Abstract
Background:
Considering the increasing trend of childhood obesity and subsequent burden of the disease in Iran and other countries and importance of early life intervention for achieving sustained effect on health of children and adolescents, this study aimed to investigate the effect of two different dose of folic acid on homocysteine (Hcy) level and insulin resistance of obese children.
Materials and Methods:
In this randomized, double-blind controlled clinical trial study, 60 obese and overweight children aged 5–12 years were enrolled. Selected obese children randomly allocated in two interventional (1 mg/day folic acid and 5 mg/day folic acid, for 8 weeks) and one control groups. Biochemical measurements including folic acid, Hcy, insulin and insulin resistance were measured between and within groups before and after trial.
Results:
In each group, 20 obese children were studied. The three groups were age and sex matched. After folic acid administration, mean of Hcy, insulin resistance and insulin decreased significantly in two groups which folic acid administrated with two different doses (P < 0.05). The reduction in studied biochemical variables was similar in two interventional groups (1 and 5 mg folic acid daily) (P > 0.05). Mean differences for Hcy, insulin resistance and insulin, in two intervention groups were significantly higher than the control group (P < 0.0001). Mean differences of Hcy, insulin resistance and insulin, in two intervention groups were not different significantly (P > 0.05).
Conclusion:
The findings of current trial showed that folic acid in two studied doses could be a safe and effective supplement for obese children to reduce Hcy level and insulin resistance, which consequently could prevent obesity-related complications including cardiovascular and metabolic disorders.
Keywords: Children, folic acid, homocysteine, insulin resistance, obese, overweight
INTRODUCTION
Childhood obesity is a chronic and complex metabolic disorder, which is associated with higher risk of various medical and psychosocial consequences.[1] Prevalence of childhood obesity is increasing rapidly and approaching epidemic proportion worldwide.[2] It seems that the rising rate is more alarming in developing countries. In a way, that it is estimated that 81% of obese children worldwide were from developing countries especially Asian ones.[3]
Cardiovascular disease (CVD) and Type 2 diabetes mellitus are the most common preventable complication of childhood obesity. Evidences indicated that the presence of some biochemical indicators such as insulin resistance, hyperinsulinemia, and hyperhomocysteinemia are associated with the early phase of mentioned conditions and proper interventions could prevent the development of such complications.[4,5]
It has been described that the high level of homocysteine (Hcy) is associated with CVD, obesity, and other clinical conditions such as hypertension and type 2 diabetes mellitus.[6,7]
The relation between plasma Hcy concentrations and insulin resistance in different groups of patients, including obese ones has been reported in some epidemiological studies.[8,9] It is well established that hyperhomocysteinemia is associated with childhood obesity and hyperinsulinism, and moreover there are evidences for increased level of the two mentioned factors among obese children.[10,11]
Evidences have documented a negative relationship between serum Hcy and folate, as well as insulin and folate.[10,12] Accordingly the concept of effectiveness of folic acid administration for reducing Hcy level and consequently its related risk factor has been developed and some studies have demonstrated the improving effect of mentioned supplement use in this regard. The usefulness of folic acid in decreasing both Hcy and insulin level among obese patients have been reported in previous studies mainly among the adult population.[13,14,15] There were few studies in this field for pediatrics population.
Thus, considering the worldwide increasing trend of childhood obesity and the subsequent burden of the disease and importance of early life interventions for preventing obesity related complication from early life period and low side effects of folic acid than other pharmacologic agents, this study aimed to investigate the effect of two different doses of folic acid on Hcy level and insulin resistance of obese and overweight children.
MATERIALS AND METHODS
In this randomized, double-blind controlled clinical trial study, 60 obese and overweight children aged 5–12 years, attended to endocrinology clinic, affiliated to Shahrekord University of Medical Sciences, were enrolled.
Children with body mass index (BMI) >85th percentile were included in the study. Those with secondary obesity (due to endocrine disorder or genetic syndromes), renal and hepatic dysfunction, history of using anticonvulsant agents, estrogen, thiazides, metformin, cholestyramine, methotrexate, fibrates, nicotinic acid, vitamin supplement (1-month before study) were excluded. In addition, those who were under weight loss diet or had not appropriate cooperation and regular follow-up were also excluded.
The protocol of the study was approved by Pediatrics Review Board and Regional Bioethics Committee of Shahrekord University of Medical Sciences. The study was registered in the Iranian Registry of Clinical Trials (IRCT), IRCT registration number (2014020116435N1). Written informed consent was obtained from all selected patients or their parents after explanation of the methods and goal of the study.
Selected obese children randomly allocated in two interventional (1 mg/day and 5 mg/day folic acid for 8 weeks) and one control groups [Figure 1].
Figure 1.
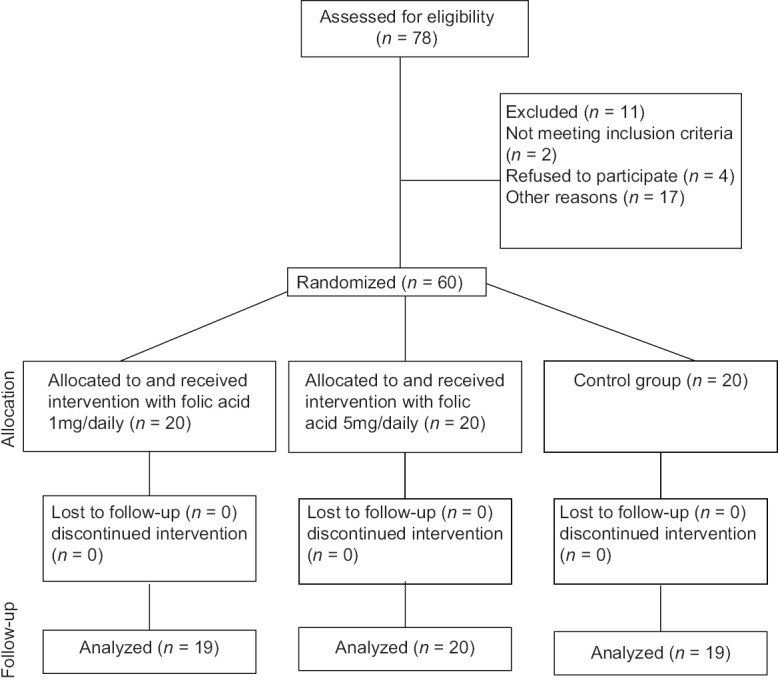
Consort diagram of the study
Folic acid (1 and 5 mg) supplied by Rouz Darou Pharmaceutical Company.
All patients examined clinically, and their demographic and anthropometrics (BMI) information recorded by a trained nurse using a questionnaire. The level of folic acid, Hcy, insulin and insulin resistance (hemostasis model assessment [HOMA]-IR) were measured in all participants before and after trial.
The levels of biochemical measurements before and after trial between and within groups were compared.
Laboratory measurements
Venous blood samples obtained from each participant after overnight fasting.
Folic acid measured using radioimmunoassay method by DRG kits (RIA-1990, Germany) kit.
Insulin measured using enzyme-linked immunosorbent assay method by using AccuBind (Costa Mesa, CA 92627 USA) kit.
Homocysteine measured by immunoassay method using Axis-Shield Diagnostics (Dundee, U.K.) kit.
Insulin resistance was measured using HOMA-IR formula (HOMA-IR = fasting insulin [µU/ml] × fasting glucose [mg/dL]/405).
Statistical analysis
Data analyzed using SPSS ver.20 (SPSS Inc., Chicago, IL, USA). The mean of studied variables before-after study and between groups compared using t-test and ANOVA, respectively.
RESULTS
In this trial 60 obese and overweight children allocated in two interventional and one control groups (20 obese children in each group). Demographics characteristic of studied populations in the three groups are presented in Table 1. The three groups were age and sex matched and mean of BMI was not different significantly (P > 0.05). Mean ± standard deviation (SD) of biochemical measurements including folic acid, Hcy, HOMA-IR, and insulin in the three studied groups before and after the trial is presented in Table 2. In Group 1 and 2 after folic acid administration for 8 weeks, mean of insulin, HOMA-IR and Hcy were decreased significantly (P < 0.001). In the control group, there was no change for folic acid, Hcy and insulin, but insulin resistance increased significantly (P < 0.05).
Table 1.
Demographic characteristics of obese and overweight children in three studied groups (folic acid 1 mg/daily, folic acid 5 mg/daily, and control group)
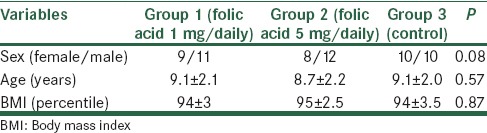
Table 2.
Mean±SD of biochemical measurements including folic acid, Hcy, HOMA-IR, and insulin in the three studied groups (folic acid 1 mg/daily, folic acid 5 mg/daily, and control group) before and after trial
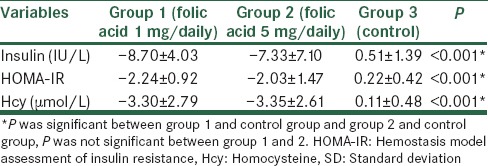
Mean ± SD differences (before-after) of studied biochemical variables in the three groups are presented in Table 3. Mean differences were significant between interventional groups and controlled one, but there were not different significantly between the two interventional, that is, 1 mg/day versus 5 mg/day folic acid groups (P > 0.05).
Table 3.
Mean±SD differences (before-after) of Hcy, HOMA-IR, and insulin in the three studied groups (folic acid 1 mg/daily, folic acid 5 mg/daily, and control group)
DISCUSSION
In this study, the effect of two different doses of folic acid on the level of Hcy and insulin resistance of obese and overweight children was evaluated. Our findings indicated that folic acid in both low and high dose administration could decrease the level of Hcy and insulin resistance among overweight and obese children.
It is well established that obesity in children is associated with increased risk of CVD in adulthood. Previous studies have indicated the higher level of metabolic risk factors among obese children.[16] There were also evidences regarding the elevated level of homocystein and insulin resistance in this group of patients.[10,11,17]
The specificity and importance of Hcy and insulin resistance in children and adolescents is due to the fact that, they are considered as independent determinant of metabolic risk which are not influenced by puberty and different Tanner stage and can identify the subjects prone to complications.[18]
Some studies indicated the positive correlation between Hcy and insulin resistance among obese children. Gallistl and associates in Austria have shown for the 1st time that the level of Hcy in obese children is significantly correlates with the level of insulin. They concluded that possibly hyperinsulinemia could result in Hcy metabolism impairment in this group of population.[19]
On the other hand, there are evidences regarding the role of some environmental factors such as dietary folate on the level of Hcy.[19]
In a study in Turkey, Atabek et al. have investigated total plasma Hcy in obese children. They reported a higher level of Hcy in this group of patients compared with control nonobese children. They also indicated that Hcy could have a crucial role in the higher risk of CVD in obese children, and it correlates with decreased folate state but not insulin. They suggested the possible role of decreased folate in the impairment of Hcy metabolism in childhood obesity.[10]
Regarding the fact that obese children have a higher level of Hcy, it seems that folate administration possibly could have a protective effect on hyperhomocysteinemia among obese children. Thus, this study was designed to evaluate the improving effect of two different doses of folic acid on mentioned risk factors.
There are some studies, which investigated the effect of folic acid on the level of Hcy and insulin resistance among the obese adult population.[13,14,15] Studies among children population were scarce. It has been hypothesized that folic acid supplementation may improve the oxidative stress and consequently reduce the risk of CVD and diabetes mellitus, possibly by decreasing the level of Hcy and insulin.[20]
The results of the current study demonstrated that both high and low dose folic acid administration for 8 weeks reduce the level of Hcy, insulin resistance, and insulin level.
Solini et al. in Italy have investigated the effect of 12 weeks folic acid treatment on insulin sensitivity and some biochemical parameters such as Hcy. After that period Hcy decreased, and insulin sensitivity increased significantly among obese adults.[13]
Peña and Associates in Austria have indicated that administration of folic acid (5 mg daily) in 53 obese children increased serum folate and decreased total Hcy.[21]
In a study in Greece, Papandreou et al. showed that oral folic acid (5 mg twice per week) could efficiently reduce serum Hcy level and increase serum folic acid level in healthy children with hyperhomocysteinemia.[22]
Gargari et al. in Tabriz-Iran showed that folic acid supplementation decreased plasma level of homocysteine and insulin resistance, as well as improved glycemic control in overweight patients with Type 2 diabetes mellitus.[14]
According to the results, of a meta-analysis regarding the effectiveness of different vitamin supplements including folic acid on Hcy level, an appropriate dose range of folic acid for this purpose is 0.5–5 mg.[23] Furthermore, Smith et al. have reported that a higher dose of folate could be harmful.[24] Though 5 mg daily folic acid is an acceptable dose, not high dose, but in this study we aimed to compare the lower dose of folic acid 1 mg/daily with its higher dose, that is, 5 mg/daily to determine whether lower dose has the same effect or not. The results of our trial demonstrated that there were not any significant differences between the effectiveness of two administrated doses of folic acid on studied variables. Both of them had a similar effect.
Studies among patients with chronic renal failure relieved that there was not any significant difference between different doses of folic acid for reducing the level of Hcy.[25,26] It seems that saturation of erythrocyte by folate prevents further reduction of Hcy level.[23] However, more studies for investigating the exact causes of the findings are necessary.
The limitations of the current study were small sample size of studied population, lack of evaluation of different metabolic risk factors and the effectiveness of folic acid on them and lack of follow-up evaluation for the long-term effect of folic acid administration in this field.
In sum, considering the similar effectiveness of high and low dose folic acid in decreasing the level of Hcy, insulin and insulin resistance, it is suggested that low dose of folic acid could be a safe and effective supplement for obese children for improving the mentioned factors, which consequently could prevent obesity-related complications including cardiovascular and metabolic disorders. For obtaining more conclusive results in this field, more studies with consideration of mentioned limitations is recommended. Further, the role of folic acid deficiency in the path physiology of obesity in children could also be investigated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
To Vice Chancellor for Research of Shahrekord University of Medical Sciences and Cellular Molecular Research Center and all health staff and the children who participated in this investigation.
REFERENCES
- 1.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond) 2009;33(Suppl 1):S60–5. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–64. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Park SB, Georgiades A. Changes in body composition predict homocysteine changes and hyperhomocysteinemia in Korea. J Korean Med Sci. 2013;28:1015–20. doi: 10.3346/jkms.2013.28.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesari M, Zanchetta M, Burlina A, Pedon L, Maiolino G, Sticchi D, et al. Hyperhomocysteinemia is inversely related with left ventricular ejection fraction and predicts cardiovascular mortality in high-risk coronary artery disease hypertensives. Arterioscler Thromb Vasc Biol. 2005;25:115–21. doi: 10.1161/01.ATV.0000149674.62430.e7. [DOI] [PubMed] [Google Scholar]
- 7.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 Suppl 1):III15–9. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis. 2003;167:105–9. doi: 10.1016/s0021-9150(02)00386-6. [DOI] [PubMed] [Google Scholar]
- 9.Gillum R. Distribution of serum total homocysteine and its association with diabetes and cardiovascular risk factors of the insulin resistance syndrome in Mexican American men: The Third National Health and Nutrition Examination Survey. Nutr J. 2003;2:6. doi: 10.1186/1475-2891-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atabek ME, Bağcı Z, Pirgon Ö, Erkul İ. Plasma total homocysteine levels in childhood obesity. Turk J Endocrinol Metab. 2004;3:107–11. [Google Scholar]
- 11.Martos R, Valle M, Morales R, Cañete R, Gavilan MI, Sánchez-Margalet V. Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children. Metabolism. 2006;55:72–7. doi: 10.1016/j.metabol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Garcia G, Trejos J, Restrepo B, Landázuri P. Homocysteine, folate and vitamin B12 in Colombian patients with coronary disease. Arq Bras Cardiol. 2007;89:71. doi: 10.1590/s0066-782x2007001400002. [DOI] [PubMed] [Google Scholar]
- 13.Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes (Lond) 2006;30:1197–202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 14.Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:33–8. doi: 10.1016/j.diabres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;98:151–8. doi: 10.1016/j.diabres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS. Consequences of childhood obesity. Ann Acad Med Singapore. 2009;38:75–7. [PubMed] [Google Scholar]
- 17.Garanty-Bogacka B, Syrenicz M, Szolomicka-Kurzawa P, Gebala A, Goral J, Krupa B. Correlation between serum homocysteine levels and selected atherosclerosis risk factors in children and adolescents with simple obesity. Przegl Lek. 2006;63:645–9. [PubMed] [Google Scholar]
- 18.Codoñer-Franch P, Murria-Estal R, Tortajada-Girbés M, del Castillo-Villaescusa C, Valls-Bellés V, Alonso-Iglesias E. New factors of cardiometabolic risk in severely obese children: Influence of pubertal status. Nutr Hosp. 2010;25:845–51. [PubMed] [Google Scholar]
- 19.Gallistl S, Sudi K, Mangge H, Erwa W, Borkenstein M. Insulin is an independent correlate of plasma homocysteine levels in obese children and adolescents. Diabetes Care. 2000;23:1348–52. doi: 10.2337/diacare.23.9.1348. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: A randomized, controlled trial. Diabetes. 2009;58:1921–8. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peña AS, Wiltshire E, Gent R, Piotto L, Hirte C, Couper J. Folic acid does not improve endothelial function in obese children and adolescents. Diabetes Care. 2007;30:2122–7. doi: 10.2337/dc06-2505. [DOI] [PubMed] [Google Scholar]
- 22.Papandreou D, Malindretos P, Arvanitidou M, Makedou A, Rousso I. Oral supplementation of folic acid for two months reduces total serum homocysteine levels in hyperhomocysteinemic Greek children. Hippokratia. 2010;14:105–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke R, Armitage J. Vitamin supplements and cardiovascular risk: Review of the randomized trials of homocysteine-lowering vitamin supplements. Semin Thromb Hemost. 2000;26:341–8. doi: 10.1055/s-2000-8101. [DOI] [PubMed] [Google Scholar]
- 24.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 25.Tamadon MR, Jamshidi L, Soliemani A, Ghorbani R, Malek F, Malek M. Effect of different doses of folic acid on serum homocysteine level in patients on hemodialysis. Iran J Kidney Dis. 2011;5:93–6. [PubMed] [Google Scholar]
- 26.Dierkes J, Domröse U, Ambrosch A, Bosselmann HP, Neumann KH, Luley C. Response of hyperhomocysteinemia to folic acid supplementation in patients with end-stage renal disease. Clin Nephrol. 1999;51:108–15. [PubMed] [Google Scholar]


