Abstract
Background:
Few reoperations are required in older patients undergoing multilevel lumbar laminectomy with noninstrumented fusions for spinal stenosis with/without spondylolisthesis/instability, and they rarely require instrumentation.
Methods:
We reviewed 336 patients averaging 66.5 years of age undergoing initial average 4.7 level lumbar laminectomies with average 1.4 level noninstrumented fusions over an average 7.1-year period (range 2.0–16.5 years). Patients uniformly exhibited spinal stenosis, instability (Grade I [195 patients] or Grade II spondylolisthesis [67 patients]), disc herniations (154 patients), and/or synovial cysts (66 patients). Reoperations, including for adjacent segment disease (ASD), addressed new/recurrent pathology.
Results:
Nine (2.7%) of 336 patients required reoperations, including for ASD, an average of 6.3 years (range 2–15 years) following initial 4.7 level laminectomies with 1.4 level noninstrumented fusions. Second operations warranted average 4.8 level (range 3–6) laminectomies and average 1.1 level non instrumented fusions addressing stenosis with instability (Grade I [7 patients] or Grade II [1 patient] spondylolisthesis), new disc herniations (2 patients), and/or a synovial cyst (1 patient).
Conclusions:
Only 9 (2.7%) of 336 patients required reoperations (including for ASD) consisting of multilevel laminectomies with noninstrumented fusions for recurrent/new stenosis even with instability; these older patients were not typically unstable, or were likely already fused, and did not require instrumentation. Alternatively, reoperation rates following instrumented fusions in other series approached 80% at 5 postoperative years. Therefore, we as spinal surgeons should realize that older patients even with instability rarely require instrumentation and that the practice of performing instrumented fusions in everyone, irrespective of age, needs to stop.
Keywords: Adjacent segment disease, diskectomy, laminectomy, low reoperation rate, lumbar surgery, multilevel laminectomy, noninstrumented fusion, spondylolisthesis, synovial cysts
INTRODUCTION
Multilevel lumbar laminectomies with 1–2 level noninstrumented fusions with/without spondylolisthesis, offer a myriad of advantages particularly for geriatric patients with more comorbid risk factors including osteoporosis. The lack of instrumentation not only avoids implant loosening but also should markedly reduce the frequency of reoperations, including for adjacent segment disease (ASD). In Park et al., the risk of reoperations largely for ASD following no fusion/noninstrumented fusions (e.g., without pedicle screw instrumentation) was 5.2–5.6% (44.8 months postoperatively) whereas ASD after transpedicular fusions occurred in 12.2–18.5% of cases (average 164 months postoperatively).[17] In this series, of 336 patients undergoing initial multilevel laminectomies with noninstrumented fusions over an average of 7.1 postoperative years, we evaluated the frequency of reoperations for spinal stenosis, including those for ASD, with/without spondylolisthesis/instability, disc herniations, and/or synovial cysts. These data were then contrasted with the published reoperation rates for multilevel laminectomies with various types of instrumented fusions, including those for ASD, which approaches in one study 9.9% at 1 year and up to 80% at 5 years.[16] Our working hypothesis was that most older patients (average age in this series was 72.4 with a range of 41–84) are not very unstable and/or may already be fused and that the majority with stenosis and instability attributed to spondylolisthesis would fuse without instrumentation.
MATERIALS AND METHODS
Data from 336 patients undergoing multilevel laminectomy with noninstrumented fusions were collected over a 16.5-year period [Table 1]. Patients averaged 72.4 years of age, with a range of 41–84 years old. The largest number of patients were between the ages of 70–74 (77 patients) followed by 65–69 and 75–79 (58 patients each). Overall in our series, there were more females (211 females) than males (47 males). Average initial 4.7 level laminectomies (range 2–10 levels) were performed, with the majority undergoing 5 (100 patients) and 6 level (90 patients) decompressions (e.g., L2–S1 or L1–S1) [Table 1]. Average 1.4 level noninstrumented fusions accompanied multilevel laminectomies; most were performed for Grade I (195 patients) spondylolisthesis whereas the remainder either exhibited no olisthy or had Grade II spondylolisthesis [Table 1]. Additional pathology included; routine disc herniations (93 patients), foramina/far lateral discs (51 patients), and synovial cysts (66 patients). During this period, the posterolateral fusion mass included lamina autograft and the use of different bone graft supplements; Inductive Conductive Matrix (Medtronic, Memphis, TN, USA: 72 patients), Vitoss (OrthoVita, Malverne, PA, USA, 213 patients), nanOss Bioactive (Regeneration Technologies, Inc., Alachua, FL, USA; 51 patients).
Table 1.
336 patients undergoing multilevel laminectomies/noninstrumented fusions

RESULTS
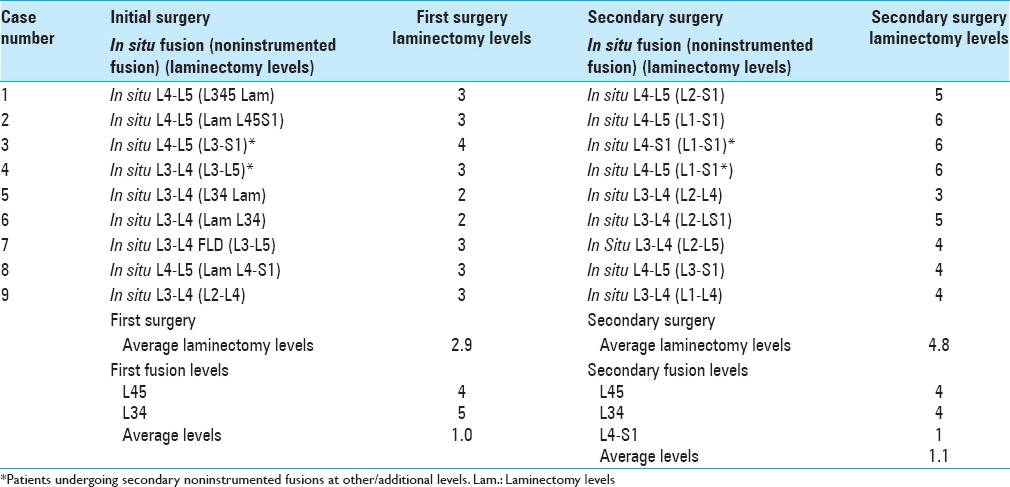
Nine (2.7%) patients required reoperations (including some for ASD) an average of 6.3 years after initial lumbar laminectomies with noninstrumented fusions [Tables 2 and 3]. They averaged 72.4 years of age when undergoing reoperations, an average of 6.3 years following initial surgery. They underwent initial average 2.9 level laminectomies (range 2–4 levels); secondary reoperations included a subset with ASD, as they typically required more extensive secondary average 4.8 level laminectomies (range 3–6 levels). Initially, these nine patients also underwent average 1.0 level noninstrumented fusions; secondary noninstrumented fusions required a slightly higher average 1.1 levels. Original fusions involved the L3–L4 (5 patients) and L4–L5 (4 patients) levels, whereas secondary fusions involved the L3–L4 (4 patients), L4–L5 (4 patients), and the L5–S1 levels (1 patient) [Tables 2 and 3]. Therefore, secondary noninstrumented fusions addressed the same levels in seven patients whereas these levels differed in only two patients. One patient who originally had an L4–L5 noninstrumented fusion required a secondary L4–L5/L5S1 fusion. A second patient who initially required an L3–L4 noninstrumented fusion required a secondary L4–L5 noninstrumented fusion.
Table 2.
Clinical data for nine patients requiring secondary noninstrumented fusions
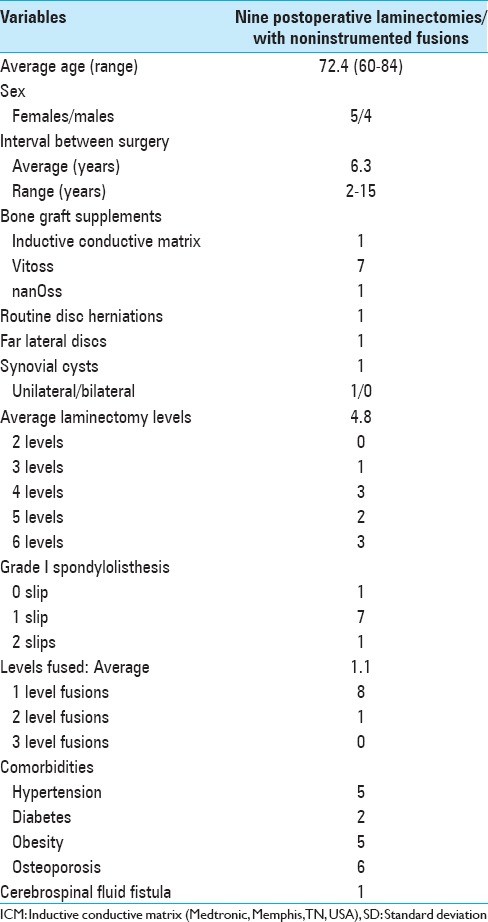
Table 3.
Nine reoperations following multilevel laminectomies with in situ/noninstrumented fusions
DISCUSSION
Summary of study
This study documented a very low 2.7% (9 patients) incidence of reoperations following 336 multilevel laminectomies with noninstrumented fusions performed in largely older patients averaging 72.4 years of age; the nine patients undergoing secondary surgery averaged 66.3 years of age [Tables 1 and 3]. For these nine patients, first and second operations, respectively, required an average of 2.9 and 4.8 level laminectomies/decompressions, and an average, respectively, of 1.0 and 1.1 level noninstrumented fusions. Additional secondary pathology included Grade I (7 patients) or Grade II (1 patient) spondylolisthesis/instability, new disc herniations (2 patients), and/or a synovial cyst (1 patient). These patients were clearly not very unstable, and/or many were already spontaneously fused; therefore, no instrumented fusions were warranted. In contrast, the literature documents a higher reoperation rate for patients undergoing a variety of instrumented fusions, including for ASD that approach 80% at 5 postoperative years.[17]
Better outcomes and low reoperation rates for lumbar laminectomy with/without predominantly noninstrumented fusions for spinal stenosis
Improved outcomes and low reoperation rates were documented for patients undergoing lumbar laminectomy with/without predominantly noninstrumented fusions for spinal stenosis.[5,6,19] Weinstein et al. in 2009 (Spine Patient Outcomes Research Trial [SPORT]) evaluated 4-year postoperative results for patients with degenerative spondylolisthesis (DS)/spinal stenosis treated with decompressive laminectomy (with or without fusion) versus nonoperative care.[19] They concluded those “…treated surgically maintain substantially greater pain relief and improvement in function for 4 years.” In 2015, Bydon et al. determined that over 4 postoperative years, reoperations were warranted for ASD in 10% of a single level and 9% of two level laminectomies performed without fusions for degenerative disease.[6] Of interest, secondary surgery required laminectomy (95%), discectomy (26%), with nearly half (49%) warranting fusion. In a second 2015 study, Bydon et al. cited an overall 5.6% complication rate and 14.4% reoperation rate (72 patients) for 500 patients undergoing initial 1–3 level laminectomies without fusions for degenerative lumbar disease (average 46.79 months).[5] Reoperations, occurring an average of 3.40 years later, required secondary decompressions only in 55.56% of patients, but also required a 44.44% incidence of decompressions with posterolateral fusions.
Low reoperation rates for noninstrumented versus high reoperation rates for instrumented fusions
Reoperation rates following noninstrumented lumbar fusions are typically lower than for instrumented fusions.[8,9,11,16,17,18] In 2004, Park et al. documented a low reoperation rate (e.g., 5.2–5.6%), including for ASD, following laminectomies with/without noninstrumented fusions (patients followed an average of 44.8 postoperative months) versus a higher 12.2–18.5% reoperation rate for instrumented fusions (followed 164 months).[17] In 2007 and 2008 studies, Epstein contrasted fusion rates for multilevel laminectomies performed with/without instrumentation.[8,9] Despite higher two-dimensional computed tomography documented fusion rates with instrumentation (e.g., 92.6% (1 level) and 91.2% [2 levels], respectively), the noninstrumented 17.3% pseudarthrosis rate led to only 1 (1.3% out of 75 patients) reoperation. When Santiago-Dieppa et al. in 2014 evaluated 376 patients undergoing noninstrumented lumbar fusions over an average 92-month postoperative period, although the rate of ASD was 18.35% (69 patients), the rate of reoperation due to failure to improve/worsening was 30.59%.[18] They attributed this to the need to perform more primary instrumented fusions but failed to provide adequate documentation to support this hypothesis. Notably, in 2015, Nakashima et al. performed 101 posterior lumbar interbody fusion (PLIF), and documented a 9.9% reoperation rate for ASD at 1 postoperative year, and a staggering 80% reoperation rate, again largely for ASD, at 5 postoperative years.[16]
Comparable or improved outcomes with noninstrumented versus instrumented fusions
Multiple studies document comparable or improved outcomes following noninstrumented versus instrumented fusions.[1,10,11,20] In 1997, Katz et al. variously managed patients with degenerative lumbar stenosis utilizing laminectomy alone (194 patients), laminectomy/noninstrumented fusion (37 patients), and laminectomy/instrumented fusions (41 patients) (followed an average of 6–24 months); although noninstrumented fusions correlated with better relief of low back pain, there were no other significant differences between any of the treatment groups.[11] In 2003, Jäger et al. concluded that comparable results were obtained after performing 16 single-level noninstrumented versus 17 instrumented posterolateral fusions in elderly patients with degenerative lumbar instability; patients in both groups improved 86.6% of the time.[10] In 2009, Abdu et al. compared outcomes for different fusion methods to treat DS over a 4-year period studying 380 patients from the SPORT database.[1] Patients underwent decompressive laminectomy with posterolateral in situ fusion (21%: Posterolateral lumbar fusion [PLF]: 80 patients), posterolateral instrumented fusion/pedicle screws (PPS) (56%; PPS: 213 patients), PPS plus interbody fusion (17%: 63 patients: 360°), or laminectomies alone (6%); they observed “no consistent differences in clinical outcomes were seen among fusion groups over 4 years.”[1] Ye et al. in their 2014 meta-analysis assessed the efficacy of lumbar noninstrumented versus instrumented fusions; they found “…the inclusion of fusion surgery with instrumentation provided no benefit as evaluated by patient-reported outcomes in patients with lumbar spondylolisthesis.”[20]
Comparable 1–2 level fusion rates for noninstrumented versus instrumented fusion
Comparable 1–2 level fusion rates have been reported utilizing either noninstrumented versus instrumented fusions.[3,14] In 1993, McGuire and Amundson found that 72% of patients undergoing in situ posterolateral arthrodesis versus 78% having internal stabilization (Steffee plate/screws) fused.[14] In 1994, Axelsson et al. documented solid fusion in 76% of patients undergoing noninstrumented PLF, particularly at a single level, including for low-grade spondylolysis-olisthesis.[3]
Instrumented fusions, especially with interbody devices, correlate with high reoperation, and complication rates versus noninstrumented fusions
Higher reoperation and complication rates were reported for instrumented fusions, particularly utilizing interbody devices versus noninstrumented fusions.[2,4,7,13,15,16] In 2009, Andersen et al. documented higher fusion rates (81% of 43 patients) for instrumented fusions versus posterolateral noninstrumented (68% of 51 patients) over a 2–7-year duration.[2] However, more patients (9 patients [20.9%]) undergoing instrumented versus those (6 patients [11.8%]) undergoing noninstrumented fusions required reoperations for ASD that correlated with poorer outcomes. In 2011, over a 5-year period, Mehta et al. documented the relatively high risk of durotomy (17% PLIF, 9% transforaminal lumbar interbody fusion [TLIF]) and nerve root injury (7.8% PLIF, 2% TLIF) occurring in 119 patients undergoing instrumented fusions utilizing interbody devices (TLIF [37% patients] and PLIF [63% patients]) addressing degenerative spinal disease or spondylolisthesis.[15] The authors concluded that “TLIF and PLIF should only be considered when the goals of surgery cannot be addressed with decompression and traditional posterolateral fusion.” Chaichana et al. in 2015 evaluated the risk of infection following 817 consecutive lumbar spinal instrumented fusions performed for degenerative lumbar disease; 37 patients (4.5%) developed postoperative infections at an average of 0.6 months.[7] Of these, 21 (57%) required reoperations. Without instrumentation, the overall infection rate would likely have been reduced, and there would have been fewer reoperations including the need for instrumentation removal. In 2015, Bydon et al. studied the safety and efficacy for older patients undergoing 1395 instrumented PLF.[4] Using The American College of Surgeons National Surgical Quality Improvement Program, they documented an overall 30-day complication rate of 11.47%; 9.04% for those under 65, and 14.05% for patients older than 65. They rightly concluded patients over 65 had significantly higher complication rates after lumbar fusions versus younger patients. In 2015, Nakashima et al. evaluated 101 patients undergoing PLIF; 10 (9.9%) patients required second operations for ASD at 1 postoperative year, whereas 80% required additional surgery for ASD at 5 postoperative years.[16] In 2015, Macki et al. evaluated whether better outcomes could be achieved in 103 patients with DS utilizing instrumented posterolateral fusion (PLF: 43.69%) alone versus instrumented posterolateral fusion PLF with an interbody device (PLF + PLIF/TLIF: 56.31%).[13] Interestingly, those undergoing PLF alone exhibited a greater clinical improvement compared with patients having PLF + PLIF/TLIF (with interbody devices) despite higher reoperation, pseudoarthrosis, and instrumentation failure rates.
Noninstrumented fusions are more cost effective than instrumented fusions
In 2000, Kuntz et al. determined the average cost of noninstrumented fusions ($56,500 per quality-adjusted year of life [QALY]) versus instrumented fusions (QALY was $82,400); although instrumented fusions cost more, there were no clear-cut clinical advantages or documented “value added” for these procedures.[12]
SUMMARY
This study documented a 2.7% (9 patients) incidence of reoperations, including some for ASD, following 336 multilevel lumbar laminectomies with noninstrumented 1–2 level fusions for spinal stenosis with/without instability. These older patients, averaging 72.4 years of age (most age 65–79) with spondylolisthesis/instability and were not very unstable or were sufficiently spontaneously fused that no instrumented fusions were warranted [Table 1]. Yet the reoperation rates for instrumented fusions, including ASD in some series, range up to 80% at 5 postoperative years, and carry higher risks and complication rates, without better clinical outcomes.[16] It is time to highlight the various advantages of noninstrumented over instrumented fusions, and to reexamine why we as spinal surgeons choose to fuse, what we use, and both when and how we fuse it. Certainly, for the vast majority of older patients with instability attributed to spondylolisthesis, adequate fusion may be achieved without the addition of instrumentation; this offers surgeons a safer, better, but less profitable alternative.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Abdu WA, Lurie JD, Spratt KF, Tosteson AN, Zhao W, Tosteson TD, et al. Degenerative spondylolisthesis: Does fusion method influence outcome.Four-year results of the spine patient outcomes research trial? Spine (Phila Pa 1976) 2009;34:2351–60. doi: 10.1097/BRS.0b013e3181b8a829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen T, Christensen FB, Niedermann B, Helmig P, Høy K, Hansen ES, et al. Impact of instrumentation in lumbar spinal fusion in elderly patients: 71 patients followed for 2-7 years. Acta Orthop. 2009;80:445–50. doi: 10.3109/17453670903170505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelsson P, Johnsson R, Strömqvist B, Arvidsson M, Herrlin K. Posterolateral lumbar fusion.Outcome of 71 consecutive operations after 4 (2-7) years. Acta Orthop Scand. 1994;65:309–14. doi: 10.3109/17453679408995459. [DOI] [PubMed] [Google Scholar]
- 4.Bydon M, Abt NB, De la Garza-Ramos R, Olorundare IO, McGovern K, Sciubba DM, et al. Impact of age on short-term outcomes after lumbar fusion: An analysis of 1395 patients stratified by decade cohorts. Neurosurgery. 2015;77:347–53. doi: 10.1227/NEU.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 5.Bydon M, Macki M, Abt NB, Sciubba DM, Wolinsky JP, Witham TF, et al. Clinical and surgical outcomes after lumbar laminectomy: An analysis of 500 patients. Surg Neurol Int. 2015;6(Suppl 4):S190–3. doi: 10.4103/2152-7806.156578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bydon M, Macki M, De la Garza-Ramos R, McGovern K, Sciubba DM, Wolinsky JP, et al. Incidence of adjacent segment disease requiring reoperation after lumbar laminectomy without fusion: A study of 398 patients. Neurosurgery. 2016;78:192–9. doi: 10.1227/NEU.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 7.Chaichana KL, Bydon M, Santiago-Dieppa DR, Hwang L, McLoughlin G, Sciubba DM, et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. Clin Neurol Neurosurg. 2015;138:117–23. doi: 10.3171/2013.10.SPINE1364. [DOI] [PubMed] [Google Scholar]
- 8.Epstein NE, Epstein JA. SF-36 outcomes and fusion rates after multilevel laminectomies and 1 and 2-level instrumented posterolateral fusions using lamina autograft and demineralized bone matrix. J Spinal Disord Tech. 2007;20:139–45. doi: 10.1097/01.bsd.0000211261.36120.3e. [DOI] [PubMed] [Google Scholar]
- 9.Epstein NE. Fusion rates and SF-36 outcomes after multilevel laminectomy and noninstrumented lumbar fusions in a predominantly geriatric population. J Spinal Disord Tech. 2008;21:159–64. doi: 10.1097/BSD.0b013e318074ddaa. [DOI] [PubMed] [Google Scholar]
- 10.Jäger M, Seller K, Raab P, Krauspe R, Wild A. Clinical outcome in monosegmental fusion of degenerative lumbar instabilities: Instrumented versus non-instrumented. Med Sci Monit. 2003;9:CR324–7. [PubMed] [Google Scholar]
- 11.Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis.Patient selection, costs, and surgical outcomes. Spine (Phila Pa 1976) 1997;22:1123–31. doi: 10.1097/00007632-199705150-00012. [DOI] [PubMed] [Google Scholar]
- 12.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976) 2000;25:1132–9. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Macki M, Bydon M, Weingart R, Sciubba D, Wolinsky JP, Gokaslan ZL, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–23. doi: 10.1016/j.clineuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 14.McGuire RA, Amundson GM. The use of primary internal fixation in spondylolisthesis. Spine (Phila Pa 1976) 1993;18:1662–72. doi: 10.1097/00007632-199309000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Mehta VA, McGirt MJ, Garcés Ambrossi GL, Parker SL, Sciubba DM, Bydon A, et al. Trans-foraminal versus posterior lumbar interbody fusion: Comparison of surgical morbidity. Neurol Res. 2011;33:38–42. doi: 10.1179/016164110X12681290831289. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima H, Kawakami N, Tsuji T, Ohara T, Suzuki Y, Saito T, et al. Adjacent segment disease after posterior lumbar interbody fusion: Based on cases with a minimum of 10 years of follow-up. Spine (Phila Pa 1976) 2015;40:E831–41. doi: 10.1097/BRS.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 17.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976) 2004;29:1938–44. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 18.Santiago-Dieppa D, Bydon M, Xu R, De la Garza-Ramos R, Henry R, Sciubba DM, et al. Long-term outcomes after non-instrumented lumbar arthrodesis. J Clin Neurosci. 2014;21:1393–7. doi: 10.1016/j.jocn.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis.Four-year results in the spine patient outcomes research trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91:1295–304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye YP, Chen D, Xu H. The comparison of instrumented and non-instrumented fusion in the treatment of lumbar spondylolisthesis: A meta-analysis. Eur Spine J. 2014;23:1918–26. doi: 10.1007/s00586-014-3453-1. [DOI] [PubMed] [Google Scholar]


