Abstract
Background:
Surgeons treating metastatic spine disease can use computed tomography (CT) imaging to determine whether lesions are osteolytic, osteoblastic, or mixed. This enables treatment that considers the structural integrity of the vertebral body (VB), which is impaired with lytic lesions but not blastic lesions. The authors analyzed CT imaging characteristics of spine metastasis from breast, lung, prostate, and renal cell carcinomas (RCCs) to determine the metastasis patterns of each of these common tumors.
Methods:
The authors identified patients with metastatic spine disease treated during a 3-year period. Variables studied included age, sex, and cancer type. Lesions from breast, lung, prostate, and RCC primary lesions were selected for imaging analysis.
Results:
Sixty-six patients were identified: 17 had breast metastasis, 14 prostate, 18 lung, and 17 RCC. Breast cancer metastasis involved 33% of VBs with 56%, 20%, and 24% osteolytic, osteoblastic, and mixed, respectively. Prostate cancer metastasis involved 35% of VBs with 14%, 62%, and 24% osteolytic, osteoblastic, and mixed, respectively. Lung cancer metastasis involved 13% of VBs with 64%, 33%, and 3% osteolytic, osteoblastic, and mixed, respectively. RCC metastasis involved 11% of VBs with 91%, 7%, and 2% osteolytic, osteoblastic, and mixed lesions, respectively.
Conclusions:
To improve surgical planning, we advocate the use of CT prior to surgery to evaluate whether spine metastases are osteolytic or osteoblastic. In cases of osteolytic lesions, the concern is of segmental instability requiring reconstruction and the risk for screw pull out should instrumentation be considered. In cases of osteoblastic lesions, surgeons should consider debulking dense bone.
Keywords: Computed tomography, osteoblastic, osteolytic, spine metastasis, surgery
INTRODUCTION
The American Cancer Society recently estimated that cancer affected approximately 1.7 million new patients in the United States in 2013.[2] Over the course of a cancer patient's disease, breast, prostate, lung, and renal cell carcinoma (RCC) patients will experience spine metastases in 73%, 68%, 36%, and 35% of the time.[4,5,7] Over 20% will present with symptoms of back pain, motor weakness, or paraplegia, and surgery may restore the ability to walk and improve overall survival.[6] Metastatic spread to the spine is characterized in three ways: Osteolytic (lytic, destructive), osteoblastic (blastic, constructive), or mixed (both lytic and blastic). Computed tomography (CT) imaging can determine whether lesions are osteolytic, osteoblastic, or mixed. In lytic lesions, structural integrity is often impaired, potentially warranting surgical reconstruction, while blastic lesions incite bone formation and may contribute to cord compression requiring decompression.
In this paper, we analyze the CT imaging characteristics of spine metastasis from breast, lung, and prostate cancers and RCC to determine whether the most common cancers incite lytic, blastic, or mixed patterns of vertebral body (VB) involvement.
METHODS
With institutional review board approval (2009–2012) we retrospectively identified all patients (surgical and nonsurgical) seen by orthopedic and neurosurgical spine surgeons at our institution. Three International Classification of Disease Ninth Edition codes were used as the search criteria: 198.5 (secondary malignancy neoplasm of bone and bone marrow), 733.13 (pathologic fracture of vertebrae), and 238 (neoplasm of uncertain behavior of other and unspecified sites and tissues). Because of the high prevalence of breast, lung, prostate, and RCC lesions, these cancer types were selected for CT imaging analysis. Other clinical data were also recorded (e.g., age, sex, and cancer type).
Radiographic review
CT and magnetic resonance imaging studies of the cervical, thoracic, and lumbar spine were reviewed to count the total number of VBs and the number of VBs involved with metastatic tumor (reviewers were not blinded to pathology). CT was used to determine whether the metastatic lesion was lytic, blastic, or mixed. Lytic lesions appeared destructive, showing loss of both cancellous and cortical bone, and were usually well circumscribed [Figure 1]. Blastic lesions appeared hyperdense, were typically expansile, and had poorly defined borders [Figure 2]. Mixed lesions showed both lytic and blastic characteristics [Figure 3]. Multiple lesions of a similar type in a single VB were counted as only one. When both osteolytic and osteoblastic lesions were present in the same VB in near-equal proportions (e.g., 40/60–50/50), they were counted as one mixed lesion [Figure 3].
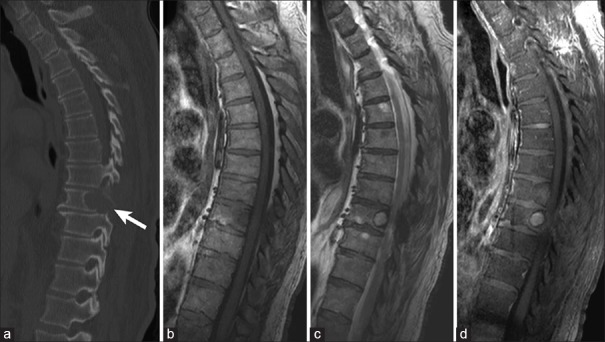
Figure 1.
Example of lytic vertebral body metastasis in a 78-year-old patient with renal cell carcinoma. (a) Sagittal computed tomography image showing mid-thoracic lytic vertebral body metastasis (arrow). Lesion is hypointense on T1-weighted magnetic resonance imaging (b), hyperintense on T2-weighted magnetic resonance imaging (c), and vividly enhances with contrast administration on T1-weighted magnetic resonance imaging (d)
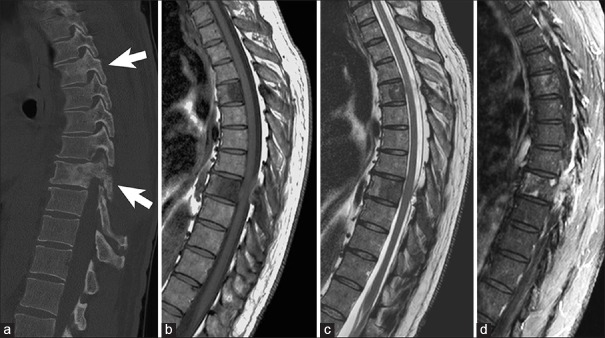
Figure 2.
Example of blastic vertebral body metastasis in a 53-year-old male patient with prostate cancer. (a) Sagittal computed tomography view depicting upper and mid-thoracic blastic vertebral body metastasis (arrows). Lesions are hypointense on T1-weighted magnetic resonance imaging (b), hypointense on T2-weighted magnetic resonance imaging (c), and have patchy contrast enhancement on T1-weighted magnetic resonance imaging (d). Note the blastic anterior and posterior vertebral body resulting in moderate thoracic spinal cord compression
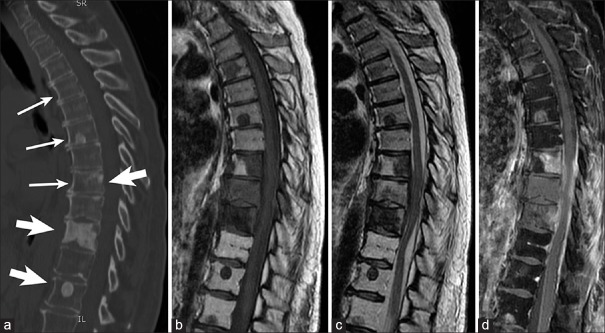
Figure 3.
Sagittal views showing lytic, blastic, and mixed vertebral body metastasis in a 60-year-old patient with breast cancer. (a) Computed tomography depicting upper and mid-thoracic lytic (small arrows), blastic (large arrows), and mixed (small and large arrow) vertebral body metastasis. Lesions are mostly hypointense on T1-weighted magnetic resonance imaging (b), hypointense on T2-weighted magnetic resonance imaging (c), with patchy contrast enhancement on T1-weighted magnetic resonance imaging (d)
RESULTS
Of 1584 VBs counted, 349 (22%) were involved with metastatic tumor with 166 (48%) lytic lesions, 120 (34%) blastic lesions, and 63 (18%) mixed lesions [Table 1 and Figure 4]. Of 66 patients identified, 17 had breast cancer (133 VBs were metastatic, of 408 counted, 33%), 14 had prostate cancer (117 of 336 metastatic VBs, 35%), 18 had lung cancer (55 of 432 metastatic VBs, 13%), and 17 had RCC (44 of 408 metastatic VBs, 11%).
Table 1.
Computed tomography analysis of breast, prostate, lung, and renal cell cancer metastatic to the spine: lytic, blastic, or mixed
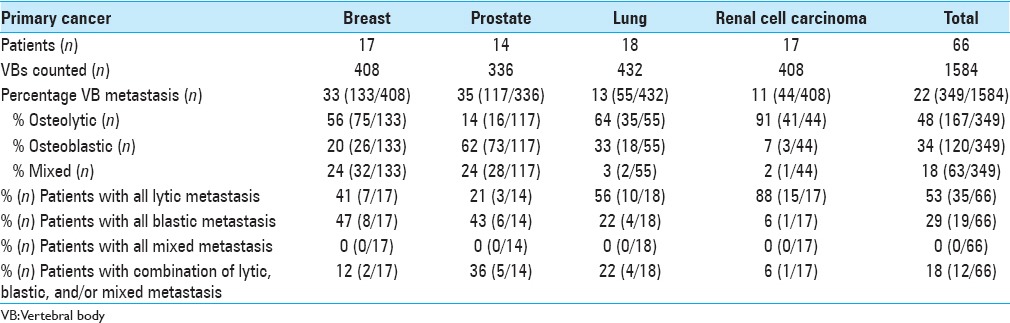
Figure 4.
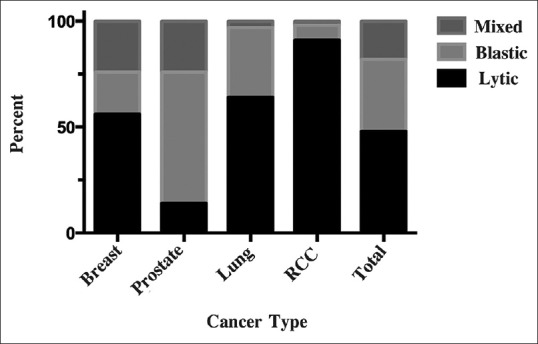
Bar graph depicting proportion of vertebral body metastasis that are lytic, blastic, or mixed for breast, prostrate, lung, renal cell cancer, and all analyzed cancers combined (total)
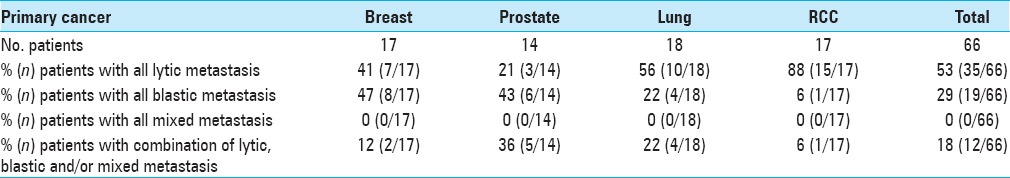
Osteolytic lesions were more common than osteoblastic or mixed in breast cancer (56%; 75/133), lung cancer (64%; 35/55), and especially RCC (91%; 40/44), whereas osteoblastic lesions were more common in prostate cancer (62%; 73/117) [Table 1]. Among the patients, the number of those with purely osteolytic, purely osteoblastic, or mixed lesions was more balanced in those with breast cancer (41 vs. 47 vs. 12%) than in those with other cancer types [Table 2]. The breakdown in RCC patients was especially unbalanced, with 88% having purely osteolytic lesions and just 6% each having osteoblastic or mixed lesions.
Table 2.
Patients with metastatic spine cancer: phenotypic computed tomography (CT) analysis of all lytic, all blastic, all mixed, or combination (lytic, blastic and/or mixed)
DISCUSSION
Surgical considerations: Osteolytic versus osteoblastic
CT scans are used to evaluate whether lesions are osteolytic or osteoblastic prior to surgery. From a surgeon's perspective, lytic and blastic metastatic lesions must be managed in dramatically different ways. Lytic lesions are associated with loss of bone and structural integrity with a risk of pathologic fracture, necessitating preoperative consideration for stabilization (e.g., graft reconstruction and planning fixation for screws).[3] In contrast, blastic lesions incite bone formation and instrumentation will likely not loosen or fail. The importance of knowing the bone quality of the metastatic lesion is further underscored by studies on osteolytic spine metastasis showing just a 2.3% risk of pathologic fracture when <50% of the cortical bone is involved, but an 80% risk of pathologic fracture when >75% of the cortical bone is involved.[3]
Bone and spine metastases: Osteolytic, osteoblastic, and mixed lesion rates
Cancer metastasizes to the spine via hematogenous, lymphatic, or direct bone invasion. In general, a patient's primary cancer can predict the prevalence of either osteolytic or osteoblastic lesions, but this has not previously been specifically evaluated in metastases to the spine. In the metastatic spine series we present, breast cancer showed a tendency toward osteolytic lesions (56%), with nearly equal lower distribution of osteoblastic and mixed (20% and 24%, respectively) [Table 1]. These results mirror whole-skeleton metastatic studies showing a predominance of lytic lesions (75–90%) with 10–25% blastic and 15% mixed activity.[1]
The prostate metastasis spine findings show a lower rate of osteoblastic lesions (62%) and higher proportion of mixed lesions (24%) compared with whole-body surveys (90–100% osteoblastic),[7,8] although combining our 24% mixed and 62% blastic lesion rates yielded a total 86% osteoblastic response rate, which more closely mirrors the higher osteoblastic rate stated by others.[7,8]
The patients in our study with lung cancer showed a significant tendency toward osteolytic lesions (64%), with 33% lytic, and with a very small number of mixed lesions (3%). Although there is not much by way of lesion statistics for lung cancer in the literature, this appears to correspond with the notation of “preference for” and “tending towards osteolytic activity” in other studies.[7]
Patients with RCC demonstrated a 91% osteolytic, 7% blastic, and 2% mixed rates, which are consistent with whole-body studies showing osteolytic rates of 71–90%, osteoblastic rates of 10–18%, and mixed rates of 11%.[8]
Surgeons making management decisions with patients who have metastatic lesions to the spine from distant sites benefit from recognizing the form of metastasis, which can have a significant effect on the structural integrity of the VBs. As blastic lesions tend to stimulate bone formation that can add to cord compression, decompression may be the key surgical goal. In contrast, with lytic lesions, surgical reconstruction may be most important to manage bone loss and potential for pathologic fracture.
CONCLUSIONS
Surgeons treating metastatic spine disease can radiographically assess the spine with CT imaging to determine whether lesions are osteolytic, osteoblastic, or mixed. We advocate the use of CT scans prior to surgery so the surgeon can plan accordingly. We believe the findings we present will enable surgeons to improve surgical planning decisions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Shirley McCartney, Ph.D. and Kristin Kraus, M.Sc., for editorial assistance, and Andy Rekito, M.S., for figure assistance.
Footnotes
Contributor Information
Justin A. Reddington, Email: reddingtonj@ohsu.edu.
Gustavo A. Mendez, Email: mendezg@ohsu.edu.
Alex Ching, Email: alexcching@gmail.com.
Charlotte Dai Kubicky, Email: charlottedai@gmail.com.
Paul Klimo, Jr., Email: atomkpnk@yahoo.com.
Brian T. Ragel, Email: ragelb@gmail.com.
REFERENCES
- 1.Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P. Biology of breast cancer bone metastasis. Cancer Biol Ther. 2008;7:3–9. doi: 10.4161/cbt.7.1.5163. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. 2013. [Last cited on 2013 Jul 08]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf .
- 3.Fidler MW. Pathological fractures of the cervical spine.Palliative surgical treatment. J Bone Joint Surg Br. 1985;67:352–7. doi: 10.1302/0301-620X.67B3.2581972. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Khan SN, Donthineni R. Surgical management of metastatic spine tumors. Orthop Clin North Am. 2006;37:99–104. doi: 10.1016/j.ocl.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 8.Swanson DA, Orovan WL, Johnson DE, Giacco G. Osseous metastases secondary to renal cell carcinoma. Urology. 1981;18:556–61. doi: 10.1016/0090-4295(81)90455-6. [DOI] [PubMed] [Google Scholar]