Abstract
Background:
In Asian population, diabetes mellitus is increasing and has become an important health problem in recent decades. In Iran, cardiovascular disease (CVD) accounts for nearly 46% of the total costs spent for diabetes-associated diseases. Because individuals with diabetes have highly increased CVD risk compared with normal individuals, it is important to diagnosis factors that may increase CVD risk in diabetic patients. The study objective was to identify predictors associated with CVD mortality in patients with type 2 diabetes (T2D) and to develop a prediction model for cardiovascular (CV)-death using a competing risk approach.
Materials and Methods:
The study population consisted of 2638 T2D (male = 1110, female = 1528) patients aged ≥35 years attending from Endocrine and Metabolism Research Center in Isfahan for a mean follow-up period of 12 years; predictors for different cause of death were evaluated using cause specific Cox proportional and subdistribution hazards models.
Results:
Based on competing modeling, the increase in blood pressure (BP) (spontaneously hypertensive rats [SHR]: 1.64), cholesterol (SHR: 1.55), and duration of diabetes (SHR: 2.03) were associated with CVD-death. Also, the increase in BP (SHR: 1.85), fasting blood sugar (SHR: 2.94), and duration of diabetes (SHR: 1.68) were associated with other death (consist of cerebrovascular accidents, cancer, infection, and diabetic nephropathy).
Conclusions:
This finding suggests that more attention should be paid to the management of CV risk in type 2 diabetic patients with high cholesterol, high BP, and long diabetes duration.
Keywords: Cardiovascular disease, cause-specific hazard model, competing risks, subdistribution hazard model, type 2 diabetes
INTRODUCTION
Nowadays, noncommunicable diseases, especially in developing countries, are considered as the biggest worldwide health problem.[1] Among which, diabetes is traditionally known as a “silent disease,” presenting no symptoms until it progresses to severe target organ damage,[2] and is the major global cause of morbidity and mortality.[3] The most recent data from the International Diabetes Federation highlighted that the Middle East and North Africa region has the highest rate of diabetes prevalence in the world.[4]
The prevalence of type 2 diabetes (T2D) ranges from 2.6% to 15.1% in the Asia-Pacific region and 3.5% to 13.1% in subjects older than 30 years or older in Iran.[5] The mortality rate in diabetic patients is higher than normal people, and this has decreased the life expectancy for 5–10 years in middle-aged diabetic patients.[6] Because diabetic patients have a higher risk of developing microvascular disease and a 2–4-fold higher risk of developing the macrovascular disease than the general population.[7] Given the rapidly escalating financial and societal costs associated with diabetes care in developing countries, where resources to address the disease are severely limited, there is an urgent need for the development, implementation, and evaluation of programs to prevent T2D and its complications.[8] T2D is a well-established risk factor for cardiovascular disease (CVD).[9] Indeed, CVDs are the most prevalent causes of mortality and morbidity among people with T2D (accounting for up to 60–80% of the deaths, word wide).[10,11]
In Iran, CVDs account for nearly 46% of the total costs spent for diabetes-associated diseases.[6] More than half the mortality and a vast amount of morbidity in people with diabetes is related to CVD, which caused researchers in the fields of diabetes and CVD to join forces to research and manage these conditions.[12] Because individuals with diabetes have increased CVD risk compared with nondiabetic individuals, it is important to diagnose factors that may increase CVD risk in diabetic patients.[13]
Several risk factors increase the risk of cardiovascular (CV) conflicts in diabetic patients. The most common factors can be cited concurrency lipid disorders in patients with diabetes. Elevated serum triglycerides (TGs), reduced high-density lipoprotein cholesterol (HDL-C), and increased low-density lipoprotein cholesterol (LDL-C). The most common lipid disorders associated with T2D have an increased incidence of CV conflicts in these patients.[13] Hypertension is an important and powerful modifiable risk factor of CVD in patients with diabetes.[14] results from Multiple Risk Factor Intervention Trial (MRFIT) and United Kingdom Prospective Diabetes Study (UKPDS) indicate that blood glucose and blood pressure (BP) might have additive effects on the CVD risk of complications in patients with T2D.[15] One factor playing an important role in determining the cause of death in people with diabetes is the duration of diabetes. Mortality risk in T2D increased with increasing diabetes duration. Longer diabetes duration is associated with an increased prevalence of classical CV risk factors (hypertension, dyslipidemia, and prevalent heart disease), which may partly explain the increase mortality risk.[16] Serum TG is a risk factor for CVD in the general population and diabetic patients, especially for those with high non-HDL-C.[17]
Hypertriglyceridemia is a prevalent risk factor for CVD and increasingly important in the setting of current obesity and insulin resistance epidemics. High TG levels are markers for several types of atherogenic lipoproteins.[18] Obesity and overweight in diabetic patients with multiple mechanisms, make them susceptible to further increase CV events.[19] Body mass index (BMI) has been identified as a potential CVD risk factor. Overweight and obesity lead to adverse metabolic effects on BP, cholesterol, TG, and insulin resistance. Risks of coronary heart disease, ischaemic stroke, and T2D mellitus increase steadily with increasing BMI, a measure of weight relative to height.[20] Most of these studies were conducted in Western populations with T2D. To the best of our knowledge, there was no study to examine the association of such variables with the entirecause and CVD-related mortality in Iranian T2D populations. As Asian populations have a different lifestyle, the main objective of this population-based study was to determine the risk factors associated with CVD and of all-cause related mortality in a diabetic type 2 Asian population.
MATERIALS AND METHODS
This is a retrospective cohort study in subjects with T2D between the ages of 35 years and older in Isfahan. Patients were selected from records in the Endocrine and Metabolism Research Center in Isfahan. The study involved 2638 T2 diabetic patients (consisted of 1110 male and 1528 female) with complete information, which was followed for 12 years from 1992 to 2004. Covariates which included in this study were: Sex, duration of diabetes, age, fasting blood sugar (FBS), cholesterol, TG, BP, and BMI.
Inclusion criteria: Males and females over the age of 35 who present with T2D
Exclusion criteria: Persons not conforming to the above inclusion criteria along with individuals who have type 1 diabetes, patients who dead from surgery, accident, unknown or others, and those with missing data.
Clinical and laboratory measurements
The baseline information including demographic data (sex, age) and duration of diabetes were collected by a trained interviewer, using a pretested questionnaire.
Height was measured using a portable inflexible bar while the participants were looking straight forward with their heels against the wall. Weight was measured, using digital scales (Seca 707, Seca Corp., Hanover, MD; range 0.1–150 kg) and recorded to the nearest 100 g. During all the weight and height measurements, the individuals were in light clothing without shoes and socks. BMI was estimated by dividing weight in kilograms by the square of the height in meters.[21] BP was measured with appropriate sized cuffs using a standardized mercury sphygmomanometer (calibrated by the Iranian Institute of Standards and Industrial Researches) on the right arm, after a 15 min rest in a sitting position; The first Korotkoff sound indicated systolic BP (SBP), and the disappearance of the sound signified diastolic BP (DBP). The mean BP after two measurements with 5 min intervals was recorded. If the difference between SBP and/or DBP in the two measurements was more than 10 mm Hg, the third measurement was recorded. After 12–14 h overnight fasting, blood samples were drawn from veins of the participants into vacutainer tubes between 7.00 and 9.00 a.m. and centrifuged within 30–45 min of collection. FBS was measured using an enzymatic colorimetric method with glucose oxidase (human Gesellschaft mit beschränkter Haftung). TG was assayed using an enzymatic colorimetric method with glycerol phosphate oxidase. Total cholesterol was assayed using the enzymatic colorimetric method with cholesterol esterase and cholesterol oxidase. All laboratory kits are supplied by Pars Azmon Inc., Tehran, Iran (with biotechnical instruments model BT-3000 Germany).
Definition of terms
Positive BP was defined as a history of antihypertensive drug use or SBP ≥140 mm Hg or DBP ≥90 mm Hg.[22] In category of BP, negative was defined as normal BP (<139.85–89 mm Hg) and positive BP was defined as hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg), according to the 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension.[23] (BP negative was regarded as the reference group).
Diabetes duration was categorized, <10 years, 10–20 years and >20 years. (<10 years was regarded as the reference group). The other diabetes risk factors were defined as follows:
Baseline BMI was defined as normal weight (<25) and abnormal weight (≥25).[21]
(Normal weight was regarded as the reference group). Because of The incidence of diabetes increases with age until about age 65 years, we categorized age as ≤65 years and >65 years.[24] (≤65 years was regarded as the reference group). We categorized total serum cholesterol as ≤240 milligrams per deciliter (mg/dl), and >240 mg/dl.[25] (≤240 mg/dl was regarded as the reference group). FBS, was categorized as ≤126 mg/dl and >126 mg/dl.[26] (FBS ≤126 mg/dl was regarded as the reference group). Also, TG was categorized as ≤200 mg/dl and >200 mg/dl.[17] (TG ≤200 mg/dl was regarded as the reference group).
Statistical analysis
Descriptive analyses were used to summarize baseline characteristics of the study subjects according to cause of death (CVD and other cause consist of cerebrovascular accident [CVA], cancer, infections, and diabetic nephropathy [DN]). Frequencies and percentages were generated for categorical variables [Table 1]. To summarize the effect of patient or disease covariates in the competing risks setting, different regression models was used as follows:
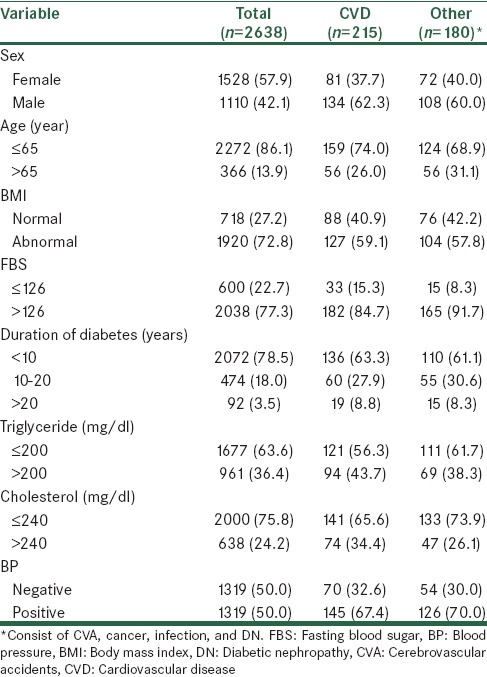
Table 1.
Baseline characteristics of 2638 study subjects according to cause of death
Cause-specific Cox hazard model
The associations between baseline covariates and various causes of death were assessed using cause specific Cox proportional hazards models that censored for the respective competing events.[27] This hazard measures the instantaneous failure rate due to one risk.[28]
Subdistribution hazard model
This model is based on the hazard of the subdistribution and provides a simple relationship between covariates and cumulative incidence.[29] The hazard of the subdistribution is interpreted as the probability of observing an event of interest in the next time interval while knowing that either the event of interest did not happen until then or that a competing risks event was observed.[30]
In this study, regression models taking competing risks into account (Cox cause-specific hazard model and Fine and Gray model based on subdistribution hazard model) were carried out to analyze the effect of covariates in T2D patients. The competing risk events were CVD related mortality, and other cause mortality (consist of CVA, cancer, infections, and DN). In examining the association between covariates and all-cause mortality, we used the Cox proportional hazards model. The statistical packages “survival,”[31] “cmprsk”[32] in R software (version 3.0.2) were used to perform the analyses, and the significance level was set at 5%.
RESULTS
The age range of 2638 T2D patients were 35–92 with a mean 54.1 ± 9.8 years. 86.1% of patients 65 years and less and 13.9% were above 65 years. 85% of patients were censored.
Table 1 provides a description of the baseline characteristics of the 2638 T2D patients. Generally, death from CVD in patients was more likely in younger (74%) and predominantly male (62%). Moreover, death from other cause mortality (consist of CVA, cancer, infection, and DN) were more likely in younger (69%) and male (60%). There were 395 (15%) deaths reported until the end of the study. Of these, 215 (54%) were CV-deaths and 180 (46%) were other death (consist of CVA, Cancer, infection, and DN).
According to Tables 2 and 3, for patient with T2D, there was an increased risk of all-cause mortality among patients with duration of diabetes >20 years (heart rate [HR]: 2.05, 95% confidence interval [CI]: 1.41–3.00, P = 0.000, <10 years as the reference group). There was also suggestion that being sex may increase the risk of all-cause death. Similarly, sex was associated with a greater risk of CVD mortality based on competing risk (spontaneously hypertensive rats [SHR]: 2.01, 95% CI 1.56–2.69, P = 0.000, female as the reference group) and Cox regression (HR: 2.06, 95% CI: 1.55–2.75, P = 0.000). Also, duration of diabetes >20 years was associated with a greater risk of CVD mortality based on competing risk (SHR: 2.03, 95% CI: 1.21–3.41, P = 0.006) and Cox regression (HR: 2.22, 95% CI: 1.33–3.69, P = 0.002). Hazard ratios for CVD-death were very similar to those of the Fine and Gray model for covariates that did not affect other death (consist of CVA, cancer, infection, and DN), that is, TG and cholesterol.
Table 2.
Results of all/cause and cause-specific mortality according to competing risks and cause-specific Cox regression models
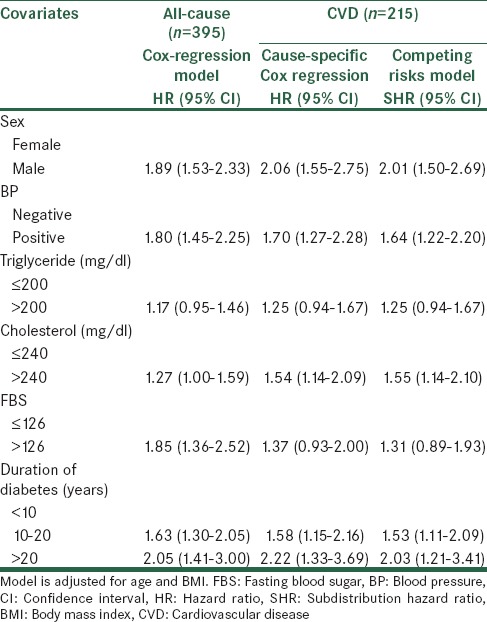
Table 3.
Results of all/cause and cause-specific mortality according to competing risks and cause - specific Cox regression models
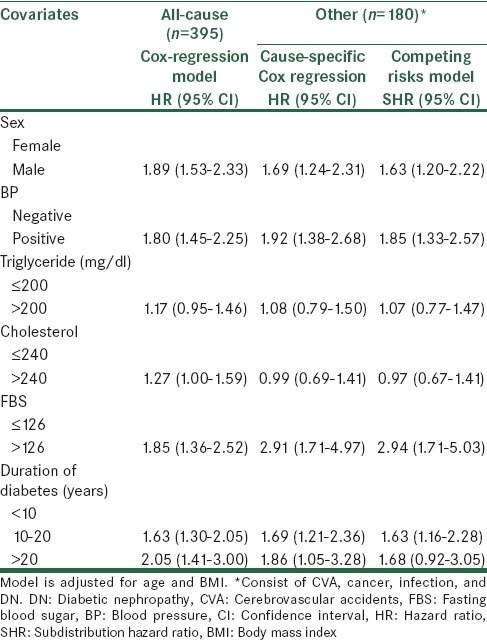
In contrast, sex, BP, FBS, and duration of diabetes were also strong predictors for other death (consist of CVA, cancer, infection and DN), and cause-specific hazard ratios for CVD were larger than in the Fine and Gray model.
Cholesterol was significantly associated with an increase in the rate of CVD-death (HR: 1.54, 95% CI: [1.14–2.09], P = 0.004, ≤240 mg/dl as the reference group), whereas it significantly decreased the rate of other death (consist of CVA, cancer, infection, and DN) (HR: 0.99, 95% CI: [0.69–1.41], P = 0.970). These results are in a column with those displayed by the cumulative incidence analyses. Indeed, cholesterol increases the probability of CVD-death (SHR: 1.55, 95% CI: [1.14–2.10], P = 0.004), whereas, reducing that of other death (SHR: 0.97, 95% CI: [0.67–1.41], P = 0.91). With considering the cause-specific and subdistribution hazard models, the risk of CVD mortality increased with sex, BP, cholesterol, and diabetes duration. Although the conclusions drawn based on the two methods were largely similar, the magnitude of effect was larger, and with wider CI based on the cause-specific Cox model as compared with the competing risk regression in most instances.
In both models, patients with positive BP, high cholesterol, and a longer diabetes duration had significantly increased the risk of CVD mortality.
Information on the estimate of the cumulative incidence and its variance for competitive events at 8 times points 24, 48, 72, 96, 120, 168, 192, and 216 months are shown in Table 4. According the results obtained, probability of death from CVD after 96 months, 0.07%, and for other causes 0.06% which after 216 months, the estimated probability for each of the outcome of CVD and other causes (consist of CVA, cancer, infection, and DN), respectively, 0.08%, and 0.07% is obtained. In Figure 1 nonparametric estimates of the cumulative incidence functions for the two competitive events at different points in time are presented.
Table 4.
The CIF estimates for two type of event with the variance
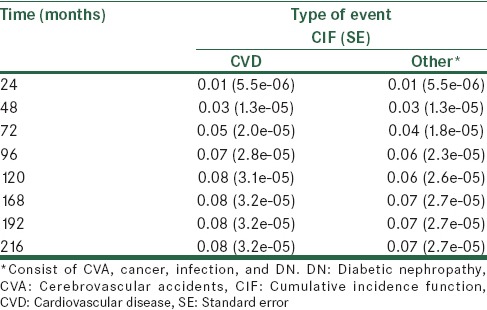
Figure 1.
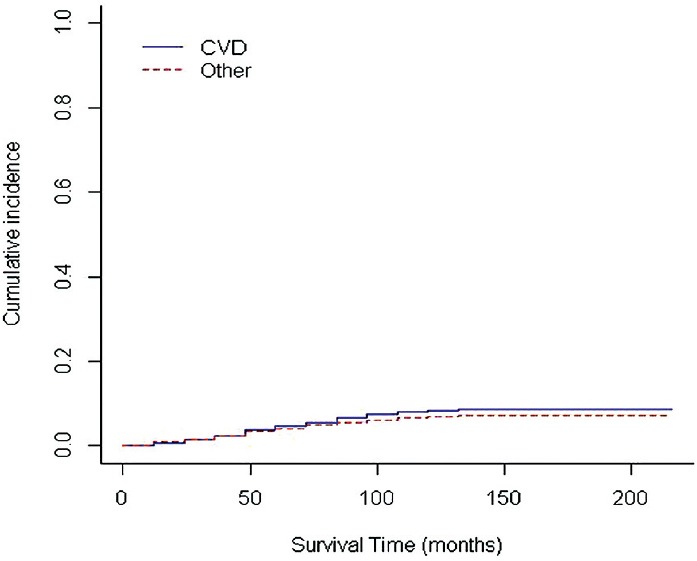
Cumulative incidence function estimates for two types of event
The probability of patient death due to one of the two causes until 48 elementary month has no difference and between 48 and 216 months, the risk of death due to CVD is higher than other (consist of CVA, cancer, infection, and DN).
DISCUSSION
In this article, we considered the cause-specific and subdistribution hazard models for evaluating the effect of one or more prognostic factors on time-to-event outcomes involving competing risks. The cause-specific Cox model censors competing events at the time when the main event occurs.
In a randomized clinical trial, its use may only be appropriate if the objective is to provide a measure of the treatment effect on a specific failure that is directly related to the treatment mechanism in isolation of other competing events. In such instances, estimates of the treatment effect on the main event can only be meaningfully interpreted by assuming that the events occur independently.
Besides, when performing a cause-specific hazard analysis, it is assumed that treatment has no effect on the hazards of competing risks.[33,34] In contrast, the subdistribution hazard model estimates the effect of a prognostic factor on the cumulative incidence of each event and is intuitively easier to understand.
Findings of this study showed that in cause-specific and subdistribution models for death from CVD, patients with BP (positive), duration of diabetes (more than 10 years), and cholesterol (more than 240 mg/dl), also for death from other (consist of CVA, cancer, infection, and DN), duration of diabetes (more than 10 years), FBS (more than 126 mg/dl), and BP, have a significant effects.
In our study, duration of diabetes was associated with the increased risk of CVD mortality in patients with T2D, in accordance with our study, some studies have noted an association between duration of diabetes and the development of CVD,[35,36] whereas, others have not.[37,38] Wannamethee et al.[39] demonstrated that participants with longer duration (≥8 years) had a significantly increased risk of CVD and total mortality compared with those with <2 years’ duration after adjustment for established and novel risk markers.
Brun et al.[40] show that CVD was the principal cause of death among people with T2D in the Verona diabetes study, and rates for natural causes of death rose with increasing duration of diabetes. Cho et al.[41] concluded that diabetes is associated with elevated total and coronary heart disease mortality. And the longer duration of diabetes is a strong predictor of death among diabetic patients.
A positive association was found between the presence of BP and CVD in our study. In accordance with our study, Chen et al.[42] found that the presence of hypertension is the strongest driver of CVD outcomes in individuals with diabetes mellitus. Lu et al.[43] reported that high BP is the main risk factor in increasing CVD risk in patients with diabetes. Hsieh et al.[44] investigated the association between BP and all-cause mortality, as well as CVD mortality in patients with T2D.
Boulanger and Hill[45] show that BP is positively associated with the risk of stroke and ischemic heart disease. Berry et al.[46] reported that good BP control in diabetes is associated with reduced risk of CVD. The UKPDS study found that tight lowering of BP markedly reduced the incidence of microvascular and macrovascular end points in patients with T2D.[47,48]
Similar to other studies Bonakdaran et al.[49] found a significant association between high BP and CVD in patients with T2D. In this study, we find a significant effect between cholesterol and CVD death, These findings are in agreement with Imperatore et al.[50] that have demonstrated for people with diabetes, high cholesterol levels increase the risk of heart disease, and stroke.
The MRFIT study showed that the absolute risk of CVD-death was at least 3 times higher for diabetic patients than for a nondiabetic population and that this relationship was amplified by serum cholesterol.[51]
Epidemiologic surveys have observed that elevated levels of total cholesterol and LDL-C are associated with increased risk of coronary heart disease.[52] Sytkowski et al.[53] explain among the numerous risk factors for CVD, the relationship between elevated serum cholesterol and CVD has long been recognized. Jousilahti in Finland showed that mortality rate from CVD among high cholesterol serum level people (over 300 mg/dl) is five folder than others and reducing of serum cholesterol level up to 10% can reduce the mortality due to CVD up to 30%.[54]
Also associated between serum cholesterol level and CVD has been shown by some studies.[55,56] Klag et al.[57] found a strong relationship between the baseline serum cholesterol level and the occurrence of coronary heart disease, as well as coronary heart disease mortality and total mortality, during subsequent 40 years.
Cox regression models for cause-specific hazards have the advantage that they are easy to fit. (Simply censor for competing events) and they provide parameter estimates which possess simple rate ratio interpretations. Such models, however, do not provide simple relationships between covariates and the easier interpretable cumulative incidences. Such simple relationships may be obtained from Fine–Gray models but the price to be paid is a set of parameter estimates which are harder to interpret.
CONCLUSION
This finding suggests that more attention should be paid to the management of CV risk in type 2 diabetic patients with high cholesterol, high BP, and long diabetes duration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lotfi MH, Saadati H, Afzali M. Prevalence of diabetes in people aged ≥30 years: The results of screen-ing program of Yazd Province, Iran, in 2012. J Res Health Sci. 2014;14:87–91. [PubMed] [Google Scholar]
- 2.Anjana RM, Ali MK, Pradeepa R, Deepa M, Datta M, Unnikrishnan R, et al. The need for obtaining accurate nationwide estimates of diabetes prevalence in India-rationale for a national study on diabetes. Indian J Med Res. 2011;133:369–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Ren X, Zhang X, Zhang X, Gu W, Chen K, Le Y, et al. Type 2 diabetes mellitus associated with increased risk for colorectal cancer: Evidence from an international ecological study and population-based risk analysis in China. Public Health. 2009;123:540–4. doi: 10.1016/j.puhe.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Javanbakht M, Abolhasani F, Mashayekhi A, Baradaran HR, Jahangiri Noudeh Y. Health related quality of life in patients with type 2 diabetes mellitus in Iran: A national survey. PLoS One. 2012;7:e44526. doi: 10.1371/journal.pone.0044526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmanian K, Shojaei M, Jahromi AS. Relation of type 2 diabetes mellitus with gender, education, and marital status in an Iranian urban population. Rep Biochem Mol Biol. 2013;1:1–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Kiadaliri A, Najafi B. Obesity in type 2 diabetes mellitus: A review of health economics’ evidences. Int J Healthc Insur Equity. 2013;1:1–8. [Google Scholar]
- 7.Vinagre I, Mata-Cases M, Hermosilla E, Morros R, Fina F, Rosell M, et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain) Diabetes Care. 2012;35:774–9. doi: 10.2337/dc11-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawal LB, Tapp RJ, Williams ED, Chan C, Yasin S, Oldenburg B. Prevention of type 2 diabetes and its complications in developing countries: A review. Int J Behav Med. 2012;19:121–33. doi: 10.1007/s12529-011-9162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444–70. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes MD. Impact of diabetes on cardiovascular disease: An update. Int J Hypertens 2013. 2013:1–15. doi: 10.1155/2013/653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan M, Wong T, Chan Y, Joseph A, Hejar A, Ng O. Predictors of cardiovascular disease in patients with type 2 diabetes mellitus. Int J Collab Res Intern Med Public Health. 2013;5:492–506. [Google Scholar]
- 12.Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 13.Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: The strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 14.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–76. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 16.Spijkerman AM, Dekker JM, Nijpels G, Jager A, Kostense PJ, van Hinsbergh VW, et al. Impact of diabetes duration and cardiovascular risk factors on mortality in type 2 diabetes: The Hoorn Study. Eur J Clin Invest. 2002;32:924–30. doi: 10.1046/j.1365-2362.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen AH, Tseng CH. The role of triglyceride in cardiovascular disease in asian patients with type 2 diabetes – A systematic review. Rev Diabet Stud. 2013;10:101–9. doi: 10.1900/RDS.2013.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13:544–52. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalangot M, Tronko M, Kravchenko V, Kulchinska J, Hu G. Body mass index and the risk of total and cardiovascular mortality among patients with type 2 diabetes: A large prospective study in Ukraine. Heart. 2009;95:454–60. doi: 10.1136/hrt.2008.150524. [DOI] [PubMed] [Google Scholar]
- 20.Alwan A. Geneva: World Health Organization; 2011. Global Status Report on Noncommunicable Diseases 2010. [Google Scholar]
- 21.Tan K. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 24.Health UDo, Services H, Control CfD, Prevention . Atlanta: GA; 2012. Prevention. Diabetes Report Card; 2012. [Google Scholar]
- 25.Roth GA, Fihn SD, Mokdad AH, Aekplakorn W, Hasegawa T, Lim SS. High total serum cholesterol, medication coverage and therapeutic control: An analysis of national health examination survey data from eight countries. Bull World Health Organ. 2011;89:92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 27.Koller MT, Raatz H, Steyerberg EW, Wolbers M. Competing risks and the clinical community: Irrelevance or ignorance? Stat Med. 2012;31:1089–97. doi: 10.1002/sim.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 29.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol. 2012;41:861–70. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pintilie M. Canada: John Wiley and Sons; 2006. Competing Risks: A Practical Perspective. [Google Scholar]
- 31.Terry M, Therneau TL. Survival Analysis. Package R; 2014. [Last accessed on 2015 Feb]. Available from: http://cran.r.project.org/web/packages/survival/survival.pdf .
- 32.Gray B. Subdistribution Analysis of Competing Risks. R Package; 2014. [Last accessed on 2015 Feb]. Available from: http://cran.r.project.org/web/packages/cmprsk/cmprsk.pdf .
- 33.Pintilie M. Dealing with competing risks: Testing covariates and calculating sample size. Stat Med. 2002;21:3317–24. doi: 10.1002/sim.1271. [DOI] [PubMed] [Google Scholar]
- 34.Latouche A, Porcher R. Sample size calculations in the presence of competing risks. Stat Med. 2007;26:5370–80. doi: 10.1002/sim.3114. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, Speizer FE, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–23. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 36.Morgan CL, Currie CJ, Stott NC, Smithers M, Butler CC, Peters JR. The prevalence of multiple diabetes-related complications. Diabet Med. 2000;17:146–51. doi: 10.1046/j.1464-5491.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 37.Jarrett RJ, Shipley MJ. Type 2 (non-insulin-dependent) diabetes mellitus and cardiovascular disease – Putative association via common antecedents; further evidence from the Whitehall Study. Diabetologia. 1988;31:737–40. doi: 10.1007/BF00274775. [DOI] [PubMed] [Google Scholar]
- 38.Haffner SM, Mitchell BD, Stern MP, Hazuda HP. Macrovascular complications in Mexican Americans with type II diabetes. Diabetes Care. 1991;14:665–71. doi: 10.2337/diacare.14.7.665. [DOI] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: Influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171:404–10. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- 40.Brun E, Nelson RG, Bennett PH, Imperatore G, Zoppini G, Verlato G, et al. Diabetes duration and cause-specific mortality in the Verona Diabetes Study. Diabetes Care. 2000;23:1119–23. doi: 10.2337/diacare.23.8.1119. [DOI] [PubMed] [Google Scholar]
- 41.Cho E, Rimm EB, Stampfer MJ, Willett WC, Hu FB. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol. 2002;40:954–60. doi: 10.1016/s0735-1097(02)02044-2. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular outcomes in framingham participants with diabetes: The importance of blood pressure. Hypertension. 2011;57:891–7. doi: 10.1161/HYPERTENSIONAHA.110.162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu WQ, Resnick HE, Jablonski KA, Jain AK, Jones KL, Robbins DC, et al. Effects of glycaemic control on cardiovascular disease in diabetic American Indians: The Strong Heart Study. Diabet Med. 2004;21:311–7. doi: 10.1111/j.1464-5491.2004.01137.x. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: A 5·5-year prospective analysis. Eur J Clin Invest. 2012;42:245–53. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 45.Boulanger JM, Hill MD. CHEP (Canadian Hypertension Educational Program). Hypertension and stroke: 2005 Canadian Hypertension Educational Program recommendations. Can J Neurol Sci. 2005;32:403–8. [PubMed] [Google Scholar]
- 46.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: Part I: Recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–42. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 47.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 48.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317:713–20. [PMC free article] [PubMed] [Google Scholar]
- 49.Bonakdaran S, Ebrahimzadeh S, Noghabi SH. Cardiovascular disease and risk factors in patients with type 2 diabetes mellitus in Mashhad, Islamic Republic of Iran. East Mediterr Health J. 2011;17:640–6. [PubMed] [Google Scholar]
- 50.Imperatore G, Cadwell BL, Geiss L, Saadinne JB, Williams DE, Ford ES, et al. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971-2000. Am J Epidemiol. 2004;160:531–9. doi: 10.1093/aje/kwh232. [DOI] [PubMed] [Google Scholar]
- 51.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 52.LaRosa JC. Understanding risk in hypercholesterolemia. Clin Cardiol. 2003;26(1 Suppl 1):I3–6. doi: 10.1002/clc.4960261303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med. 1990;322:1635–41. doi: 10.1056/NEJM199006073222304. [DOI] [PubMed] [Google Scholar]
- 54.Jousilahti P, Vartiainen E, Pekkanen J, Tuomilehto J, Sundvall J, Puska P. Serum cholesterol distribution and coronary heart disease risk: Observations and predictions among middle-aged population in eastern Finland. Circulation. 1998;97:1087–94. doi: 10.1161/01.cir.97.11.1087. [DOI] [PubMed] [Google Scholar]
- 55.Szklo M, Chambless LE, Folsom AR, Gotto A, Jr, Nieto FJ, Patsch W, et al. Trends in plasma cholesterol levels in the atherosclerosis risk in communities (ARIC) study. Prev Med. 2000;30:252–9. doi: 10.1006/pmed.1999.0612. [DOI] [PubMed] [Google Scholar]
- 56.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 57.Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY, et al. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med. 1993;328:313–8. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]


