Abstract
Background:
Methicillin-resistant Staphylococcus aureus (MRSA) is a frequent cause of infections. The changing epidemiology of MRSA became evident in the 1990s when CA-MRSA cases were first reported. Nasal carriage of CA-MRSA is associated with an increased risk for development of infections in various populations.
Materials and Methods:
Anterior nares culture for the presence of methicillin-susceptible Staphylococcus aureus (MSSA) and MRSA was taken from 345 children attending kindergartens, who didn’t have any known risk factor for MRSA colonization. Also, children demographic variables were recorded. Identification of SA and community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) with standard microbiological test was performed. Finally, the susceptibility of isolated to various antibiotics determined. The data were analyzed with Whonet 5.6 software.
Results:
Of 345 children, 20 children (5.8%) were colonized with CA-MRSA, 86 children (24.9%) with MSSA and 239 cases (69.3%) didn’t have SA colonization. The highest rate of MSSA and MRSA colonization was obtained at the age of 6 years. The frequency distribution of SA (MSSA and MRSA) colonization prevalence didn’t have any significant differences based on age, gender and the admission time (P > 0.05); but it was significantly different in the urban areas (P < 0.001). The lowest resistance rate of CA-MRSA isolates, with a frequency of 10%, was detected with gentamicin, rifampin, and trimethoprim-sulfamethoxazole.
Conclusions:
In summary, CA-MRSA colonization was observed in child care centers remarkably. Therefore, by facing various infections due to SA especially in areas of low socio-economic status, it must be considered. Based on antibiogram test, empirical treatment with rifampin, gentamicin and ciprofloxacin is recommended during CA-MRSA infections.
Keywords: Community-acquired, methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, methicillin-susceptible Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus (SA) is a commensal micro-organism that can be found in healthy people.[1] It is frequently found on the skin, axilla, perineum and in the nares of healthy individuals.[2,3] Its carriage in healthy individuals is mostly situated in the nose. Carriage of SA rarely causes disease in healthy individuals, but it is associated with an increased risk for the emergence of infections in various populations.[1,2,4] SA infections are common in hospitals and community settings.[4] Its infections cause substantial morbidity and mortality. Studies have showed that the risk of SA infections is higher in male gender, very young and elderly individuals. In addition, two studies identified that the most important risk factor is dialysis, either peritoneal or hemodialysis. Other risk factors of invasive SA infections include diabetes, cancer, rheumatoid arthritis, HIV infection, intravenous drug use, or alcohol abuse. Nevertheless, one of the most important factors that independently added to these predisposing conditions is chronic SA nasal carriage. Whether they are in the hospital or in the community, patients mostly become infected with their own carriage strain.[5] Since the introduction of antibiotics, SA has quickly become resistant to many significant antibiotics such as the β-lactams and macrolides.[5,6] Methicillin resistance is the most significant antibiotic resistance of SA.[5] Methicillin resistance is mediated by penicillin binding protein (PBP)-2a, a PBP encoded by mecA gene that permits the organism to grow in the presence of methicillin and other β-lactam antibiotics. The mecA gene is located on a mobile genetic element called Staphylococcal Chromosome Cassette (SCC mec).[5,6,7] methicillin-resistant SA (MRSA) first appeared in the 1960s and has been a hospital-related phenomenon for a few decades.[1,2,4] Healthcare-associated MRSA (HA-MRSA) has usually been linked to persons with HA risk factors such as hospitalization or nursing home care, chronic dialysis, and antibiotics treatment.[2,6] Community associated methicillin-resistant S. aureus (CA-MRSA) infections emerged in persons having none of the risk factors associated with MRSA in the 1990s.[1,2,6] The epidemiology of MRSA changed through the emergence of community-acquired MRSA (CA-MRSA).[8] An increase in the number of cases of MRSA infection and colonization in community will influence the precaution to be taken against infections and the recommended empirical treatment.[9] CA-MRSA was also shown to have higher expression levels of toxin, phenol soluble modulins, and hemolysins, suggesting that these isolates are more virulent than HA-MRSA.[5] Today CA-MRSA has become the most frequent cause of skin and soft-tissue infections acquired in the community. Group with high-intensity physical contact is especially affected this, includes competitive athletes, children in daycare centers, military recruits, injecting drug users, jailed inmates, and homosexual men. In addition, CA-MRSA has started to spread from the community into the hospital, where outbreaks of infections with typical CA-MRSA have occurred. In spite of HA-MRSA, CA-MRSA is susceptible to many none β-lactam antibiotics.[10] The primary reservoirs of SA are the anterior nares, but the organism can be isolated from multiple sites. Therefore, the purpose of this study was to survey prevalence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) carriers in children from 2 to 6 years without any known risk factors for MRSA colonization that performed in child care centers (kindergartens) of Isfahan in 2013–2014. We also studied methicillin-susceptible SA (MSSA) and MRSA colonization according to age, sex, urban areas and the average time of attendance at childcare centers (years). In addition, the susceptibility of isolated CA-MRSA to various antibiotics was determined.
MATERIALS AND METHODS
This surveillance was conducted in 15 child care centers (kindergartens) of Isfahan. This research was a descriptive study that conducted on 345 children aged from 2 to 6 years, from March 2013 to March 2014. At first, 15 kindergartens in a different part of Isfahan were randomly selected. Then, sampling of 345 children (2–6 years old) who didn’t have any known risk factor for MRSA colonization was done. Data on age, sex, the average time of attendance at childcare centers and urban areas (based on Isfahan education and training center classification) were recorded. About the urban areas, it should be mentioned that because of very close distance of regions 1 and 2 and lack of childcare centers in region 2, they were considered as one. Children with a history of skin infection or hospitalization in 3 months ago and children whose parents worked in healthcare centers were not included in the study.
The swabs were obtained from both nares. Cultures in trypticase soy broth + NaCl 6.5% were performed and incubated at 35°C for 24 h. Then 10 µl of trypticase soy broth was cultured on Mannitol Salt agar, incubated for 24 h; and identification of SA with standard microbiological test was performed on growth bacteria’s, then MRSA was identified by disk diffusion method with 30 µg disk of cefoxitin and also oxacillin screen agar method. Finally, the susceptibility of isolated to various antibiotics determined with disk diffusion method based on Clinical Laboratory Standards Institute 2013 guidelines. The data were analyzed with Whonet 5.6 software (WHO Collaborating Centre).
RESULTS
In this study, 345 children with no known risk factors for colonization with MRSA were studied from 15 child care centers. The subjects were aged between 2 and 6 years with a mean of 5.38 (±0.89) years. In the study group, 182 (52.8%) of children were males and 163 (47.2%) were female. The mean age of boys and girls was 5.35 (±0.94) and 5.41 (±0.83) years respectively; and according to t-test, there was no statistically significant difference between the two genders (P = 0.5). In Figure 1, the age distribution of children is shown based on sex. The average time of attendance at childcare centers was 1.47 (±0.87) years in the subjects ranged from 1 to 6 years. Out of the 345 studied children, 20 cases (5.8%) were colonized with MRSA, 86 cases (24.9%) with MSSA and 239 cases (69.3%) didn’t have SA colonization [Figure 2].
Figure 1.
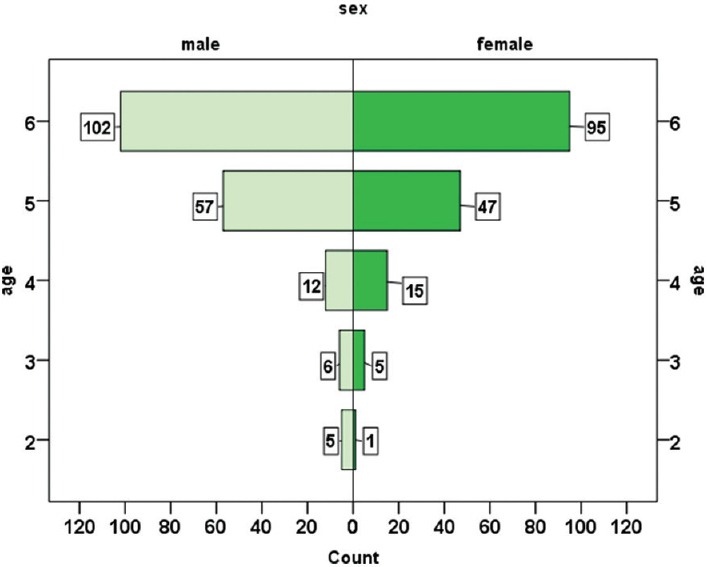
The distribution of age and sex
Figure 2.
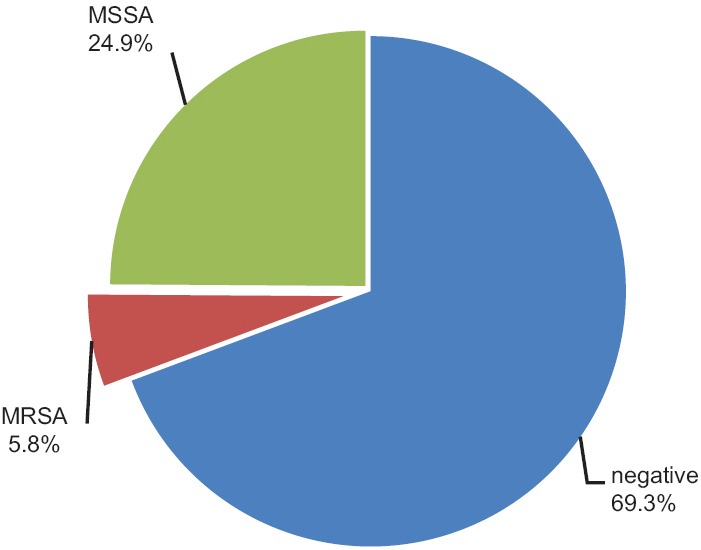
Frequency of Staphylococcus aureus colonization
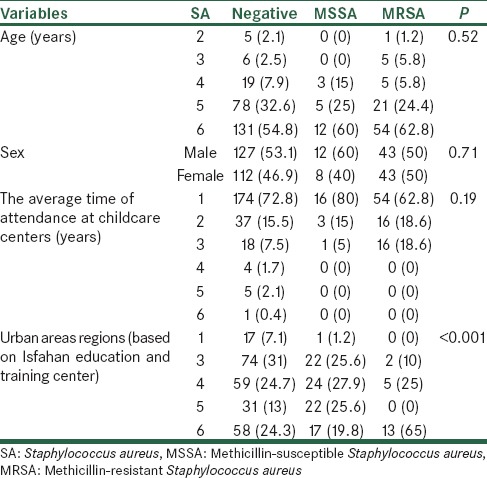
In Table 1, the frequency distribution of SA colonization (MRSA and MSSA) based on the demographic variables of children is shown. Based on the above table, the highest rate (60%) of MRSA colonization was obtained at the age of 6, so that, 12 of all MRSA discovered at this age. In addition, the highest MSSA colonization rate with the prevalence of 54 cases (62.8%) was found at the same age. Nevertheless, Fisher’s exact test, showed no significant association between SA colonization and age (P = 0.52). In terms of gender distribution, 12 cases of MRSA observed in the boys and 8 cases in the girls (60% vs. 40%) and MSSA colonization was equal in both sexes; according to Chi-square test, there was no association with the SA colonization in the two sexes (P = 0.71). According to Chi-square and Fisher’s exact tests, frequency distribution of SA colonization prevalence didn’t have any significant differences based on age, gender and the time of attendance at childcare centers (P > 0.05). Most cases of MRSA positive (16 cases) were children who had the history of 1-year attendance at daycare centers and also the most MSSA colonization with frequency of 54 (62.8%), were observed in this group, but according to Fisher’s exact test, the frequency distribution of SA colonization in terms of the time of attendance at childcare centers, didn’t show any significant differences (P = 0.19). On the other hand, according to our results, the frequency distribution of SA colonization (MSSA and MRSA) was significantly different in the urban areas (P < 0.001). The highest prevalence (65%) of CA-MRSA colonization was found in the sixth urban area (n = 13). The second-ranked region was the fourth area with frequency of 5 (25%) cases.
Table 1.
Frequency distribution of SA based on the demographic variables
Evaluation of antibiotic resistance in isolated CA-MRSA strains showed that, all isolated CA-MRSA strains were resistant to penicillin and cefoxitin. Also, 45% of them were resistant to erythromycin and tetracycline. Resistant to clindamycin was 20% and the lowest resistance rate, with a frequency of 10%, was detected with gentamicin, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMZ). In Figure 3, the frequency of antibiotic-resistant of discovered CA-MRSA strains is shown.
Figure 3.
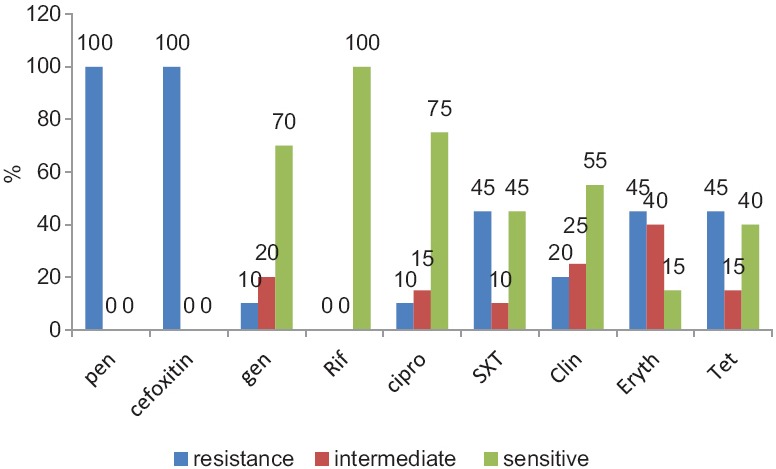
Frequency of antibiotic-resistant strain of methicillin-resistant Staphylococcus aureus discovered
DISCUSSION
The emergence of MRSA in the community is of paramount importance and is the subject of multiple studies in a variety of clinical settings and from many parts of the world. This study was designed to answer several specific questions. At first, how much is the actual rate of SA nasal colonization whether sensitive or resistant to methicillin in child care centers children? Our study was conducted in Isfahan. According to this study, 24.9% and 5.8% of children in kindergartens were colonized with MSSA and CA-MRSA, respectively. In 2004, Erdenizmenli et al. conducted a study on children under 10 years, the prevalence of MSSA colonization was 19.1%, but none has been MRSA.[9] In a national study conducted in 2008 in Hamadan, 22.3% of children admitted to the pediatric wards of Hamadan hospital were colonized with SA; the results of this study showed that 13.5% of these children had MRSA colonization.[11] In 1385, Loghmany conducted a study on kindergarten children of Isfahan, 35.6% of children at age 2–6 years had nasal colonization with SA that 7% of them were CA-MRSA. In 2010, Singh et al. studied SA (MSSA and MRSA) colonization in physical and mental handicapped children of India, the frequency of SA and MRSA, was reported 11.3% and 0%, respectively.[12] A study in the USA found that 32.4% of the population carried SA and 0.8% MRSA.[1] The prevalence of colonization with SA in our society is approximately similar to other countries, but the prevalence of CA-MRSA is higher. By comparison with the previous study in Isfahan that conducted in 2007, the prevalence of SA and CA-MRSA carriage has declined. The reason for this finding is not clear. It is necessary for the empirical treatment of infections due to SA, CA-MRSA prevalence to be considered. In addition, preventive health measures should be taken in this regard.
Second, there is evidence that CA-MRSA is intrinsically different to that of hospital origin. These differences manifest in antibiotic susceptibility patterns. The community strains are more sensitive to various anti-staphylococcal agents than those of hospital origin. These antibiotics include clindamycin, erythromycin, TMP/SMZ, gentamicin, rifampin, and fluoroquinolones.[1,5,7,10] A study conducted in Hamedan in 2008, resistance of CA-MRSA isolates were 33.3% against erythromycin, 11.1% against clindamycin.[11] In a study conducted in 2012 by the Etok et al., the MRSA isolates exhibited 31.1% resistance to erythromycin, 20% to clindamycin, 29.4% to ceftriaxone, 35.6% to ciprofloxacin, 51.7% to TMP-SMZ and 22.2% to amoxicillin-clavulanic acid.[13] In 2007, Tiwari et al. studied 162 strains of SA were isolated from various clinical specimens. Out of 162 isolates, 112 (69.1%) were found to be methicillin-resistant. Of the 112 MRSA isolates, 45 (41%) were multi-drug resistant. Very high degrees of resistance were observed with penicillin (100%), amoxicillin (91.8%), ampicillin (90%), co-trimoxazole (72.7%), and cephalexin (66.03%); relatively lower degrees of resistance were observed with amikacin (40%), ciprofloxacin (45.8%), and norfloxacin (43.4%).[14] Kaplan noted that most of the CA-MRSA isolates are susceptible to vancomycin, gentamycin, rifampicin, cotrimoxazole, clindamycin, doxycycline, and linezolid.[15] In the Sobhya study, 100% of the CA-MRSA isolates were resistant to fusidic acid.[16] Our evaluation of antibiotic resistance in CA-MRSA isolates showed that, all CA-MRSA isolates were resistant to penicillin and cefoxitin. Also, 45% of them were resistant to erythromycin and tetracycline. Resistant to clindamycin was 20% and the lowest resistance rate, with a frequency of 10%, was detected with gentamicin, rifampin, and TMP-SMZ. Frequency of CA-MRSA isolates resistance to rifampin was 0%. Antibiotic sensitivity patterns of the CA-MRSA strains isolated by us are different from those mentioned in the literature. These findings must be considered when empirical antibiotics treatment begins during SA infections.
Third, studies have shown that most children at a very young age in contact with the organism to be colonized. So if the mother is colonized, the baby will be colonized shortly after birth. Colonization rate increases with increasing age of children. But after 10 years, colonization rate will be reduced. Hence, the maximum rate of sustain colonization is seen in children under 10 years. The results of our study showed that the frequency distribution of SA colonization prevalence didn’t have any significant differences based on age, gender and the time of attendance at childcare centers. On the other hand, according to our results, the frequency distribution of SA colonization (MSSA and MRSA) was significantly different in the urban areas (P < 0.001). The highest prevalence (65%) of CA-MRSA colonization was found in the sixth urban area (n = 13). The second-ranked region was the fourth area with frequency of 5 (25%) cases. Both the 4 and 6 urban regions are crowded areas with a low socioeconomic level.
Considering the obtained results in this study and comparison with other studies, the general conclusion that can be found from this study is that CA-MRSA among children under 6 years has relatively high prevalence. It is necessary to determine the degree of persistent in contrast to intermittent colonization, the incidence of infectious complications induced by CA-MRSA in colonized patients, specifying of various infectious complications, identifying geographic pathology and comparing it to other parts of the world, the age conversion from carrier to noncarrier and following noncarriers annually to detect rate of new colonization. On the other hand, due to the sensitivity of CA-MRSA to the numbers of non-β-lactam antibiotics (unlike HA-MRSA), it is recommended to determine sensitivity pattern of the organism against non-β-lactam antibiotics. It helps to avoid indiscriminate use of antibiotics, such as vancomycin, linezolid, and etc., reduce medical costs and the risk of resistance to these drugs in the future.
In summary, CA-MRSA colonization is observed in child care centers remarkably. Therefore, by facing various infections due to SA especially in low socioeconomic areas, this point must be considered. Based on our findings, empirical treatment with rifampin, gentamicin and ciprofoxacin is recommended during CA-MRSA infections. Other questions regarding the frequency of temporary versus permanent colonization in children, the frequency of colonization with increasing age, the incidence of infectious complications in colonized children and geographic pathology of CA-MRSA infections should be answered through prospective cohort study of this population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cohen J, Powderly WG, Opal SM. 3rd ed. London: Mosby Elsevier Science; 2010. Infectious Diseases. [Google Scholar]
- 2.Davis JP, Fox BC. Hospital and Clinics Community Associated Methicillin Resistant Staphylococcus aureus (CA MRSA) Guidelines for Clinical Management and Control of Transmission PPH 42160, University of Wsconsin, October, 2005 [Google Scholar]
- 3.Salmenlinna S, Lyytikäinen O, Vuopio-Varkila J. Community-acquired methicillin-resistant Staphylococcus aureus, Finland. Emerg Infect Dis. 2002;8:602–7. doi: 10.3201/eid0806.010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 5.Gerald L. 7th ed. London: Churchill Livingstone; 2010. Principles and Practice of Infectious Diseases. [Google Scholar]
- 6.Rolo J, Miragaia M, Turlej-Rogacka A, Empel J, Bouchami O, et al. High genetic diversity among community-associated Staphylococcus aureus in Europe: Results from a multicenter study. PLoS One. 2012;7:e34768. doi: 10.1371/journal.pone.0034768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DJ, Sexton DJ, Baron EL. Epidemiology of methicillin-resistant Staphylococcus aureus infection in adults-UptoDate version 19.3, 2011 [Google Scholar]
- 8.Faria NA, Oliveira DC, Westh H, Monnet DL, Larsen AR, Skov R, et al. Epidemiology of emerging methicillin-resistant Staphylococcus aureus (MRSA) in Denmark: A nationwide study in a country with low prevalence of MRSA infection. J Clin Microbiol. 2005;43:1836–42. doi: 10.1128/JCM.43.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdenizmenli M, Yapar N, Senger SS, Ozdemir S, Yuce A. Investigation of colonization with methicillin-resistant and methicillin-susceptible Staphylococcus aureus in an outpatient population in Turkey. Jpn J Infect Dis. 2004;57:172–5. [PubMed] [Google Scholar]
- 10.Samileh N, Siadati A, Mohammad F, Shahnaz R, Tabatabaei A. Methicillin resistant Staphylococcus aureus in children. Iran J Pediatr Soc. 2007;1:24–30. [Google Scholar]
- 11.Sedighi I, Yousefi MR, Pak N, Seif MA. D-test method for detection of inducible clindamycin resistance in Staphylococcus aureus. Iran J Pediatr. 2009;19:293–7. [Google Scholar]
- 12.Pathak A, Marothi Y, Iyer RV, Singh B, Sharma M, Eriksson B, et al. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatr. 2010;29:100. doi: 10.1186/1471-2431-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etok CA, Ochang EA, Inyang V, Onwuezobe IA, Asuquo EE. Antibiogram of nasal methicillin resistant Staphylococcus aureus (MRSA) from antenatal clinic attendees in a tertiary hospital, South-South Nigeria. IJAPA. 2012;2:79–82. [Google Scholar]
- 14.Tiwari HK, Das AK, Sapkota D, Sivrajan K, Pahwa VK. Methicillin resistant Staphylococcus aureus: Prevalence and antibiogram in a tertiary care hospital in western Nepal. J Infect Dev Ctries. 2009;3:681–4. doi: 10.3855/jidc.86. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan SL. Washington, DC: 2005. Clinical Implications of Community-acquired Methicillin-Resistant Staphylococcus aureus Program and Abstracts of the Pediatrics Academic Societies Annual Meeting May 14-17. Course # 5102. [Google Scholar]
- 16.Sobhy N, Aly F, Abd El Kader O, Ghazal A, Elbaradei A. Community-acquired methicillin-resistant Staphylococcus aureus from skin and soft tissue infections (in a sample of Egyptian population): Analysis of mec gene and staphylococcal cassette chromosome. Braz J Infect Dis. 2012;16:426–31. doi: 10.1016/j.bjid.2012.08.004. [DOI] [PubMed] [Google Scholar]


