Abstract
Purpose
To describe a single institution experience with adrenal metastasectomy, and to elucidate factors that may bear prognostic significance.
Methods
Single center, retrospective review of patients with adrenal metastasis who underwent adrenalectomy performed with curative intent between 2000–2012. Kaplan-Meier method was utilized to evaluate overall survival from time of adrenalectomy to death or last follow-up. Primary endpoint was death from any cause. Clinical variables were examined for association with survival.
Results
Study included 62 patients with mean age of 60 (± 12) years. 55% (34/62) were male, 85% (53/62) presented with isolated adrenal metastasis, and 82% (51/62) had metachronous disease with median DFI of 22 months (range 6–217). Non-small cell lung cancer (NSCLC) was the most common primary comprising 50% of cases. Median survival for the study population was 30 months (range 1–145) and 5-year survival was 31%. Patients with NSCLC had significantly shortened survival compared to non-NSCLC with median and 5-yr survival of 17 vs. 47 months and 27% vs. 38%, respectively (p=0.033). Synchronous metastasis (p=0.028) and DFI <12 months (p=0.038) were also associated with worse survival outcome, though male gender (p=0.69) and oligometastatic disease (p=0.62) were not.
Conclusion
Adrenal metastasectomy resulted in median survival of 30 months and 5-year survival of 31%. Shorter survival was associated with lung primary, short disease-free interval, and synchronous metastasis, but not with the presence of oligometastatic disease provided that the primary cancer and additional metastatic lesions were adequately controlled and amenable to resection.
Introduction
Metastasis to the adrenal gland can occur from a variety of extraadrenal malignancies, and is a common finding at autopsy occurring in up to 27% of cases.1 Adrenal metastasis is typically reflective of disseminated disease, and palliative therapy is often instituted. In contrast, solitary adrenal metastatic lesions are relatively uncommon, but are being increasingly diagnosed in the context of an otherwise resectable or controlled primary malignancy due to the improvement and more widespread use of radiographic imaging in cancer workup and surveillance.2–4
As a result, there has been growing interest in the therapeutic value of adrenal metastasectomy in patients with limited, potentially curable metastatic disease. To date, several small series and case reports have demonstrated potential survival benefit compared to historical stage-matched controls. However, the surgical indications and prognostic factors for improved outcome remain undefined due in part to the relative infrequency of this condition and heterogeneity of previous studies.5–12 The purpose of this investigation, therefore, is to 1) report survival data for a single-institution series of adrenal metastasectomies performed with curative intent, and 2) identify potential clinical variables that have significant impact upon patient survival.
Methods
Under an institutional QA/QI approved protocol (QIIRB1101) we conducted a single center, retrospective, descriptive analysis of survival in a consecutive series of patients with adrenal metastasis who had adrenalectomy performed with curative intent between 2000 and 2012. Curative intent was defined as local R0 resection without evidence of residual metastatic or primary disease after surgery, except in the case of a planned staged resection of the primary tumor to occur after adrenalectomy. Therefore, patients were included who underwent 1) concurrent resection of primary tumor and synchronous adrenal metastasis, and 2) concurrent and complete resection of other metastatic lesions.
Initial electronic medical record query yielded 791 patients who underwent adrenalectomy during the time period of interest. After review of individual pathology reports, 712 patients were excluded due to 1) primary adrenal pathology, 2) adrenalectomy performed as part of radical nephrectomy for renal cell carcinoma, 3) direct tumor extension into the adrenal gland, or 4) adrenal gland evaluation at autopsy. Of the 79 remaining patients, 17 were further excluded after review of operative reports and radiographic data when it was determined that adrenalectomy was performed for symptom relief in the face of incurable, disseminated cancer and was therefore palliative in nature. This resulted in 62 patients who had adrenalectomy performed with curative intent.
Patient follow-up and death information was retrieved from the patient medical record and verified with the Social Security Death Index at www.genealogybank.com/gbnk/ssdi (accessed 9/1/12). Synchronous metastasis refers to adrenal lesions detected within 6 months of primary cancer resection or diagnosis. DFI refers to the time period between the date of primary cancer diagnosis or resection to the date of adrenalectomy, and thus was adrenal-specific and not cancer-specific, as it did not take into account other treated metastases. Overall survival refers to the time period between date of adrenalectomy to date of last follow-up or death.
Survival analysis was conducted utilizing the Kaplan-Meier method. The primary endpoint (failure) was death from any cause, and the primary outcome variable was overall survival. Clinical variables including gender, primary tumor type, and clinical presentation of adrenal metastasis (isolated vs. oligometastatic, synchronous vs. metachronous, DFI) were examined for association with overall survival by testing equality of survivor functions with the log-rank test. Statistical analysis was performed with Stata version 12.1 (College Station, TX). P-values less than 0.05 were considered significant.
Results
A total of 62 patients met inclusion criteria and thus were included in the final analysis, yielding an approximate incidence for adrenal metastasectomy with curative intent of 8% (62/791) among all patients requiring adrenal surgery during the study period. Clinical characteristics of the study population are outlined in Table 1. Most patients underwent laparoscopic adrenalectomy, however, conversion to open was required in 7% (3/41). Clinically, the most commonly observed primary malignancy was non-small cell lung cancer (NSCLC), accounting for 50% of cases, and the majority presented with isolated metastasis to the adrenal gland (85%). Of the patients with oligometastatic disease, all but one underwent simultaneous resection of other metastatic lesions in liver (3), spleen (2), colon (1), lung (1), and breast (1), at the time of adrenalectomy. The remaining patient completed successful cyberknife therapy for brain metastasis immediately prior to adrenalectomy.
Table 1.
Clinical characteristics of study population (n=62). Categorical data reported as n (%), continuous data reported as mean (± sd) or median (range).
| Variable | |
|---|---|
|
| |
| Age, years | 60 (±12) |
|
| |
| Gender | |
| Male | 34 (55) |
| Female | 28 (45) |
|
| |
| Primary malignancy | |
| Lung | 31 (50) |
| Renal | 8 (13) |
| Melanoma | 4 (6) |
| Colon | 4 (6) |
| Other1 | 15 (24) |
|
| |
| Procedure type | |
| Laparoscopic | 41 (66) |
| Conversion | 3 (7) |
| Open | 17 (27) |
| Robotic-assisted | 4 (6) |
|
| |
| Isolated adrenal metastasis | 53 (85) |
| Oligometastatic disease | 9 (15) |
|
| |
| Synchronous adrenal metastasis | 11 (18) |
| Metachronous adrenal metastasis | 51 (82) |
|
| |
| Disease-free interval | 22 (6–217) |
| Metachronous <12 months | 9 (18) |
| Metachronous ≥12 months | 42 (82) |
Other: ovarian (3), esophageal (3), unknown (3), neuroendocrine (2), sarcoma (2), liver (1), and ampullary (1).
With respect to timing of diagnosis, most patients (82%) presented with metachronous disease with median DFI of 22 months. The remaining 11 patients had synchronous adrenal metastasis: 8/11 had simultaneous diagnosis of primary and adrenal tumor, and 3/11 had development of adrenal disease within 6 months of primary cancer resection. All 8 patients who presented with stage IV disease had primary lesions that were deemed resectable at the time of adrenalectomy, however, 5/8 experienced unexpected progression of their primary cancer after adrenalectomy and thus did not undergo previously planned resection. Of the remaining 3/8 patients with synchronous metastasis who did eventually undergo primary resection: 1 patient with NSCLC had no evidence of disease at 125 months, 1 patient was alive at 11 months with recurrence in the form of a pathologic femur fracture at 7 months post-adrenalectomy, and 1 patient was dead with recurrent disease at 25 months.
Follow-up information was available for 59/62 patients: 1 patient was lost to follow-up immediately after adrenalectomy, and there were 2 patient deaths in the immediate perioperative period for an operative mortality rate of 3% (2/62). Of the 2 perioperative deaths, 1 patient who underwent conversion from laparoscopic to open adrenalectomy died intraoperatively from cardiopulmonary arrest, and 1 patient who underwent laparotomy and simultaneous resection of hepatic and adrenal lesions died on post-operative day 10 from progressive hepatic failure. In the 59 evaluable patients: 27 died of disease progression, 5 died but had unknown disease status at the time of death, 7 were alive with disease at last follow-up (median 24 months, range 7–100), and 20 had no evidence of disease at most recent follow-up (median 29 months, range 6–145). All living patients had documented follow-up in the medical record within the previous 6 months.
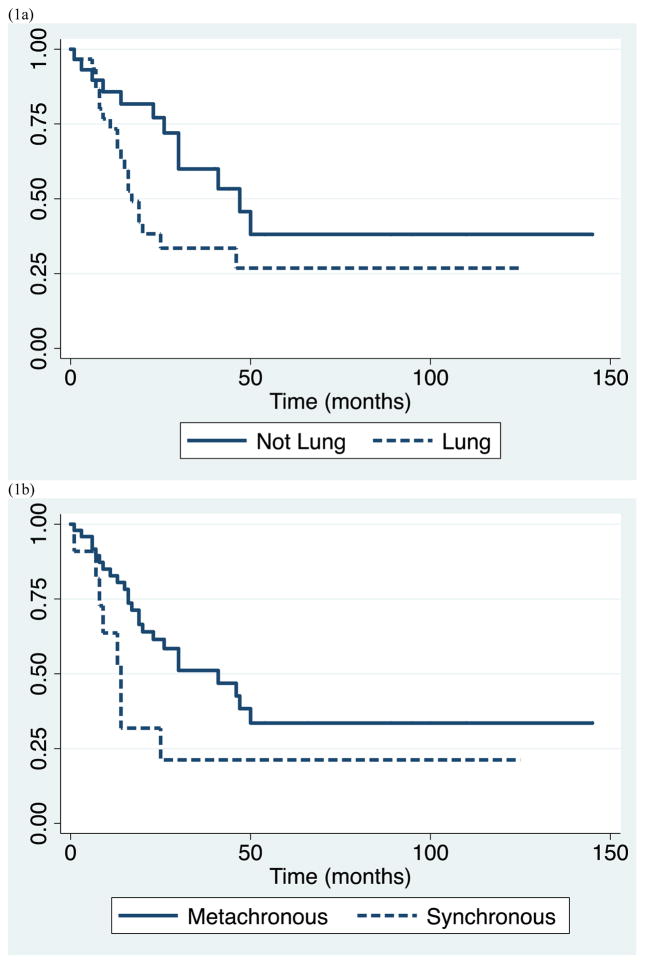
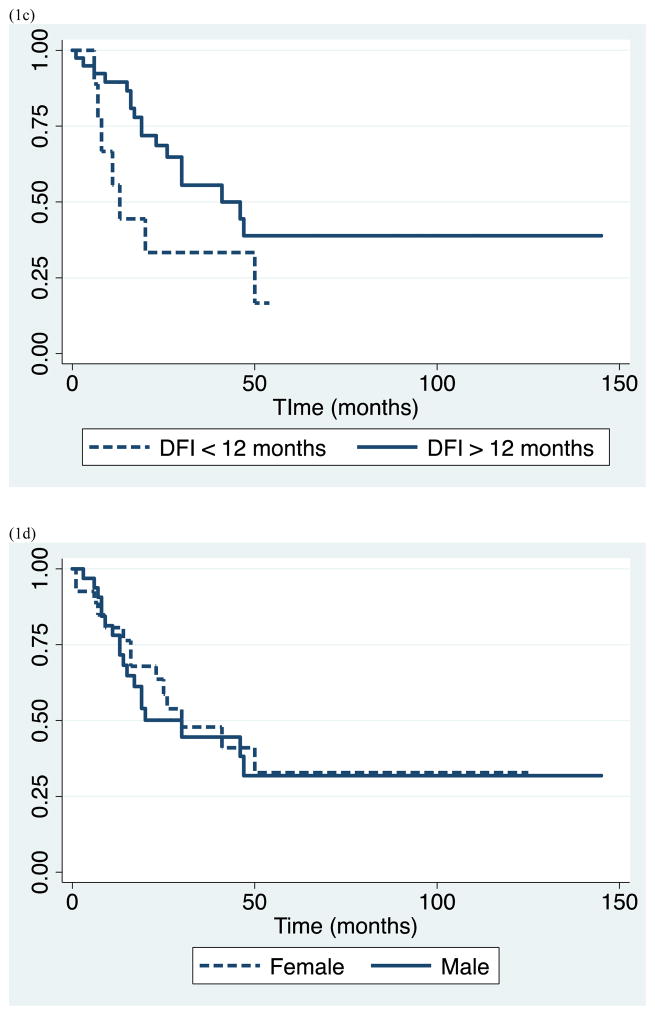
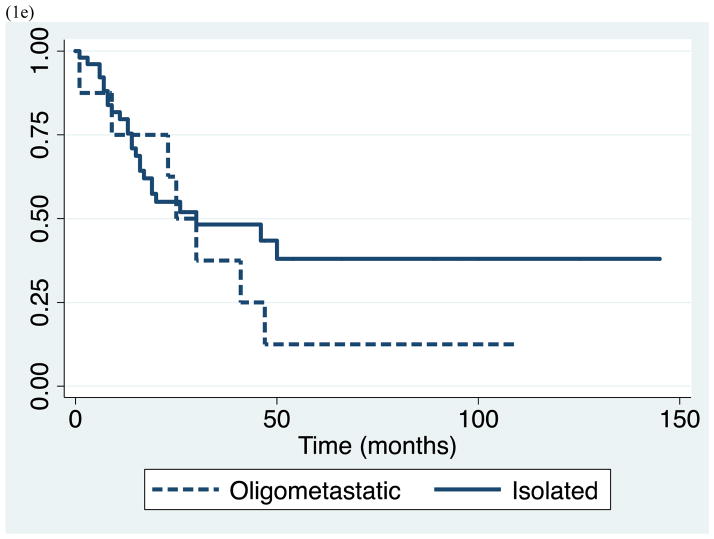
Survival data for the study population as a whole is demonstrated in Table 2. We observed median overall survival of 30 months (range 1–145), and 1-, 3-, and 5-year survival of 79% (95% CI 0.66–0.88), 45% (95% CI 0.31–0.59), and 31% (95% CI 0.17–0.47), respectively. Subsequently, we evaluated clinical parameters for association with survival including: lung primary tumor type, synchronous metastasis, short DFI <12 months, oligometastatic disease, and gender. Patients with lung primary had significantly shorter survival with median survival of 17 months and 5-year survival of 27%, compared to patients with other primary cancers who had median survival of 47 months and 5-year survival of 38% (p=0.033). Similarly, patients who presented with synchronous metastasis had significantly shorter survival than those who presented with metachronous lesions, with median survival of 14 vs. 41 months, and 5-year survival of 21% vs. 34 % (p=0.028). In addition, patients who had DFI <12 months had worse outcome compared with those who had DFI ≥12 months with median survival of 13 vs. 41 months, and 5-year survival of 0% vs. 39% (p=0.038). In contrast, there was no significant difference in survivor functions between patients of different gender, and patients with isolated versus metastatic disease. Patients with oligometastatic disease experienced median survival equivalent to those with isolated disease of 25 months and 5-year survival of 13%. (Table 2 and Figure 1)
Table 2.
Kaplan-Meier survival analysis for study population.
| Overall Survival | N (%) | Median (months) | 1-year | 3-year | 5-year | P value |
|---|---|---|---|---|---|---|
| All1 | 59 (100) | 30 | 79% | 45% | 31% | N/A |
| Lung Primary | 29 (49) | 17 | 73% | 33% | 27% | 0.033 |
| Other Primary | 30 (51) | 47 | 86% | 60% | 38% | |
| Synchronous | 11 (19) | 14 | 64% | 21% | 21% | 0.028 |
| Metachronous | 48 (81) | 41 | 83% | 51% | 34% | |
| DFI <12 months2 | 9 (19) | 13 | 56% | 33% | 0% | 0.038 |
| DFI ≥12 months2 | 39 (81) | 41 | 90% | 56% | 39% | |
| Male | 32 (54) | 30 | 78% | 45% | 32% | 0.69 |
| Female | 27 (46) | 30 | 81% | 48% | 33% | |
| Oligometastatic | 8 (14) | 25 | 75% | 38% | 13% | 0.62 |
| Isolated | 51 (86) | 30 | 80% | 48% | 38% |
Total n excludes 2 perioperative deaths, and 1 patient lost to follow-up.
Proportion includes only patients with metachronous diagnosis (n=48).
Figure 1.
Kaplan-Meier survival estimates for study population by (a) lung vs. other primary malignancy, (b) synchronous vs. metachronous metastasis, (c) disease free interval < 12 vs. ≥ 12 months, (d) gender, and (e) isolated vs. oligometastatic disease.
Discussion
The presence of adrenal metastasis generally portends a poor prognosis as it is most often associated with disseminated, incurable metastatic disease. In these circumstances, surgical resection is likely futile, and patients are referred for palliative and supportive therapy. In contrast, survival associated with resection of adrenal metastasis in the face of isolated or limited extra-adrenal disease is not well characterized. As such, in this study we sought to describe survival in a relatively large series of patients who underwent adrenalectomy with curative intent. In the present investigation, we did not include patients who underwent adrenalectomy for symptomatic pain relief, though there is also evidence of benefit for this indication.12–13 Our primary outcome measure was overall survival, and thus we restricted our analysis to cases in which the intent was to cure as previous studies have demonstrated differential outcome for palliative and curative resections.13–15
Herein, we report overall median and 5-year survival of 30 months and 31% respectively. Similarly, the 2007 Memorial Sloan-Kettering Cancer Center (MSKCC) experience (n=94) which reported identical median and 5-year survival of 30 months and 31%, respectively, also had a large proportion of lung cancer patients (41%), but limited inclusion to patients with isolated adrenal metastasis and excluded those with oligometastatic disease.16 Likewise, a single-institution series of 65 patients from France reported median survival of 48 months and 5-year survival of 45%. These survival figures are higher than those reported in other studies and is likely due in part to the predominance of patients with renal cell carcinoma (35%), who had significantly longer survival compared to other tumor types in both univariable and multivariable analysis (p=0.007 and p=0.009).18
The clinical variables that were associated with shortened survival in our analysis included lung primary, synchronous metastasis, and DFI < 12 months. The adrenal gland is a common site of metastasis from NSCLC and two recent pooled analyses have demonstrated 5-year survival between 25–33% after resection of isolated adrenal lesions.20–21 We found that patients with NSCLC had median survival 17 months and 5-year survival of 27%. Though this compares more favorably to the dismal prognosis typically associated with stage IV NSCLC, patients with primary non-lung cancers had longer median and survival of 47 months and 5-year survival of 38% (p=0.033). This is corroborated by some, but not by others.14–15, 18–19 One potential theory that may partially explain the discordance relates to the pathophysiology of lung cancer spread to the adrenal glands. The lymphatic drainage between the lung and retroperitoneum has been well described and it is postulated by some that ipsilateral isolated adrenal metastasis may occur through this mechanism, and thus represent a less aggressive form of regional spread.22 In contrast, contralateral adrenal metastasis occur through the hematogenous route, and therefore reflect more aggressive, distant dissemination.20, 22 Our study did not examine this specifically and the hypothesis is speculative at this time.
Our patients who presented with synchronous metastasis also had significantly shorter survival compared to those with metachronous disease. The exception was a 40-year old woman with simultaneous diagnosis of NSCLC and an isolated adrenal metastasis. Following resection of both, her survival time from adrenalectomy to last follow-up was 125 months. In theory, patients with metachronous disease could be viewed as having more indolent tumors because detection of metastasis occurs at least 6 months after initial cancer diagnosis.
Similar reasoning would suggest that longer DFI for metachronous lesions would also portend improved prognosis if DFI represents a surrogate for tumor aggressiveness. However, there has been conflicting evidence with respect to this factor likely due in part to the significant heterogeneity in the definition and cut-off length of DFI between individual studies.7, 14, 16–17 Typically, as is the case in this study, DFI is defined as the time interval between primary diagnosis and detection of adrenal metastasis without taking into account other treated metastasis. The only study that utilized a strict definition of DFI as the time period the patient was actually tumor-free prior to recognition of adrenal metastasis, reported that DFI >12 months was a positive prognostic factor with mean survival of 57 months compared with 21 months (p=0.028). We also found that patients with metachronous disease and DFI ≥12 months had significantly longer median survival compared to those with DFI < 12 months (41 vs. 13 months), and further, that no patients with DFI <12 months were alive at 5-years whereas those with DFI >12 months had 39% survival at 5 years.
Of particular note, there was no significant difference between patients with oligometastatic disease and isolated adrenal metastasis with median survival of 25 and 30 months, respectively. 15% of our study cohort included patients with oligometastatic disease who had resection of their primary cancer, and had limited, well-controlled extra-adrenal metastasis that were amenable to concurrent curative resection or definitive non-surgical therapy. This finding is consistent with Zerrweck et al, who found no differences in survival between the patients with and without extra-adrenal metastasis. Up to one-third of their patients had oligometastatic disease that was either resected simultaneously or prior to adrenalectomy, and the comparable survival observed in this group supported the fact that adrenal metastasectomy could still be indicated in patients with advanced, but controlled disease burden.18 As the options for safe adrenalectomy and extirpation of oligometastatic disease expands, careful patient selection and understanding of disease progression becomes increasingly important and can be facilitated by a multidisciplinary approach.
Our study has a few notable limitations. First, we did not incorporate the use of systemic therapy into our analysis. This was primarily due to the heterogeneity of primary tumor types represented in our study, and resultant variability and efficacy of chemotherapy regimens that may also often be administered outside of our institution. Second, we focus solely on survival as our primary endpoint and do not specifically examine disease recurrence after adrenalectomy. The frequency of recurrence and time-to-recurrence in this selected patient population is of particular interest to surgeons in assessing the appropriateness of resection, however, the ability to accurately provide this information in a retrospective medical record review is limited. Finally, there is inherent selection bias. Undoubtedly, patients who underwent adrenalectomy were selected for aggressive surgical therapy based on some combination of favorable tumor characteristics and good performance status. It can be argued that the long-term survival observed in these patients is reflective of their underlying tumor biology and not the surgical resection. The only way to overcome this obstacle is to perform a randomized trial comparing observation to surgical resection, but given the low occurrence of isolated adrenal metastasis, this is likely not feasible. This limitation was partially addressed by Vazquez et al, who compared their retrospective series of 166 patients to Surveillance Epidemiology and End Results (SEER) database stage-matched controls that underwent primary resection without resection of distant adrenal disease. Comparisons to SEER data were made for patients with sarcoma, kidney, pancreatic and lung primaries, and adrenalectomy improved survival at 1, 2, and 3 years in patients with all tumor types. Further, their study patients overall had median survival of 26 months and 5-year survival rate of 31%, which is comparable to our findings.
In summary, we report survival data for our institutional experience of patients who underwent adrenal metastasectomy with curative intent. Importantly, we did not find shorter survival in association with oligometastatic disease provided the primary cancer and additional metastatic lesions were adequately controlled and amenable to resection. Further, our analysis suggests that patients with a lung primary, short DFI <12 months, and synchronous disease fare worse, and thus, more cautious consideration of resection should be undertaken in these groups of patients.
Synopsis.
Adrenal metastasectomy performed with curative intent resulted in median survival of 30 months and 5-year survival of 31% in our single institution experience. Shorter survival was associated with lung primary, short disease-free interval, and synchronous metastasis.
References
- 1.Abrams HL, Spiro R, Goldstein N. Metastasis in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Lam KY, Lo CY. Metastatic tumors of the adrenal glands: a 30-year experience in a teaching hospital. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 3.Sancho JJ, Triponez F, Montet X, et al. Surgical management of adrenal metastases. Langenbecks Arch Surg. 2012;397:179–194. doi: 10.1007/s00423-011-0889-1. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell IC, Nwariaku FE. Adrenal masses in the cancer patient: surveillance of excision. Oncologist. 2007;12:168–74. doi: 10.1634/theoncologist.12-2-168. [DOI] [PubMed] [Google Scholar]
- 5.Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996;62:1614–16. doi: 10.1016/s0003-4975(96)00611-x. [DOI] [PubMed] [Google Scholar]
- 6.Pfannschmidt J, Schlolaut B, Muley T, et al. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer. 2005;49:203–207. doi: 10.1016/j.lungcan.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Porte H, Siat J, Guibert B, et al. Resection of adrenal metastases from non-small cell lung cancer: A multicenter study. Ann Thorac Surg. 2001;71:981–5. doi: 10.1016/s0003-4975(00)02509-1. [DOI] [PubMed] [Google Scholar]
- 8.Raz DJ, Lanuti M, Gaissert H, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg. 2011;92:1788–93. doi: 10.1016/j.athoracsur.2011.05.116. [DOI] [PubMed] [Google Scholar]
- 9.Mourra N, Hoeffel C, Duvillard P, et al. Adrenalectomy for clinically isolated metastasis from colorectal carcinoma: Report of eight cases. Dis Colon Rectum. 2008;51:1846–49. doi: 10.1007/s10350-008-9235-2. [DOI] [PubMed] [Google Scholar]
- 10.Collinson FJ, Lam TK, Bruihn WMJ, et al. Long-term survival and occasional regression of distant melanoma metastases after adrenal metastasectomy. Ann Surg Oncol. 2008;15:1741–49. doi: 10.1245/s10434-008-9836-y. [DOI] [PubMed] [Google Scholar]
- 11.Haigh PI, Essner R, Wardlaw JC, et al. Long-term survival after complete resection of melanoma metastatic to the adrenal gland. Ann Surg ONcol. 1999;6:633–39. doi: 10.1007/s10434-999-0633-z. [DOI] [PubMed] [Google Scholar]
- 12.Zeiger MA, Thompson GB, Duh Q-Y, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas. Endocrine Practice. 2009;15(Suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 13.Lo CY, vanHeerden JA, Soreide JA, et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83:528–31. doi: 10.1002/bjs.1800830432. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez BJ, Richards ML, Lohse CM, et al. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg. 2012;36:1400–1405. doi: 10.1007/s00268-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 15.Muth A, Persson F, Jansson S, et al. Prognostic factors for survival after surgery for adrenal metastasis. EJSO. 2010;36:699–704. doi: 10.1016/j.ejso.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Strong VE, D’Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14:3392–3400. doi: 10.1245/s10434-007-9520-7. [DOI] [PubMed] [Google Scholar]
- 17.Sarela AI, Murphy I, Coit DG, et al. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol. 2003;10:1191–6. doi: 10.1245/aso.2003.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Zerrweck C, Caiazzo R, Clerquin B, et al. Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann Surg Oncol. 2012;19:3621–6. doi: 10.1245/s10434-012-2464-6. [DOI] [PubMed] [Google Scholar]
- 19.Valeri A, Bergamini C, Tozzi F, et al. A multi-center study on the surgical management of metastatic disease to adrenal glands. J Surg Oncol. 2011;103:400–405. doi: 10.1002/jso.21843. [DOI] [PubMed] [Google Scholar]
- 20.Beitler AL, Urschel JD, Velagapudi SRC, et al. Surgical management of adrenal metastases from lung cancer. J Surg Oncol. 1998;69:54–57. doi: 10.1002/(sici)1096-9098(199809)69:1<54::aid-jso11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26:1142–47. doi: 10.1200/JCO.2007.14.2091. [DOI] [PubMed] [Google Scholar]
- 22.Karolyi P. Do adrenal metastases from lung cancer develop by lymphogenous or hematogenous route? J Surg Oncol. 1990;43:154–6. doi: 10.1002/jso.2930430306. [DOI] [PubMed] [Google Scholar]