Abstract
Background:
Hepatitis B virus infection (HBV) and its complications is one of the most serious problems of the health system in many parts of the world. In the present study, we will assess chronic and occult HBV and isolated anti-Hepatitis B core antigen whose screening and evaluation is not routine in different populations.
Materials and Methods:
This descriptive analytical study was conducted on 213 patients undergoing chemotherapy - radiotherapy referred to the hematology - oncology clinics of Isfahan, Iran in 2012. In order to determine the serum levels of hepatitis B surface antigen (HbSAg), Hepatitis B Antigen and Antibody (HBCAb), aspartate aminotransferase (AST), alanine transaminase (ALT) and Alkaline phosphatase (ALK.P), venous blood samples were obtained. If the HBCAb sample was positive, another sample of the serum was sent to the laboratory to perform polymerase chain reaction and to determine viral load.
Results:
The mean age of the patients was 47.7 ± 9 years, with an age range of 27 -73 years; 98 (46%) and 115 (54%) cases were male and female, respectively, with mean age of 51.9 ± 8.3 and 44.1 ± 8.1 years, and there was no significant difference (P < 0.001). The mean level of liver enzymes including AST, ALT and ALK.P were 34.2 ± 36.02, 38.9 ± 47.1 and 252.1 ± 234.7, respectively. Two cases were HbSAg positive (0.9%) and six cases were HBCAb positive (2.8%) and HbSAg negative. Three cases had a high viral load at the rate of starting treatment among positive anti-HBC patients.
Conclusion:
Because occult hepatitis is investigated less commonly in routine studies, it seems that screening and evaluating its prevalence is useful in the management of patients.
Keywords: Hepatitis B virus, chemo - radiotherapy, liver enzymes, occult hepatitis
INTRODUCTION
Hepatitis B virus infection (HBV) and its complications is one of the most serious problems in many parts of the world. Reports indicate that more than 1/3 of the world’s population has had the serologic evidences of infection with HBV in the past or present and that there are 350–400 million carriers of hepatitis B.[1] In endemic areas, chronic Hepatitis B infection has a carrier rate of 8–20%. Its average rate of prevalence is 2–7% in Mediterranean, Central Asian and the Middle Eastern countries.[2,3] Because of chemo - radiotherapy, reactivation of hepatitis B is a serious cause of morbidity and mortality in patients with cancer. In patients with cancer, the onset chemo - radiotherapy is associated with several complications, such as change in liver transaminases and jaundice, that differentiating between it and hepatitis B reactivation is difficult and encounters the management of treatment and organization of patient with problems in the hard periods of chemo - radiotherapy. For this reason, the knowledge of viruses such as Hepatitis B and liver transaminases can give a clearer picture of the created complication. This screening and evaluation includes chronic Hepatitis B (HbSAg) performed routinely and also occult hepatitis and isolated anti-HBC studied less frequently, which have not been screened yet in many populations despite its high prevalence and being the source of transmission of Hepatitis B. Several reports have described the risk of flaring up of chronic Hepatitis B (positive HbSAg) in patients treated by chemo - radiotherapy for hematologic and solid malignancies, with the highest risk of flaring up occurring when chemotherapy is discontinued.[4,5,6] In various studies, the risk of reactivation of Hepatitis B (positive HbSAg) is between 20% and 50%.
In some cases, the core Ab of Hepatitis B is also positive, indicating the healed patients with previous infection; however, because the virus genome has been integrated with the human genome, it is exposed to reactivation with immunesuppression, specifically when rituximab is used alone or in combination with steroids.[7,8,9]
If the patient prone to flaring up has a high viral load due to immunosuppression, pre-emptive therapy with nucleoside analogues will be required during chemotherapy for 12 months after discontinuing the immunesuppressive therapy. Patients with positive anti-HBC (negative HbSAg) having undetectable viral load should be studied in terms of ALT and HBV-DNA level within chemo - radiotherapy.[10,11,12] Given the lack of previous sufficient studies on the significant prevalence of chronic and occult Hepatitis B in the patients, the present study mentions the importance of screening occult hepatitis and its prevalence that help to make decisions.
MATERIALS AND METHODS
This is a descriptive - analytical study conducted on patients undergoing chemo - radiotherapy referred to the hematology - oncology clinics of Isfahan, Iran in 2012.
The inclusion criteria were patients with solid or hematologic malignancies undergoing chemo - radiotherapy and patients’ consent for participating in the study. In the absence of consent, the patient was excluded from the study.
Using the formula of estimating the sample size for the prevalence studies and considering a confidence level of 95%, and also accepting an error rate of 0.05, the sample size required for the study was estimated as 195 patients in order to ensure that at least 213 cases were studied. In this study, the sampling method was an easy method. The collected data were analyzed by SPSS-22 software. The statistical tests applied for data analysis included the Chi-square and T-tests.
The method was such that the a venous blood sample was obtained from patients undergoing chemo - radiotherapy and referred to the hematology - oncology clinics of Isfahan, Iran, in 2012, which were then sent to the laboratory to determine the HbSAg (Hepatitis B surface antigen), ALT (alanine aminotransferase), AST (aspartate aminotransferase) and ALK.P (alkaline phosphatase) levels. A Spanish bio-system kit was used in the biochemical determination and the same kit and an ELISA test were used to determine the antibody concentration.
The serum sample of patients with positive HBCAb that was negative for HbSAg was obtained and sent to the laboratory for evaluating and determining the viral load (HBV-DNA polymerase chain reaction [PCR]). A ROCHE kit and reverse transcriptase (RT)-PCR test were used to determine the viral load.
Viral load (HBV-DNA) more than 2000 IU/mL was considered as the positive sample for treatment of occult hepatitis. The level of viral load higher than 45 mg/dL was considered for abnormal liver enzymes.
RESULTS
Among the 213 cases studied, the mean age of the patients was 47.7 ± 9 years (range, 27–73 years) and 98 (6%) and 115 (54%) cases were male and female, respectively. The mean age of the men and women were 51.9 ± 8.3 and 44.1 ± 8.1 years, respectively, and, according to the statistical results obtained from the T-test, the age difference between the male and female patients was significant (P < 0.001).
The most common type of cancer in the studied patients was breast cancer, with a frequency of 62 (29.1%) cases, followed by gastrointestinal cancer with a frequency of 42 (19.7%) cases, head and neck cancer with a frequency of 29 (13.6%) cases and cancer of the genitourinary system (prostate, bladder and kidney) with a frequency of 28 (13.1%) cases [Figure 1].
Figure 1.
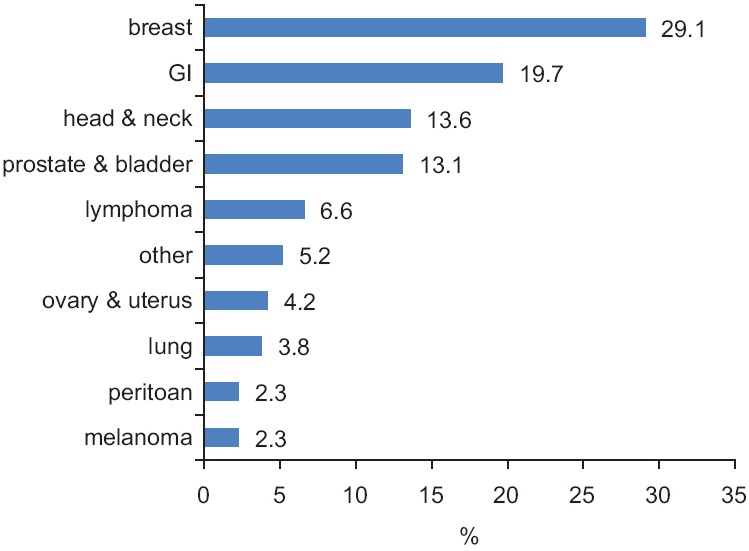
The frequency percentage of cancer type in the studied patients
The most common type of treatment offered to the patients was chemo - radiotherapy, with a frequency of 102 (47.9%) cases. Also, the treatment plan of radiotherapy was for 33 (15.5%) patients and chemotherapy was for 30 (14.1%) cases [Figure 2].
Figure 2.
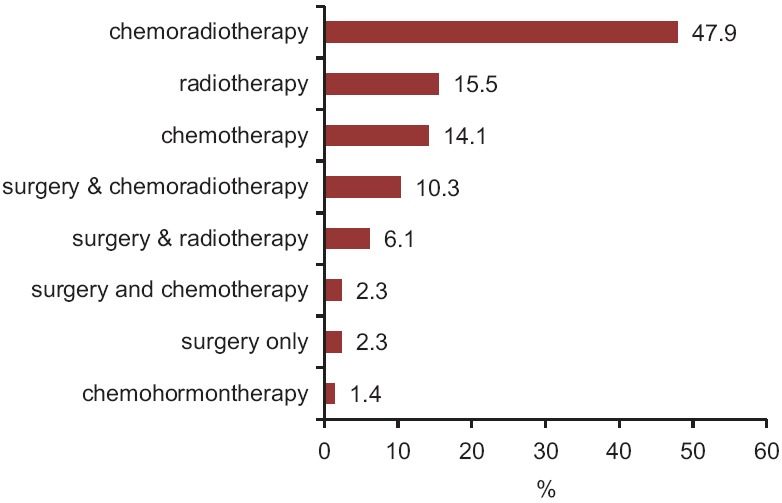
The frequency percentage of treatment type provided for the patients in the studied patients
The mean level of liver enzymes including AST, ALT and ALK.P were 34.2 ± 36.02, 38.9 ± 47.1 and 252.1 ± 234.7, respectively.
Of the 213 cases studied, two (0.9%) cases were HbSAg positive, six (2.8%) cases were HBCAb positive and the remainders of the patients were negative in terms of the above factors [Figure 3].
Figure 3.
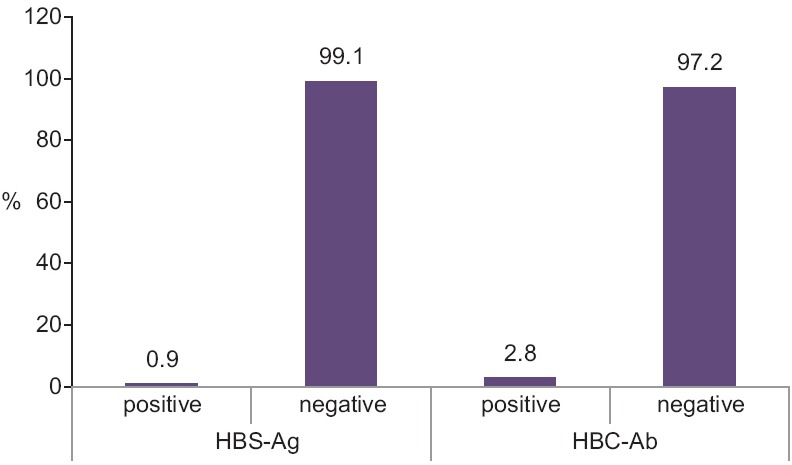
The frequency percentage of HBSAg and HBCAb in the studied patients
In terms of T-test, patients with positive and negative HBCAb had similar mean age (P = 0.21). The sex and job distribution of patients had no significant differences (P = 0.76 and P = 0.99). Performing the Fisher test on the above data showed that there was no significant relationship between the type of treatment and the result of the HBCAb test (P > 0.05) [Table 1].
Table 1.
Distribution of cancer type and treatment methods in terms of the status of HBCAb
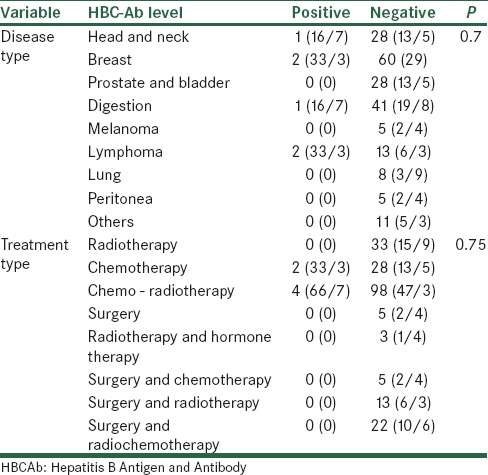
In terms of the future exposure of patients with radiotherapy and chemotherapy, the frequency distribution of the result of the HBCAb test indicated that none of patients undergoing radiotherapy were positive for HBCAb, while the therapy recommended for two (33.3%) cases (out of patients with HBCAb positivity) was chemotherapy and for four (66.7%) cases was chemo - radiotherapy. However, in terms of the Fisher’s exact test, the distribution of the result of the HBCAb test had no significant difference (P = 0.39) [Table 2].
Table 2.
Distribution of frequency of treatment plan in terms of the results of the HBCAb test
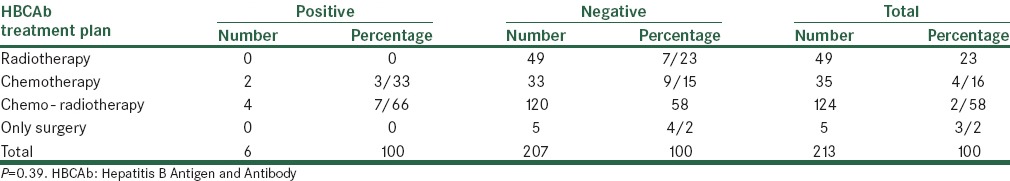
According to the PCR test, the average of viral load in patients with positive HBCAb was 866666.67 ± 305505, with a range of 600,000–1200,000 IU/mL. Also, in patients with positive and negative HBCAb, the mean serum level of AST was 54.33 ± 30.29 and 33.6 ± 36.1, respectively, and, according to the T-test, the difference between these two groups was not significant (P = 0.17).
In the two groups of positive and negative HBCAb, the mean serum level of ALT was also 78.8 ± 60.2 and 37.8 ± 226.2, respectively, and, in terms of the T-test, the difference between these two groups was significant (P = 0.035).
In the positive and negative groups of HBCAb, the mean serum level of alkaline phosphatase was 387.2 ± 343.1 and 248.1 ± 230.9, respectively, and the difference between the two groups was not significant (P = 0.15). From the results obtained, 37 (17.4%) patients had a high level of AST, 46 (21.6%) patients had a high level of ALT and 36 (16.9%) patients had a high level of alkaline phosphatase. There was at least one of the above-mentioned enzymes in 66 (31%) patients and, in 17 (8%) patients; the levels of all the three mentioned enzymes were higher than normal.
DISCUSSION
The overall objective of this study was to screen, evaluate and determine the prevalence of occult hepatitis, positive isolated anti-HBC and chronic hepatitis in patients undergoing chemo - radiotherapy for cancer to prevent the induced reactivation due to immunesuppression. Of the 213 cases studied, two (0.9%) cases had positive HbSAg (treated because of high viral loads) and six (2.8%) cases had positive HBCAb, while only three cases (50% of positive anti-HBC patients) had high HBV-DNA level at the rate of starting pre-emptive treatment, while the rest should be followed by ALT and viral load levels. Several studies have been performed on patients with positive HbSAg undergoing chemo - radiotherapy. In a study conducted by Orhan et al. (2009) in Turkey, among 1826 studied patients, 59 (3.2%) cases were HbSAg positive and, with chemotherapy, nine patients suffered from reactivation of Hepatitis B.[13]
On anti-HBC, studies are limited to patients undergoing chemo - radiotherapy and have been performed more commonly in groups of blood donor, dialyses and transplantation and hemophilia, etc.
In the study by Kumar et al. on 2552 blood donors screened for anti-HBC, 10 cases had a positive test result.[14]
Among blood donors, the rate of prevalence of isolated Hepatitis BCAb has been reported as 0.4–1.7% in geographical regions with a low prevalence of HBV and 10–20% in endemic areas by the Center of Hepatic Studies in Washington in 2012.
In the study by Fontenele et al. in Brazil, the prevalence of occult Hepatitis B was considered highly variable, and it was in the range of 0–58% among hemodialysis patients. In this study, an Iranian study has been mentioned in which a high rate of HBV-DNA in 50% of patients with isolated anti-HBC was mentioned.[15]
In a study in Isfahan, Iran, in 2004 on blood donors, of 545 donated blood samples, 43 (8%) cases had positive anti-HBC and, by evaluation using PCR, five (11.6%) samples had positive HBV-DNA.[16]
In other studies conducted by Alavian et al. (hepatitis monthly), the prevalence of occult hepatitis among patients with positive anti-HBC and the total prevalence of occult hepatitis B in various settings such as blood donors, dialyses, hemophilia, immunosuppression and the general population were 0–17% and 0.05–13%, 6.4–64.7% and 0–58%, 6–100% and 5.3–51.2%, 37.8–62.3% and 3.3–37.8% and 6.1–51% and 0.7–34%, respectively.[17]
Given the comparison of the results obtained from the present study with those of other studies, the reactivation risk of occult hepatitis is higher in the field of immunosuppression and also chemo -radiotherapy and, considering the next probable problems in the field of differentiation of medicinal hepatitis, it seems that screening and evaluating the virus is essential before the onset of immunosuppressive therapy.
CONCLUSION
According to the findings of the studies conducted and the effect of chemo - radiotherapy on flaring up of occult hepatitis (which is considered and studied less), and given its prevalence in our study and the importance of initiating pre-emptive therapy to prevent viral reactivation, it seems likely that screening and evaluating isolated anti-HBC and, if necessary, in terms of occult hepatitis, is essential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 2.Arababadi MK, Nasiri Ahmadabadi B, Yousefi Daredor H, Kennedy D. Epidermiology of occult hepatitis B infection among thalassemic, hemophilia, and hemodialysis patients. Hepat Mon. 2012;12:315–9. doi: 10.5812/hepatmon.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y, Shang QL, Liu JY, Li D, Xu WZ, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patient and healthy people in China. J Infect. 2009;58:383–8. doi: 10.1016/j.jinf.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Perrillo RP. Acute flares in chronic hepatitis B: The natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–22. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JC, Liu MC, Tsai SY, Fang WT, Jer-Min Jian J, Sung JL. Unexpectedly frequent hepatitis B reactivation by chemoradiation in postgastrectomy patients. Cancer. 2004;101:2126–33. doi: 10.1002/cncr.20591. [DOI] [PubMed] [Google Scholar]
- 6.Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic transplantation. Blood. 2002;99:2324–30. doi: 10.1182/blood.v99.7.2324. [DOI] [PubMed] [Google Scholar]
- 7.Ji D, Cao J, Hong X, Li J, Wang J, Chen F, et al. Low incidence of hepatitis B virus reactivation during chemotherapy among diffuse large B-cell lymphoma patients who are HBsAg-negative/HBcAb-positive: A multi center retrospective study. Eur J Haematol. 2010;85:243–50. doi: 10.1111/j.1600-0609.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 8.Even AM, Jovanovic BC, Su YC, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoporoliferative disease: Meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–80. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok SF. Occult hepatitis B virus infection: Diagnosis, implication and management? J Gastroenteral Hepatol. 2004;19:S114–7. [Google Scholar]
- 10.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with hematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 11.Cornberg M, Protzer U, Petersen J, Wedemeyer H, Berg T, Jilg W, et al. Prophylaxis, diagnosis and therapy of hepatitis B virus infection-the German guideline. Z Gastroenterol. 2011;49:871–930. [Google Scholar]
- 12.Ahmed A, Keeffe EB. Lamivudine therapy for chemotherapy-induced reactivation of hepatitis B virus infection. Am J Gastroenterol. 1999;94:249–51. doi: 10.1111/j.1572-0241.1999.00808.x. [DOI] [PubMed] [Google Scholar]
- 13.Eren OO, Artac M, Boruban MC, Yavas O, Arslan U, Basaranoglu M. Chemotherapy-induced Hepatitis B virus reactivation in HbsAg positive cancer patient: A single center experience. Med Oncol. 2009;26:386–92. doi: 10.1007/s12032-008-9133-4. [DOI] [PubMed] [Google Scholar]
- 14.Kumar CH, Gupta LC, Jaiprakash BM. The role of anti-HBC IgM in screening of blood donors. Med J Arm Forc Ind. 2007;63:350–2. doi: 10.1016/S0377-1237(07)80013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontenele AM, Filho NS, Ferreira AS. Occult hepatitis B in patients on hemodialysis: A review. Ann Hepatol. 2013;12:527–31. [PubMed] [Google Scholar]
- 16.Arababadi MK, Poor Azar A, Salehi M, Jaafarzadeh A. Evaluation of occult hepatitis B virus infection in blood donor with negative HbSAg and positive anti HBC. Shahgid Sadoughi Journal of Yaz University of Medical Sciences. 2007:1–74. Persian. [Google Scholar]
- 17.Alavian SM, Miri SM, Hollinger FB, Jazayeri SM. Occult hepatitis B (OBH) in clinical settings. Hepat Mon. 2012;12:e6126. doi: 10.5812/hepatmon.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]


