Abstract
Background:
Intralesional injection of autologous blood-derived products has recently gained attention as a potential treatment for plantar fasciitis (PF). We compared platelet-rich plasma (PRP) and whole blood (WB) for the treatment of chronic PF.
Materials and Methods:
Patients with chronic PF received either an intralesional injection of 3 cc PRP prepared by double centrifuge technique or WB (n = 17 in each group). Overall, morning and walking pain severity were assessed by 11-point numerical rating scale, and function was assessed by the Roles and Maudsley score (RMS) at baseline and 1-month and 3 months after treatment. Ultrasonography was performed to measure plantar fascia thickness at baseline and 3 months after treatment.
Results:
Pain scores were reduced over the study in the PRP (mean change = −5.00 ± 1.17 to −5.47 ± 1.46) and WB groups (mean change = −5.29 ± 2.56 to −6.47 ± 2.83), with no difference between groups (P > 0.05). One month and 3 months after treatment, successful treatment (RMS of ≤ 2) was respectively observed in 29.4% and 82.3% of the PRP and in 47.1% and 76.4% of the WB groups (P > 0.05). Also, fascia thickness was decreased in both the PRP and WB groups (mean change = −1.74 ± 1.11 vs. −1.21 ± 0.73 mm, respectively, P = 0.115).
Conclusions:
Significant improvement in pain and function, as well as decrease in plantar fascia thickness, was observed by intralesional injection of the PRP and WB in patients with chronic PF. The study results indicate similar effectiveness between PRP and WB for the treatment of chronic PF in short-term.
Keywords: Growth factors, plantar fasciitis, platelet-rich plasma, ultrasonography
INTRODUCTION
Plantar fasciitis (PF) is the most common cause of heel pain in adults with a lifetime prevalence of about 10% and a peak incidence in the middle age. The etiology of PF is not completely understood and seems to be multifactorial. The high incidence in runners suggests a role for biomechanical overuse injury and repetitive micro-trauma in this regard.[1] Although PF is a self-limiting condition, the resolution may require several months. Moreover, pain may become chronic and disabling in a subset of patients with a significant impact on patients’ quality of life[2] and associated healthcare burden.[3]
Conservative treatments such as relative rest, activity modification, oral analgesics, and stretching techniques are found successful for the initial management of PF in most patients.[4] Those who are nonresponsive to such treatments may require more invasive procedures. Injection of corticosteroids into the proximal plantar fascia is commonly used in the treatment of chronic PF and often results in short-term pain relief.[5] However, recurrence is common after corticosteroids’ injection, it is not complication free, and may increase the risk of fat pad atrophy and plantar fascia rupture.[6] Although surgical intervention such as plantar fasciotomy is beneficial for recalcitrant cases, potential complications as well as the need for postoperative cares limit its efficacy to the last resort.[7]
Intralesional injection of autologous blood-derived products (ABDPs) such as platelet-rich plasma (PRP) has recently gained attention as a potential treatment for PF. The PRP, produced via centrifuged blood, is rich with platelets that release a variety of growth factors and cytokines which can stimulate the natural healing process in traumatic and degenerative tissues.[8] It has been shown effective for the management of osteoarthritis,[9] muscle injury, and tendinopathies.[10] Current evidence also has shown promising results for the use of PRP in the treatment of PF, though a limited number of controlled trials are yet conducted in this regard.[5] Available data have shown that injection of PRP is more effective and durable than corticosteroids in providing pain relief for patients with chronic PF.[11,12]
A number of studies showed that injection of the whole blood (WB) provides comparable results to PRP for the treatment of tendinopathies.[13,14,15,16] Although intralesional WB injection is shown to improve pain in patients with chronic PF, it has not been as effective as corticosteroids’ injection in some studies.[17,18] However, there is no evidence on a direct comparison between PRP and WB in the treatment of patients with PF. If shown to be noninferior than PRP, the WB can be easily applied, without the need for specific equipment, and with less costs than PRP. Considering the lack of data in this regard, we aimed to compare autologous WB with PRP in the treatment of patients with chronic PF. We hypothesized that WB is as effective as PRP in reducing pain and improving function in these patients.
MATERIALS AND METHODS
Patients and settings
This single-blinded (outcome assessors) comparative clinical trial was conducted on patients with PF referring to the physical medicine and rehabilitation clinic of the Alzahra University Hospital (Isfahan city, Iran) between October 2013 and March 2015. The inclusion criteria were age of ≥ 18 years, diagnosis of PF by a physiatrist based on current guidelines,[19] duration of symptoms for at least 3 months before the study, and lack of response to conservative treatments such as nonsteroidal anti-inflammatory drugs (NSAIDs) and physiotherapy. Patients with any of the following characteristics were not included in the study: Received corticosteroids in the preceding 6 weeks, history of surgical interventions on the ankle/heel, consumed aspirin or NSAIDs in the previous week, history of stroke within the last 3 months, pregnancy or breastfeeding, and evidence of malignancy, anemia, diabetes, hypothyroidism, peripheral neuropathy, acute infection, and coagulopathies. Sample size was calculated as 17 cases in each group considering type I error probability of 0.05, study power of 0.8, and expecting at least 2 points reduction in the numeric pain rating scale (NPRS) as the suggested minimal clinically important difference for this scale.[20] The study was approved by the Ethics Committee of the Isfahan University of Medical Sciences, and informed consent was obtained from patients. Also, the study protocol was registered at the Iranian Registry for Clinical Trials (www.irct.ir, registration number: IRCT2015041821830N1).
Interventions and study design
Autologous PRP was prepared based on the double centrifuge technique and using available commercial kit (Rooyagen Co., Tehran, Iran). Briefly, 40 cc venous blood was collected in tubes containing citrate and heparin. Then, erythrocytes were separated at first spin step (centrifugation at 1600 rpm, for 12 min), and leukocyte-reduced PRP was collected after the second spin (centrifugation at 3500 rpm, for 7 min). This protocol resulted in 3 cc of PRP with about 6- to 8-fold increase in platelet levels. Blood collection, PRP preparation, and injection steps were all performed in a same room with a maximum interval of 30 min between blood collection and PRP injection. The WB was prepared by collecting 3 cc of venous blood in heparin-containing blood tubes just before the injection.
The study was not able to be conducted as a double-blinded trial because of the appearance of the drugs and technical processes. Patients were randomized into the PRP and WB groups. All injections were done by a single physiatrist resident with the patient at the supine position and the ankle in neutral position. The skin at the site of injection was appropriately prepped and draped. Injection was done in sterile condition, using a 22G needle (included in the PRP kit, Rooyagen Co., Tehran, Iran), at the maximal tenderness point, and with the medial approach. No local anesthetic was used in any group. To manage pain at the injection site, patients were instructed to elevate the lower extremity, avoid weight bearing activity for 3 days, avoid running for 10 days, use cold pack, and consume acetaminophen as needed. Also, patients were educated to schedule the program of stretching the Achilles tendon and plantar fascia. However, using corticosteroids or NSAIDs was prohibited.
Measurements
A physiatrist resident who was not aware of the allocation sequence (single-blinded design) interviewed with each patient and assessed pain severity and activity limitation at baseline and then 1-month and 3 months after intervention. Pain severity was assessed using the 11-point NPRS (0 = no pain, 10 = the worst imaginable pain) in three states of (a) at early morning, (b) over a day in average, and (c) by walking. Also, patients were interviewed for the Roles and Maudsley score (RMS), which evaluates activity limitation due to pain with a 4-point scale; excellent (1) = no pain, full movement and activity; good (2) = occasional discomfort, full movement and activity; acceptable (3) = some discomfort after prolonged activity; and poor (4) = pain limits activities.[21] The RMS of 1 or 2 at 1-month and 3 months after the intervention was defined as treatment success based on the previous studies.[22] At the second and third visits, patients were also interviewed, and the heel was examined for any possible side effect or complication.
A radiologist performed two-dimensional real-time B-mode ultrasonography using a 10 MHz linear array transducer (SONOLINE G60, Siemens, Munich, Germany) at baseline and then 3 months after intervention. Plantar fascia thickness was measured at about 2 cm distal of the medial calcaneal tuberosity. Also, plantar fascia appearance was evaluated for echogenicity and biconvexity. The radiologist was not aware of the patients’ allocation sequence (single-blinded design).
Study outcomes
The study primary outcomes were the change in pain score after treatment and treatment success as defined by the RMS. Secondary outcomes were the change in plantar fascia thickness and ultrasonographic characteristics.
Statistical analysis
Data were analyzed using the SPSS software for windows (version 16.0, SPSS Inc., Chicago, IL, USA). Descriptive analyses are presented as mean ± standard deviation or number (%). Normal distribution of quantitative data was checked using the Kolmogorov–Smirnov test. Then, the independent sample t-test was applied for comparison of quantitative data between the two groups. The Mann–Whitney U-test was used for comparison of quantitative data without normal distribution as well as ordinal variables. Qualitative data were compared using the Fisher’s exact test. Changes in outcome variables were analyzed using repeated measure test (for pain score), Friedman test (for RMS), paired t-test (for plantar fascia thickness), and McNemar’s test for other ultrasonographic parameters. Correlations between study outcomes were checked by Pearson or Spearman’s test. A P value of ≤ 0.05 was considered statistically significant in all analyses.
RESULTS
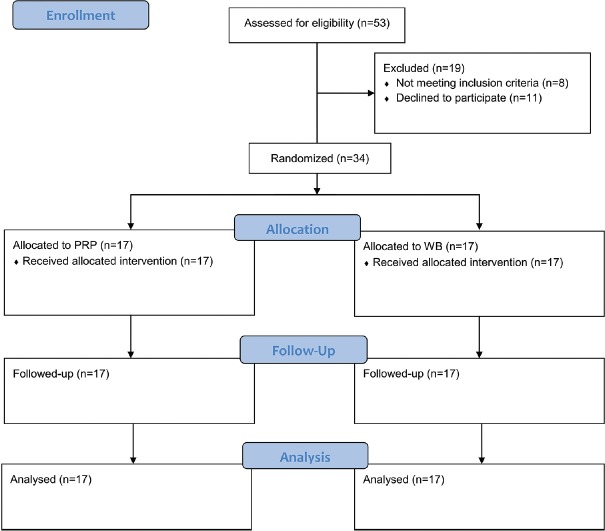
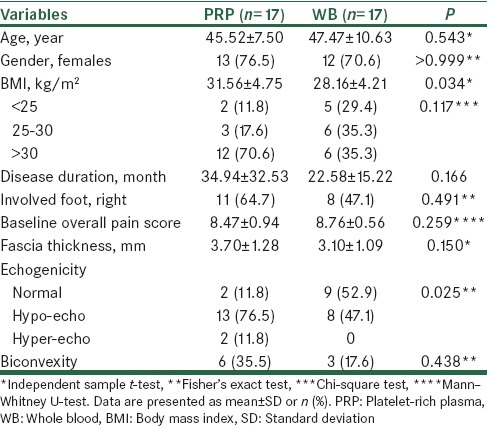
A total of 53 patients were evaluated during the study from which 34 were eligible for the study and were allocated into the study groups, all of whom completed the study [Figure 1]. Patients’ baseline characteristics are summarized in Table 1. The BMI was higher (by 3.4 ± 1.5 kg/m2, P = 0.034) and abnormal echogenicity at plantar fascia was more frequent in the PRP compared with the WB group (88.2% vs. 47.1%, P = 0.026).
Figure 1.
Patients’ flow diagram
Table 1.
Comparison of baseline characteristics between the two groups
Comparing trend of changes in pain scores over the study between the two groups is summarized in Table 2. Overall pain, morning pain, as well as walking pain were all improved over the study in both the PRP (F = 65.4–88.4, P < 0.001) and WB (F = 53.0–67.9, P < 0.001) groups, Figures 2–4. There was no significant difference between the two groups regarding the amount of changes in pain scores by 3 months after treatment (P > 0.05). Also, the repeated measure test found no significant effect for the type of treatment on the trend of changes in pain scores over the study (P > 0.05). There was only significant interaction between treatment type and trend of change in walking pain scores over the study (F = 4.1, P = 0.021). However, after controlling for baseline scores, which was higher in the WB than PRP group (9.23 ± 0.90 vs. 8.05 ± 1.02, P = 0.003), such effect became nonsignificant (F = 2.5, P = 0.088). Because BMI was correlated with baseline morning pain scores (r = 0.454) and there was a difference between the two groups in BMI, this factor was then included into the repeated measure as a covariate. There was no change in the overall test’s results after controlling for BMI.
Table 2.
Comparison of trend of change in pain scores over the study between the two groups
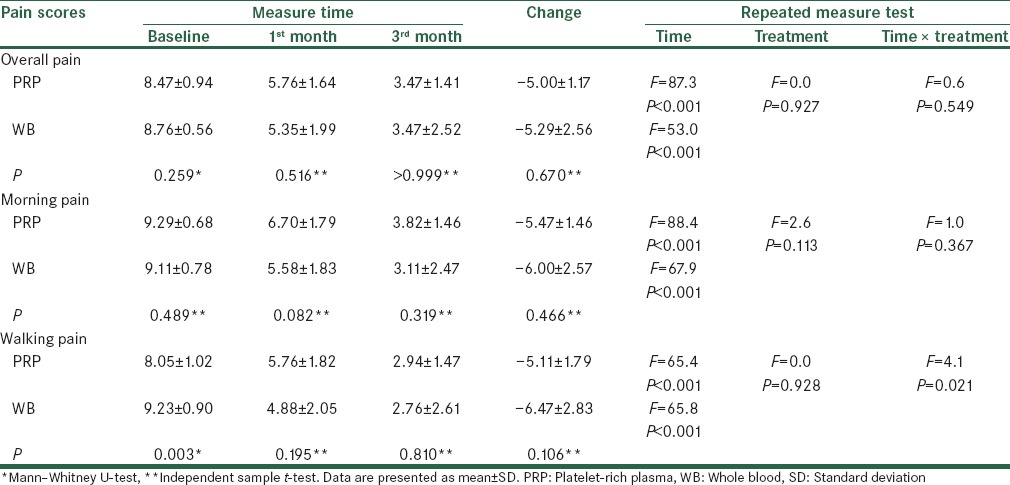
Figure 2.
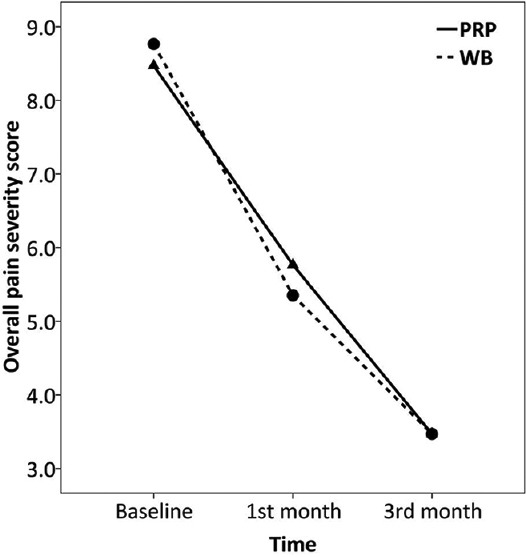
Trend of changes in overall pain severity score over the study
Figure 4.
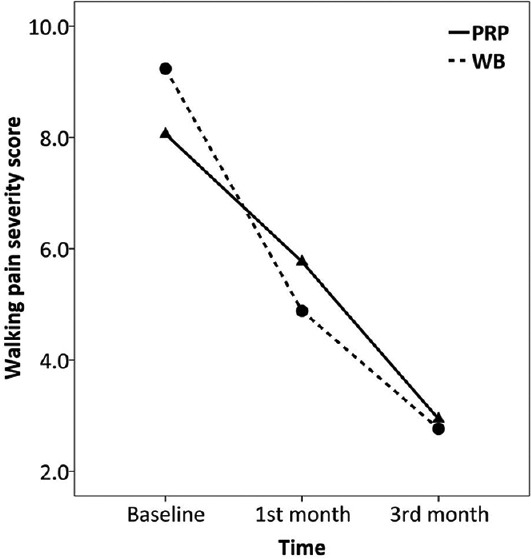
Trend of changes in walking pain severity score over the study
Figure 3.
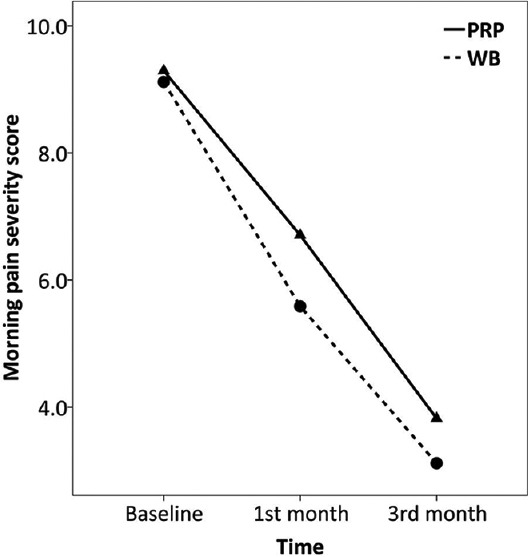
Trend of changes in morning pain severity score over the study
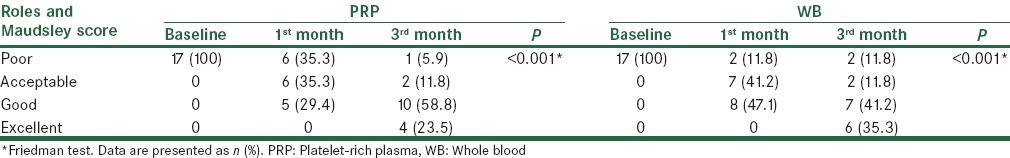
All patients had RMS of 4 (poor) at baseline. One month after treatment, 5 (29.4%) and 8 (47.1%) patients of the PRP and WB groups, respectively, had treatment success based on the RMS (between groups comparison, P = 0.481). Three months after treatment, success was observed in 14 (82.3%) and 13 (76.4%) patients of the PRP and WB groups, respectively (between groups comparison, P > 0.999) [Table 3].
Table 3.
Comparison of pain-related disability over the study between the two groups
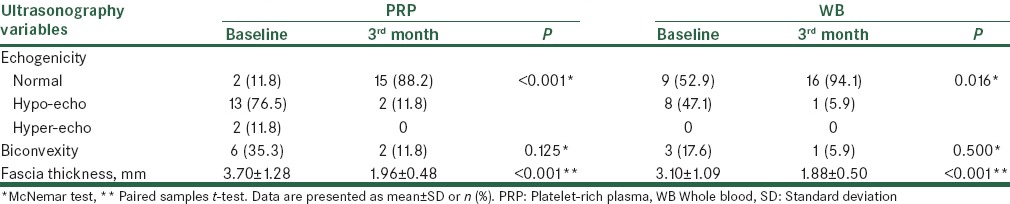
Echogenicity of the plantar fascia was normalized 3 months after treatment in 86.6% (13/15) of the PRP and 87.5% (7/8) of the WB groups (between groups comparison, P > 0.999). Also, fascia thickness was decreased in both the PRP and WB groups with no significant difference between the two groups (mean change = −1.74 ± 1.11 vs. −1.21 ± 0.73 mm, respectively, P = 0.115) [Table 4]. A weak and statistically nonsignificant correlation was found between reduction in fascia thickness and decrease in overall pain (r = 0.272, P = 0.119), morning pain (r = 0.212, P = 0.229), and walking pain in all patients (r = 0.263, P = 0.133).
Table 4.
Comparison of ultrasonographic outcomes between the two groups
Side effects
No severe side effect or complication was reported by the patients or observed by the physiatrist over the study period.
DISCUSSION
Various nonsurgical interventions are proposed for the treatment of chronic PF; however, the optimal treatment is not yet identified.[4] Recent studies have shown promising results by intralesional injection of ABDPs for the treatment of patients with chronic PF. To our knowledge, our study is the first direct comparison between PRP and WB in these patients. We found significant and similar improvement in pain and function by the intralesional injection of the PRP and WB. Also, fascia thickness was decreased, and echogenicity of the fascia was normalized similarly after both treatments. These results showed that at least in short-term, intralesional injection of PRP and WB are similarly effective for treatment of patients with chronic PF.
A limited number of controlled clinical trials are conducted on the efficacy of ABDPs for PF. Comparative studies with 6 weeks to 6 months follow-up periods have found better improvement in pain and function by the intralesional injection of PRP compared to corticosteroids in patients with chronic PF.[12,23,24] In a long-term study, Monto found improvement by the corticosteroid injection that last for about 6 months, but pain rebounded to the baseline after 12–24 months. However, the improvement observed by PRP maintained for up to 24 months after treatment.[11] In contrast to these reports, a 6-month trial by Aksahin et al. reviled similar improvement in pain and function with both PRP and corticosteroids.[25] Although we did not compare ABDPs with corticosteroids, the observed pain relief by PRP and WB in our study (pain reduction of between 5 and 6.4 scores out of 10) was comparable to previous reports (pain reduction of between 3.4 and 7.8 scores out of 10).[11,12,24,25] In overall, available evidence shows better outcomes for intralesional injection of PRP compared to corticosteroids. When the potential complications of corticosteroids are also taken into account, PRP injection seems to be a better treatment option than corticosteroids for treatment of chronic PF. Comparison of PRP with other treatments such as dextrose prolotherapy[26] and extracorporeal shock wave therapy (ESWT)[27] also showed equal or better improvement with PRP. With regards to the WB, it is shown to improve pain in patients with chronic PF, but it has not been as effective as a corticosteroid injection.[17,18] However, we found similar short-term results between PRP and WB for the treatment of PF. Considering a limited number of head-to-head trials, further comparative studies are required in this regard.
The therapeutic effects of ABDPs for PF are not only evident by subjective assessment of pain but also through objective measures. The thickness of the plantar fascia, as measured by ultrasonography, is proposed as an objective outcome measure for the assessment of treatment response in PF.[28] In this regard, Ragab and Othmanreported about 2.3 mm reduction in the plantar fascia thickness 3 months after treatment with PRP. Also, these investigators found changes in the echogenicity of the plantar fascia after PRP injection.[29] In another study, Chew et al. found reduction in plantar fascia thickness of about 1.5 mm by 6 months after PRP injection which was greater than that observed after ESWT.[27] Similar to these studies, we found between 1.2 and 1.7 mm reduction in plantar fascia thickness and also alterations in the echogenicity of the plantar fascia after injection of ABDPs. However, we found no strong and significant correlation between changes in pain severity and changes of the plantar fascia thickness which may be attributed to the small sample size of the study. Using objective measures such as plantar fascia thickness in future studies can provide better data on the effectiveness of ABDPs for the treatment of PF.
Besides our study, there is no other evidence on a direct comparison between PRP and WB in the treatment of PF. Previous comparative trials in this regard were mostly conducted for the treatment of tendinopathies (e.g., tennis elbow). In the study by Zhao et al., patients with chronic tennis elbow received either PRP or WB and were followed for 8 weeks. Authors found significant and similar improvement in pain and function in both groups after 4 weeks, but PRP resulted in better outcomes than WB after 8 weeks.[16] The study by Raeissadat et al. also found similar improvement by PRP and WB in patients with chronic tennis elbow in short-term (4 weeks), but better outcomes by PRP after 8 weeks.[14] However, in another trial by the same investigators, a similar improvement in pain and function was observed for PRP and WB over a 1-year follow-up of the patients.[15] The study by Creaney et al. also found improvement in pain and function which was similar between PRP and WB over a 6-month follow-up period.[13] Considering the higher concentration of platelets’ products, it may be expected that PRP would result in better healing than WB. To date, however, evidence has shown similar outcomes by administration of various types of ABDPs for the treatment of tendinopathies.[30] Similarly, we found comparable short-term outcomes between PRP and WB in the treatment of PF that was evident by both subjective and objective measures. Whether such outcomes are the same between PRP and WB after a longer follow-up period should be investigated by further studies.
The mechanisms behind therapeutic effects of ABDPs for PF are not completely understood yet. Platelets contain various growth factors and proteins that are vital in many stages of tissue healing.[31] The PRP contains a concentrated amount of such products that can augment the native healing processes at the site of injury by enhancing fibroblast proliferation and migration, stimulating vascularization, and increasing collagen deposition in the tissue.[31] It is shown that PRP promotes differentiation of tendon stem cells into active tenocytes and also increases tenocyte proliferation in the healing area leading to increased production of collagen which is required for repair of the injured tendons.[32,33,34] Moreover, a number of studies have shown that the regenerative properties of the PRP are dose-dependent, and the concentrated growth factors work in a synergetic manner to initiate a healing process.[35,36,37,38] Accordingly, expecting a better improvement by PRP compared with WB is justified by these mechanisms. On the other hand, the activity of growth factors may be affected through interaction by other blood cells (e.g., leukocytes) presented in WB.[30] It may explain why the WB has been as effective as the PRP for the treatment of tendinopathies and PF. However, considering the lack of data, a clear conclusion cannot be made in this regard yet. Measuring biomarkers of tissue healing after injection of ABDPs can provide better data regarding the underlying mechanisms of these promising therapies for PF.
Our study has a number of limitations. The study sample size was small, which affected the randomization quality as well, and the follow-up was only for 3 months. Due to the nature of the interventions, blinding of patients and treatment providers were not easily possible though the outcome assessors were not aware of the assigned treatments in our study. Also, the study has no placebo control group and the observed therapeutic effects in our study cannot be completely attributed to the ABDPs injection.[30] However, in addition to subjective pain assessment, we also evaluated objective measures, that is, plantar fascia thickness. The decrease in plantar fascia thickness after active interventions has not been observed by placebo in previous studies.[39,40,41] Accordingly, our study results are less likely to be largely affected by placebo effects.
In summary, we found significant improvement in pain and function and reduction in plantar fascia thickness by intralesional injection of ABDPs in patients with chronic PF. The study results indicate the similar effectiveness of an intralesional injection of PRP and WB for the treatment of chronic PF in short-term. Compared with PRP, injection of WB does not require special equipment, the costs are relatively low, and the technique can be easily applied in various clinical settings. However, our study results need to be confirmed by further studies with a larger sample of patients and longer follow-up duration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are thankful to Dr. Ahmad Moradi who helped us in data gathering and Dr. Ali Gholamrezaei for statistical analyses and editing this report.
REFERENCES
- 1.Buchbinder R. Clinical practice. Plantar fasciitis. N Engl J Med. 2004;350:2159–66. doi: 10.1056/NEJMcp032745. [DOI] [PubMed] [Google Scholar]
- 2.Irving DB, Cook JL, Young MA, Menz HB. Impact of chronic plantar heel pain on health-related quality of life. J Am Podiatr Med Assoc. 2008;98:283–9. doi: 10.7547/0980283. [DOI] [PubMed] [Google Scholar]
- 3.Tong KB, Furia J. Economic burden of plantar fasciitis treatment in the United States. Am J Orthop (Belle Mead NJ) 2010;39:227–31. [PubMed] [Google Scholar]
- 4.Thomas JL, Christensen JC, Kravitz SR, Mendicino RW, Schuberth JM, Vanore JV, et al. The diagnosis and treatment of heel pain: A clinical practice guideline-revision 2010. J Foot Ankle Surg. 2010;49(3 Suppl):S1–19. doi: 10.1053/j.jfas.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao MY, Hung CY, Chang KV, Chien KL, Tu YK, Wang TG. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: A network meta-analysis. Rheumatology (Oxford) 2015. doi: 10.1093/rheumatology/kev010. In Press: doi: 10.1093/rheumatology/kev010. [DOI] [PubMed] [Google Scholar]
- 6.Tatli YZ, Kapasi S. The real risks of steroid injection for plantar fasciitis, with a review of conservative therapies. Curr Rev Musculoskelet Med. 2009;2:3–9. doi: 10.1007/s12178-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tweed JL, Barnes MR, Allen MJ, Campbell JA. Biomechanical consequences of total plantar fasciotomy: A review of the literature. J Am Podiatr Med Assoc. 2009;99:422–30. doi: 10.7547/0990422. [DOI] [PubMed] [Google Scholar]
- 8.Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24:173–82. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 9.Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br J Sports Med. 2015;49:657–72. doi: 10.1136/bjsports-2014-094036. [DOI] [PubMed] [Google Scholar]
- 10.Andia I, Maffulli N. Platelet-rich plasma for muscle injury and tendinopathy. Sports Med Arthrosc. 2013;21:191–8. doi: 10.1097/JSA.0b013e318299972b. [DOI] [PubMed] [Google Scholar]
- 11.Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014;35:313–8. doi: 10.1177/1071100713519778. [DOI] [PubMed] [Google Scholar]
- 12.Say F, Gürler D, Inkaya E, Bülbül M. Comparison of platelet-rich plasma and steroid injection in the treatment of plantar fasciitis. Acta Orthop Traumatol Turc. 2014;48:667–72. doi: 10.3944/AOTT.2014.13.0142. [DOI] [PubMed] [Google Scholar]
- 13.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: A prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–71. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 14.Raeissadat SA, Sedighipour L, Rayegani SM, Bahrami MH, Bayat M, Rahimi R. Effect of platelet-rich plasma (PRP) versus autologous whole blood on pain and function improvement in tennis elbow: A randomized clinical trial. Pain Res Treat 2014. 2014 doi: 10.1155/2014/191525. 191525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is Platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: One year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao LL, Tong PJ, Xiao LW, Zhu QL, Xu B, Yan MH. Case-control study on local injection of autoallergic platelet rich plasma or whole blood for the treatment of tennis elbow. Zhongguo Gu Shang. 2014;27:908–11. [PubMed] [Google Scholar]
- 17.Lee TG, Ahmad TS. Intralesional autologous blood injection compared to corticosteroid injection for treatment of chronic plantar fasciitis. A prospective, randomized, controlled trial. Foot Ankle Int. 2007;28:984–90. doi: 10.3113/FAI.2007.0984. [DOI] [PubMed] [Google Scholar]
- 18.Kalaci A, Cakici H, Hapa O, Yanat AN, Dogramaci Y, Sevinç TT. Treatment of plantar fasciitis using four different local injection modalities: A randomized prospective clinical trial. J Am Podiatr Med Assoc. 2009;99:108–13. doi: 10.7547/0980108. [DOI] [PubMed] [Google Scholar]
- 19.McPoil TG, Martin RL, Cornwall MW, Wukich DK, Irrgang JJ, Godges JJ. Heel pain – Plantar fasciitis: Clinical practice guildelines linked to the international classification of function, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38:A1–18. doi: 10.2519/jospt.2008.0302. [DOI] [PubMed] [Google Scholar]
- 20.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011. 63(Suppl 11):S240–52. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 21.Roles NC, Maudsley RH. Radial tunnel syndrome: Resistant tennis elbow as a nerve entrapment. J Bone Joint Surg Br. 1972;54:499–508. [PubMed] [Google Scholar]
- 22.Haake M, Buch M, Schoellner C, Goebel F, Vogel M, Mueller I, et al. Extracorporeal shock wave therapy for plantar fasciitis: Randomised controlled multicentre trial. BMJ. 2003;327:75. doi: 10.1136/bmj.327.7406.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omara AS, Ibrahima ME, Ahmedb AS, Saida M. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: Randomized clinical trial. Egypt Rheumatologist. 2012;34:43–9. [Google Scholar]
- 24.Shetty VD, Dhillon M, Hegde C, Jagtap P, Shetty S. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: A preliminary report. Foot Ankle Surg. 2014;20:10–3. doi: 10.1016/j.fas.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Aksahin E, Dogruyol D, Yüksel HY, Hapa O, Dogan O, Celebi L, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781–5. doi: 10.1007/s00402-012-1488-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6:152–8. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Chew KT, Leong D, Lin CY, Lim KK, Tan B. Comparison of autologous conditioned plasma injection, extracorporeal shockwave therapy, and conventional treatment for plantar fasciitis: A randomized trial. PM R. 2013;5:1035–43. doi: 10.1016/j.pmrj.2013.08.590. [DOI] [PubMed] [Google Scholar]
- 28.Mohseni-Bandpei MA, Nakhaee M, Mousavi ME, Shakourirad A, Safari MR, Vahab Kashani R. Application of ultrasound in the assessment of plantar fascia in patients with plantar fasciitis: A systematic review. Ultrasound Med Biol. 2014;40:1737–54. doi: 10.1016/j.ultrasmedbio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065–70. doi: 10.1007/s00402-012-1505-8. [DOI] [PubMed] [Google Scholar]
- 30.Kampa RJ, Connell DA. Treatment of tendinopathy: Is there a role for autologous whole blood and platelet rich plasma injection? Int J Clin Pract. 2010;64:1813–23. doi: 10.1111/j.1742-1241.2010.02432.x. [DOI] [PubMed] [Google Scholar]
- 31.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–94. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38:2477–86. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 33.Baksh N, Hannon CP, Murawski CD, Smyth NA, Kennedy JG. Platelet-rich plasma in tendon models: A systematic review of basic science literature. Arthroscopy. 2013;29:596–607. doi: 10.1016/j.arthro.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y, et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2020. In Press: doi:10.1002/term. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N. The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J Bone Joint Surg Br. 2008;90:966–72. doi: 10.1302/0301-620X.90B7.20235. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173:258–66. doi: 10.1016/j.jss.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40:1035–45. doi: 10.1177/0363546512437525. [DOI] [PubMed] [Google Scholar]
- 38.Xian LJ, Roy Chowdhury S, Bin Saim A, Bt Hj Idrus R. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17:293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 39.McMillan AM, Landorf KB, Gilheany MF, Bird AR, Morrow AD, Menz HB. Ultrasound guided corticosteroid injection for plantar fasciitis: Randomised controlled trial. BMJ. 2012;344:e3260. doi: 10.1136/bmj.e3260. [DOI] [PubMed] [Google Scholar]
- 40.Schulhofer SD. Short-term benefits of ultrasound-guided corticosteroid injection in plantar fasciitis. Clin J Sport Med. 2013;23:83–4. doi: 10.1097/JSM.0b013e31827e9ec9. [DOI] [PubMed] [Google Scholar]
- 41.Vahdatpour B, Sajadieh S, Bateni V, Karami M, Sajjadieh H. Extracorporeal shock wave therapy in patients with plantar fasciitis. A randomized, placebo-controlled trial with ultrasonographic and subjective outcome assessments. J Res Med Sci. 2012;17:834–8. [PMC free article] [PubMed] [Google Scholar]