemer M. Emotion regulation in mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. 2nd ed. New York, NY: Guilford Press; 2014. pp. 413–427. [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine & Tobacco Research. 2010;12(7):781–785. doi: 10.1093/ntr/ntq083. doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kun B, Demetrovics Z. Emotional intelligence and addictions: a systematic review. Substance Use and Misuse. 2010;45(7–8):1131–1160. doi: 10.3109/10826080903567855. doi: 10.3109/10826080903567855. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA Journal of The American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology. 2014;82(1):122–129. doi: 10.1037/a0035046. doi: 10.1037/a0035046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative reinforcement: Possible clinical implications of an integrative model. In: Kassel JD, editor. Substance abuse and emotion. Washington, DC: American Psychological Association; 2010. pp. 15–42. doi: 10.1037/12067-001. [Google Scholar]
- McLaughlin KA, Mennin DS, Farach FJ. The contributory role of worry in emotion generation and dysregulation in generalized anxiety disorder. Behaviour Research and Therapy. 2007;45(8):1735–1752. doi: 10.1016/j.brat.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Holoway RM, Fresco DM, Moore MT, Heimberg RG. Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behavior Therapy. 2007;38:284–302. doi: 10.1016/j.beth.2006.09.001. doi: 10.1016/j.beth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mennin DS, McLaughlin KA, Flanagan TJ. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. Journal of Anxiety Disorders. 2009;23(7):866–871. doi: 10.1016/j.janxdis.2009.04.006. doi: 10.1016/j.janxdis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH State-of-the-Science Panel. National institutes of health state-of-the-science conference statement: Tobacco use: Prevention, cessation, and control. Annals of Internal Medicine. 2006;145(11):839–844. doi: 10.7326/0003-4819-145-11-200612050-00141. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Further evidence for the role of psychosocial factors in depression chronicity. Clinical Psychology: Science and Practice. 2000;7(2):224–227. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78(1):13–23. doi: 10.1037/a0018065. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CS, Cohen LM, Morrell HR, Watson NL, Low BE. Treating depressed and anxious smokers in smoking cessation programs. Journal of Consulting and Clinical Psychology. 2013;81(2):263–273. doi: 10.1037/a0027793. doi: 10.1037/a0027793. [DOI] [PubMed] [Google Scholar]
- Riley H, Schutte NS. Low emotional intelligence as a predictor of substance-use problems. Journal of Drug Education. 2003;33(4):391–398. doi: 10.2190/6DH9-YT0M-FT99-2X05. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. doi: 10.1111/j.0022-3506.2005.00364. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91(2–3):159–168. doi: 10.1016/j.drugalcdep.2007.05.017. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: effects on craving and on smoking behavior. Journal of Abnormal Psychology. 2013;122(1):264–280. doi: 10.1037/a0028339. doi: 10.1037/a0028339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997;65(2):292–300. doi: 10.1037/0022-006x.65.2.292.a. doi: 10.1037/0022-006X.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gyns M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. doi: 10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72(2):192–201. doi: 10.1037/0022-006X.72.2.192. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ, US: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Szasz PL, Szentagotai A, Hofmann SG. Effects of emotion regulation strategies on smoking craving, attentional bias, and task persistence. Behaviour Research and Therapy. 2012;50(5):333–340. doi: 10.1016/j.brat.2012.02.010. doi: 10.1016/j.brat.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! Journal of Personality and Social Psychology. 2001;80(1):53–67. doi: 10.1037/0022-3514.80.1.53. [PubMed] [Google Scholar]
- Tull MT, Roemer L. Emotion regulation difficulties associated with the experience of uncued panic attacks: evidence of experiential avoidance, emotional nonacceptance, and decreased emotional clarity. Behavior Therapy. 2007;38(4):378–391. doi: 10.1016/j.beth.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Vine V, Aldao A. Impaired emotional clarity and psychopathology: A transdiagnostic deficit with symptom-specific pathways through emotion regulation. Journal of Social and Clinical Psychology. 2014;33(4):319–342. doi: 10.1521/jscp.2014.33.4.319. [Google Scholar]
- Weiss NH, Tull MT, Davis LT, Dehon EE, Fulton JJ, Gratz KL. Examining the association between emotion regulation difficulties and probable posttraumatic stress disorder within a sample of African Americans. Cognitive Behavior Therapy. 2012;41:5–14. doi: 10.1080/16506073.2011.621970. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Riley WT. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An anxiety sensitivity reduction smoking cessation program for Spanish-speaking smokers. Cognitive and Behavioral Practice. 2014;21:350–363. [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Feldner MT. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008a;10(8):1415–1427. doi: 10.1080/14622200802238951. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Yartz AR, Gregor K, Gonzalez A, Bernstein A. Interoceptive exposure-based cessation intervention for smokers high in anxiety sensitivity: A case series. Journal of Cognitive Psychotherapy. 2008b;22(4):346–365. doi: 10.1891/0889-8391.22.4.346. [Google Scholar]