Abstract
Peak bone mass, the maximum amount of bone accrued at the end of the growth period, is an important predictor of future risk of osteoporosis and fracture. Hence, the contribution of genetic factors influencing bone accrual is of considerable interest to the osteoporosis research community. In this article, we review evidence that genetic factors play an important role in bone growth, describe the genetic loci implicated so far and briefly discuss lessons learned from the application of genome-wide association studies. Moreover, we attempt to make the case for genetic investigations of bone mineral density in paediatric and young adult populations, describing their potential to increase our knowledge of the process of bone metabolism throughout the life course, and in turn, identify novel targets for the pharmacological treatment of osteoporosis.
Introduction
Peak bone mass is defined as the maximum amount of bone accrued throughout the life course.1 It accounts for more than half of the variability in bone mineral density (BMD) in the elderly and as such, represents an important predictor of future risk of osteoporosis and fracture.2 It has been estimated that a 10% increase in peak bone mass could decrease subsequent fracture risk in postmenopausal women by up to 50%.3 Therefore, optimising peak bone mass represents a promising intervention strategy for preventing osteoporosis. Epidemiological studies have identified numerous environmental factors (e.g. physical activity, nutrition and lifestyle behaviours) that modulate bone acquisition.4 In addition, they have demonstrated that intervention strategies targeting these modifiable risk factors result in gains in peak bone mass that persist into later life.4 However, individuals at high risk of osteoporosis are often only identified after they present with low trauma fracture, minimising the impact of the above-mentioned interventions. Furthermore, the majority of pharmacological treatments for osteoporosis function as anti-resorptives that halt further bone loss, but fail to fully restore bone quantity and quality. Only one osteoanabolic drug (i.e. Teriparatide) is presently FDA approved; however, it is far from ideal as it is expensive and requires daily administration via injection to ensure adequate bone formation.5 For these reasons, there is considerable scope for identifying novel anabolic pathways that could in principle be targeted by new pharmacotherapies.
Genetic studies, and in particular genome-wide association studies (GWAS), offer one means to discover biological mechanisms relevant to osteoporosis pathophysiology. For example: the GEnetic Factors for Osteoporosis (GEFOS) consortium recently performed a GWAS of adult BMD that encompassed up to 84 000 adults; and detected 56 loci associated with this trait, including several regions containing genes (or their pathways) targeted by existing pharmacotherapies.6 Despite this success, only ∼5.8% of the estimated heritability of adult BMD has been accounted for,6 suggesting that many more genetic variants remain to be discovered, thereby creating further opportunities to identify novel drug targets. It is conceivable that larger GWAS of adult and elderly individuals would provide such an opportunity. However, a complementary strategy would be to perform GWAS of BMD in cohorts of children and/or young adults. This strategy may prove valuable in finding additional loci, mainly due to increased power to target specific loci regulating bone acquisition and peak bone mass attainment, whose effects can be masked in elderly populations due to the accumulation of differing environmental influences over several years.7,8,9 In addition to identifying a complementary set of variants, studies involving younger individuals may also provide a better understanding of the genetic architecture underlying variation in BMD across the life course.10,11
In this review, we attempt to make the case for genetic investigations of BMD in paediatric and young adult populations. In so doing, we summarise the current knowledge of the genetic architecture of BMD in young individuals. We discuss lessons learned through the study of BMD in these populations, including the discovery of BMD-associated variants that display marked age heterogeneity and/or skeletal site specificity. Furthermore, we provide an outline of the GWAS results of bone-related phenotypes of paediatric and young adult populations. Finally, we discuss the success of these endeavours in identifying molecular mechanisms that influence bone growth and bone mineral acquisition and highlight some of the emerging genetic methodologies and resources that may improve our understanding of bone accrual and osteoporosis pathophysiology.
Genetic architecture of paediatric and adolescent BMD
Twin and family studies indicate that BMD is a highly heritable trait, with estimates ranging from 50 to 85%.12,13,14 There is some evidence to suggest that the heritability of BMD may be greater at younger ages.15 However, estimates can vary depending on the analytical model used, the skeletal site measured and the population under study. As a consequence, the genetic architecture underlying the normal variation in paediatric and adolescent BMD is still the subject of study. Recent methodological developments have provided considerable empirical evidence to suggest that a substantial proportion of the heritability of peak bone mass attainment may be present in the form of many variants of a small (yet real) effect scattered across the genome. Specifically, results of a new statistical methodology known as GREML (i.e. genetic restricted maximum likelihood16) have indicated that between one third to one half of the variance in paediatric BMD is tagged by common single nucleotide polymorphisms (SNPs) that are present on commercially available genome-wide genotyping arrays (i.e., termed the SNP heritability).10
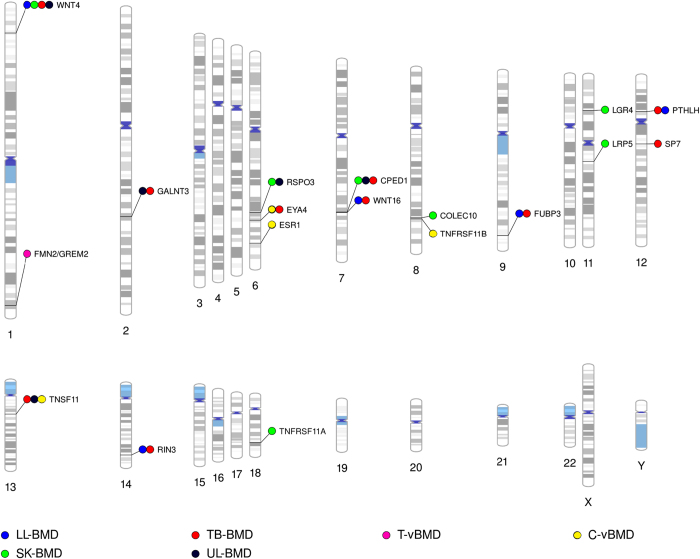
In 2009, the first BMD GWAS involving a paediatric population was reported. Although underpowered, it implicated an osteoblast transcription factor Osterix (SP7) as a possible determinant of total-body (less head) derived BMD (that is, TB-BMD).17 Suggestive associations between variants in SP7 and BMD had previously been observed in a larger meta-analysis of adults.18 More recently, the association was confirmed by the GEFOS consortium in a subsequent GWAS of adult BMD.6 Collectively these results imply that GWAS of paediatric BMD might be an alternative method of identifying BMD-related loci. Subsequently, up to 15 different loci have been robustly associated with BMD in children and young adults (Figure 1). It is not surprising that the majority of these loci are associated with BMD in adults.6,19,20, as BMD, irrespective of the age when measured, is considered to be a function of the peak bone mass accrued. Remarkably, adult GWAS studies were performed on sample sizes that were at least twice the size of GWAS encompassing younger individuals who had not yet attained peak bone mass.
Figure 1.
Phenogram of all bone-related loci identified by GWAS in children and/or young populations to date. Each locus is named according to either the most biologically relevant candidate gene in the region, the gene that is physically closest to the most strongly associated SNP, or in the case of intergenic regions, the cytogenic band containing the association. Note that in the vast majority of instances neither the identity of the true functional variant(s) nor the particular gene responsible for the association is known with certainty. Results from the following studies were used to generate the phenogram: Medina-Gomez et al.11 (PMID: 22792070), Kemp et al.10 (PMID: 24945404), Paternoster et al.8 (PMID: 21124946) and Paternoster et al.36 (PMID: 23437003). BMD (bone mineral density); C-vBMD (cortical volumetric BMD); LL (lower limbs); SK (skull); TB (total-body less head); T-vBMD (trabecular volumetric BMD) and UL (upper limbs).
Age-dependent effects
BMD reflects a combination of physiological processes that act across the life course. These include: (i) the acquisition of bone mass from early childhood to mid-adulthood, mediated mainly by bone modelling, (ii) the subsequent maintenance of bone mass from mid to late adulthood, via bone remodelling and (iii) the progressive loss of bone in later life, when less bone is formed than resorbed.21,22 It is possible that genetic variants related to BMD display age-dependent effects. That is, some bone associated variants might be more strongly related to developmental processes that occur in childhood and adolescence as compared with those that occur during adulthood.11 Consequently, GWAS of paediatric and adolescent BMD may offer a more powerful locus detection setting (as compared with adults). Furthermore, BMD measured early in the life course may be less influenced by the cumulative effect of non-genetic factors attributable to lifestyle and environment. As such, when analysing paediatric and adolescent BMD gains in power may be achieved via increased effect sizes and/or a reduction in the residual variance.
Robust evidence suggesting that some genetic variants display age-dependent effects on BMD was first reported by a study that encompassed ∼2200 6-year-old children from the Generation R Study and additional 5 cohorts that represented distinct age groups, ranging from 10 to 75 years (n=11 052).11 Variants mapping to CPED1 [also known as C7Orf58, (7q31.31)] showed a larger effect on skull BMD in children as compared with older individuals. Although the role of CPED1 in bone biology remains to be elucidated, efforts involving functional follow-up of the locus in animal models are underway.
To date, the largest GWAS meta-analysis of paediatric BMD was completed in 2014, and comprised ∼9395 children aged between 5 and 11 years.10 Six adult-BMD-associated loci (WNT4, WNT16, TNFSF11, GALNT3, PTHLH and FUBP3) and a novel locus encompassing RIN3 were robustly associated with TB-BMD. Variants within or in the neighbourhood of RIN3 have not been implicated in adult GWAS of hip and spine BMD to date6,19 possibly due to the existence of age-dependent effects. However, since paediatric and adult BMD data were obtained at different skeletal sites in these studies, we cannot exclude the possibility that variants at RIN3 operate in a site-dependent manner. Furthermore, it should be noted that in terms of bone research, RIN3 is not a novel locus as variants within this locus have previously been associated with Paget's disease of bone,23 a late-onset disorder of the skeleton.
To date, 63 independent genetic variants have been associated with BMD in adults.6 To evaluate the role of these variants in paediatric and adolescent BMD, Medina-Gúmez et al.24 used a genetic risk score (GRS) approach and found that the variants collectively explained ∼2.5% of the TB-BMD variation in two independent, multi-ethnic paediatric cohorts. Moreover, Warrington et al.9 also investigated the association between the rate of change in TB-BMD (spanning an 8-year period ranging from 9 to 17 years of age) and the GRS. Their analysis indicated that each adult-BMD-lowering allele was robustly associated with a mean decrease in BMD (centred at age 13) and an overall reduction in the rate of bone acquisition across childhood and adolescence. Analyses of individual loci making up the risk score, found that SNPs in 11 loci (AXIN1, FUBP3, SPTBN1, RSPO3, ABCF2, WNT16, CPED1, ZBTB40, WNT4, WLS and RPS6KA5) exerted detectable effects on BMD at age 13. Furthermore, three loci influenced the rate at which BMD accrued (KIAA2018, ESR1 and ZBTB40).9
Two recent studies by Mitchell et al.25 report interactions between adult-BMD-associated loci and BMD/BMC Z-scores with chronological age or sexual maturation26 using a relatively small sample (n∼800) of children and adolescents from the Bone Mineral Density in Childhood Study, which were followed up over a 6-year period. In the first study, different GRS were generated using adult-BMD-associated loci, of which three were composed of all loci, loci that contain genes involved in the WNT signalling pathway and loci robustly associated with increased fracture risk. All three GRS showed association with lower Z-scores at hip, femur, spine and total body. Further, an interaction with chronological age was observed for the fracture GRS at all sites, being more strongly associated with increased age. In the second study, individual adult-BMD-associated loci were investigated using forearm, hip, spine and total-body BMD Z-scores. Evidence of an interaction with pubertal stage was detected for 23 of these loci. Interestingly, GRS–sex interactions were also observed in both studies.
Altogether, the results of these studies, suggest that while the effects of a number of BMD-associated loci are age dependent, the effect of the majority is detectable throughout the life course, indicating that their role in bone growth and mineral acquisition early in the life course contributes to the variation in adult BMD. This is plausible, considering that peak bone mass is thought to account for more than half the variability in adult bone mass.2 Alternatively, it may also suggest that these loci continue to regulate bone acquisition throughout the life course, perhaps a consequence of the continued expansion of bone via periosteal apposition and their ability to change shape and size in response to mechanical loading (i.e. modelling).
Skeletal site-specific effects
GWAS of adult BMD have reported evidence of heterogeneity, in which specific loci are more strongly associated with BMD at the femoral neck than at the lumbar spine or vice versa.6,19 This heterogeneity may be a consequence of a number of factors including the different types of bone measured at the sites (i.e. the proportion of cortical versus trabecular bone) or differences in biomechanical response (i.e. mechanical loading). It is possible that this form of heterogeneity is also present at other sites across the body.27,28,29 Studies of paediatric BMD represent an ideal setting in which to test this hypothesis as total-body dual-energy X-ray absorptiometry (DXA) scans are typically used to measure BMD in children, whereas most adult studies are limited to measurement of BMD at the hip, spine and forearm. Total-body DXA measurements can be partitioned into distinct skeletal sub-regions, including the skull, upper- and lower-limbs. This is extremely advantageous as it enables the investigation of skeletal sites that differ in terms of their exposure to loading [i.e. skull (low), upper limbs (intermediate) and lower limbs (high)]. Furthermore, partitioning permits the investigation of molecular mechanisms regulating growth and development that may differ across sites. For example, the vault of the skull arises mainly through intramembranous ossification and is primarily made up of flat dermal bones that are cortical in nature.30 In contrast, upper- and lower-limbs consist of long bones that are made up of broadly equivalent amounts of cortical and trabecular bone that collectively develop from a cartilaginous template during endochondral ossification.31
To determine whether genetic factors contribute to the skeletal site-specific differences mentioned above, GREML analysis was used to investigate the genetic contribution to BMD measured at the skull, upper- and lower-limbs in a cohort of ∼5300 10-year-old children.10 SNP heritability estimates indicated that the common variants present on genotyping arrays, explained a larger proportion of the overall variance of BMD at the skull, when compared with BMD measured at the appendicular sites (i.e. upper- and lower-limbs).10 These differences possibly reflect the differential exposure of each skeletal site to varying environmental stimuli that influence BMD. For example, mechanical loading may influence the skull to less of an extent when compared to the limbs. To explore this possibility further, residual correlation across the different skeletal sites (i.e., the correlation between BMD measures at sites due to environmental factors and other sources of variation not tagged by SNPs on the array) was also estimated. Results suggested that while the environmental (and other residual) factors influencing the appendicular sites were moderately similar to each other, they appeared to be appreciably different from the factors influencing the skull. Taken together, the SNP heritability, coupled with a high residual correlation between the two appendicular sites, may reflect the greater exposure of these sites to loading and muscular stimulation, when compared with the skull. Likewise, estimates of the genetic correlations indicated that the limbs shared a more similar genetic architecture with each other than the skull,10 possibly reflecting the composition of bone at each skeletal site and/or the biological processes that govern their growth and maintenance.
To further explore the basis for the above-mentioned differences in genetic architecture, we performed GWAS meta-analyses of sub-regional TB-DXA data, and identified SNPs in 15 loci that exceeded the genome-wide significance threshold at one or more skeletal sites (i.e. SNPs at WNT4, GALNT3, CPED1, WNT16, FAM3C, RSPO3, FUBP3, PTHLH, TNFSF11, TNFRSF11B, TNFRSF11A, LRP5, LGR4, RIN3 and EYA4).10 A comparison of the effects of all 15 loci across each skeletal site echoed the findings from the GREML analyses, and supported the idea that although the underlying genetic architecture influencing BMD appears to be largely similar it varies according to skeletal site. Variants at TNFRSF11A, TNFRSF11B, EYA4, RSPO3 and LGR4 showed some evidence for site specificity, being most strongly associated with BMD at the skull, suggesting a stronger effect in the absence of habitual mechanical loading. Other patterns of site specificity were observed that are more difficult to explain. For example, variants at CPED1 were associated with BMD at the skull and upper limbs, but not with lower limbs, whereas variants at WNT16 were most strongly related to upper limbs when compared with the lower limbs and skull.
Further phenotypic refinement
DXA measures of BMD are only partially corrected for bone size. As a consequence, DXA-derived BMD also reflects differences in bone growth and overall skeletal size, making it difficult to evaluate the independent effects of true bone density. In addition to this limitation, DXA is unable to differentiate trabecular from cortical bone and therefore fails to account for true volumetric density and other geometric and micro-architectural properties that primarily determine bone strength and quality in younger populations (i.e., periosteal expansion, cortical density and thickness and trabecular number and thickness).32,33 For these reasons alternative-imaging technologies, including peripheral quantitative computer tomography (pQCT), are increasingly being used to identify novel determinants of bone strength. The primary advantage of pQCT over DXA is its ability to measure different constituents of bone mass separately [i.e. cortical and trabecular bone volumetric density (vBMD)], while fully adjusting for skeletal size by measuring bone slices of fixed thickness.34 As a result, pQCT measures offer distinct advantages over DXA in terms of identifying genetic correlates of refined bone phenotypes, especially considering that the genetic underpinnings of both traits is pronounced, with larger heritability estimates reported for trabecular BMD when compared with cortical vBMD.35
Paternoster et al.8,36 recently performed a GWAS of cortical and trabecular vBMD in a cohort of adolescents and young adults, with subsequent replication in elderly individuals. Three known adult hip and spine BMD-associated loci displayed associations with cortical vBMD (i.e. TNFSF11, ESR1 and TNFRSF11B) and two novel loci (i.e., EYA4 and GREM2/FMN2) displayed strong associations with cortical and trabecular vBMD, respectively.8 Subsequent analysis using high-resolution pQCT measures of bone microarchitecture of male adolescents found that the cortical vBMD association with TNFSF11 reflected a change in cortical porosity, whereas the association of trabecular vBMD with GREM2/FMN2 reflected a change in trabecular number and thickness. Interestingly, a separate GWAS combining data from 5878 European individuals (with ages between 13 and 80 years), reported a strong association between variants in the WNT16 locus and cortical bone thickness.37 Altogether, these findings demonstrate how refined measures of adolescent bone traits might provide better understanding of how these loci influence bone acquisition. For example, it is likely that the WNT16 association with cortical thickness reflects its role in bone modelling, whereas associations between ESR1, EYA4, TNFSF11 and TNFRSF11B and cortical density reflect their role in bone remodelling.
Biological pathways implicated in bone growth and accrual
The primary motivation behind GWAS of paediatric and adolescent BMD is to increase our fundamental understanding of the molecular pathways that regulate bone growth and/or accrual. When viewed retrospectively, an evaluation of the collective findings reported here suggests that GWAS of paediatric and adolescent bone traits have achieved this aim with remarkable success. For example, genes in four well-known bone signalling pathways [i.e., canonical WNT (LRP5, RSPO3, LGR4, AXIN1, RSPO3, WNT4, WNT16 and WLS), parathyroid hormone (PTHLH), oestrogen (ESR1) and RANK/RANKL/OPG (TNFRSF11A, TNFSF11 and TNFRSF11B)] show robust associations with paediatric and adolescent bone traits. While it is beyond the scope of this review to provide an in-depth description of each of these pathways, their role in bone homoeostasis is well-documented (reviewed elsewhere38,39).
Novel attributes of existing pathways have been uncovered as a consequence of these investigations. Most notably, it has become evident that adult-BMD-associated variants located near or within TNFRSF11A, TNFSF11 and TNFRSF11B influence paediatric and adolescent BMD6,10, suggesting that bone resorption may play an important role in bone growth and accrual. This notion is consistent with reports outlining the critical role of bone resportive cells in endochondral bone growth, in addition to observations that periods of rapid growth (i.e. puberty) are associated This notion is consistent with reports outlining the critical role of bone resorptive cells in endochondrial bone growth,40 in addition to observations that periods of rapid growth (that is, puberty) are associated with marked increases in markers of resorption and formation.41 To examine this hypothesis further, the relationship between bone modelling and bone resorption was investigated in a cohort of adolescents.42 Variants in the above-mentioned genes were associated with increased bone resorption [i.e. serum β-C-telopeptides of type I collagen (CTX)], reduced cortical thickness and cortical vBMD, and increased periosteal circumference. These relationships may imply that higher bone resorption to be permissive for greater periosteal expansion (i.e. modelling), and that this relationship reflects a compensatory mechanism that occurs during growth, whereby periosteal expansion increases in response to endosteal resorption in an effort to retain bone strength by limiting cortical thinning.43
Several genetic association studies mentioned in this review highlight the role of WNT16 in bone mass acquisition. As a consequence, a number of functional studies characterising its role in skeletal regulation have been performed, including a study by Movérare-Skrtic et al.44 that demonstrate that Wnt16-deficient mice suffer from spontaneous fractures as a result of reduced cortical thickness and high cortical porosity. Although no trabecular bone phenotype was evidenced in this study, the same group recently demonstrate that Wnt16 overexpression results in increased TB-BMD that is mostly attributed to increases in trabecular bone mass.45 Notably, a further study demonstrated that Wnt16 mediates mechanical loading-induced stimulation of periosteal bone formation via canonical Wnt signalling pathways.46
Identification of putative anabolic drug targets
Sanseau et al.47 recently reported that the genes identified through GWAS studies are likely to be amenable as targets for therapeutic intervention. Therefore, the findings reported by paediatric and adolescent studies of BMD may aid the discovery of novel drug targets for bone restorative pharmacotherapies. Although we are not yet in a position to determine the implication of these recent findings (in terms of improving treatment and prevention of osteoporosis), a retrospective review of the literature illustrates the merit of this strategy at identifying clinically validated drug targets. For example, several existing drugs used to treat osteoporosis target receptor proteins that are encoded by genes robustly associated with paediatric and adolescent BMD. These include: denosumab (TNFSF11), romosozumab and blosozumab (SOST), and several oestrogen analogues (ESR1). Importantly it has recently been noted that WNT16 may represent a novel osteoporosis target, as pharmacological overexpression of WNT16, increases trabecular bone mass45 and its depletion has strong consequences on cortical thickness.48 It should also be mentioned that RIN3 could hold significant therapeutic potential, especially when considering its differential expression in osteoporotic bone,10 likely role in osteoclast function and association with Paget's disease susceptibility.23
Future prospects
The implementation of a recent extension of the GREML method,49 described previously, suggests that almost all of the heritable variation in complex traits like height can be explained by the aggregate additive effects of genetic variants across the genome. Thus, assuming that the genetic architecture of paediatric and young adult BMD is similar, it should be possible, in theory, to identify the vast majority of individual genetic variants that are responsible for the variation in bone acquisition, by performing a combination of GWAS and whole-genome sequencing studies that involve very large samples of children and young adults. A study following this strategy has already proved successful, identifying a rare coding variant in EN1 associated with BMD and fracture risk in adults.19 In the following section, we highlight alternative GWAS approaches that may further our understanding of bone metabolism.
Life course approaches
It is evident that a better understanding of the genetic complexities underpinning skeletal development, maturation and senescence can be achieved when studying BMD throughout the life course. As a result, the GEFOS consortium recently established a new effort in which 49 300 individuals from 24 different studies with TB-DXA measurements have been collected and are presently being analysed across (and within) 3 different age groups [i.e., 0–15 years (n=11 200); 15–45 years, (n=9600); and >45 years, (n=28 500)].50 We expect that the results of this study will provide interesting insights into questions related to age heterogeneity.
Multivariate association methods
GWAS of paediatric and adult BMD traditionally involve univariate genetic association analysis of BMD. Nevertheless, it is plausible that some genetic factors primarily influence bone growth. If genetic variants simultaneously affect bone mineral content (BMC) and bone area (BA), their effect may only be detectable in genetic association studies of BA or BMC, as BMD (the ratio of these measures) may be unaffected. Thus, a promising alternative is to analyse BMC and BA simultaneously using multivariate genetic association methods, taking advantage of the correlation between these traits. Simulation studies and statistical theory suggest that they can be more powerful than genetic association analysis of univariate measures.51,52 A high degree of correlation exists between different components of body mass (that is, bone mass, fat mass and lean mass), and there is growing interest in their interdependence. Multivariate modelling may represent an exciting strategy to better understand these complex relationships and further identify genetic variants with pleiotropic effects. To explore these prospects, we are presently conducting two separate investigations. The first involves a GWAS of BMD, BMC and BA in a sample of 12 713 children and adolescents, and the second involves the evaluation of total-body lean mass and BMD using a bivariate GWAS approach.54
Trans-ethnic studies
Racial differences in BMD are well-documented and partially explain differences in osteoporosis and fracture risk across populations. Individuals of Sub-Saharan African ancestry tend to have higher BMD and lower fracture risk compared with other populations,55,56 even before achieving peak bone mass.24,57,58,59,60 A recent multi-ethnic cohort study showed that the frequency of alleles associated with increased BMD was systematically elevated in individuals of Sub-Saharan African ancestry, consistent with their higher BMD.24 The inclusion of ethnic groups other than European, as well as admixed populations in GWAS studies is occurring more frequently following the need to extrapolate findings to non-European populations, fine-map existing BMD loci, discover new associations and increase statistical power. Paediatric BMD GWAS have started to pursue this goal10,11,61 and new trans-ethnic studies are on their way.
Summary and conclusions
Genomic investigations of BMD in young individuals (i.e. prior to attaining peak bone mass) indicate that between a third and half of variation in bone growth and mineral accretion are tagged by common genetic variants that are assayed on commercially available genotyping chips. GWAS in these populations have successfully identified variants at more than 15 loci, some of which influence paediatric and adolescent BMD in an age-, skeletal site- and/or trait-specific manner. Disentangling these differences is providing valuable insights as to how molecular pathways influence bone growth and accrual. Genetic variants discovered so far implicate well-known bone metabolism pathways, but also point to novel genes and pathways not previously implicated in bone metabolism. Although the therapeutic significance of these findings is yet to be determined, the study of young individuals appears to be a promising strategy to identify novel targets for osteoporosis treatment. For all these reasons, we suggest that GWAS investigations of paediatric and adolescent BMD have made a significant contribution to our understanding of the genetic determinants of bone acquisition and osteoporosis and represent a powerful strategy for the identification of novel genetic loci that complement genetic studies in elderly individuals.
Acknowledgments
DME is supported by an Australian Research Council Future Fellowship (FT130101709) and a Medical Research Council Programme Grant (MC_UU_12013/4). MCMG and FR are partially supported by The Netherlands Organization for Scientific Research (NWO-ZonMW grant VIDI 016.136.367).
Footnotes
The authors declare no conflict of interest.
References
- Ott SM. Attainment of peak bone mass. J Clin Endocrinol Metab 1990; 71: 1082A–11082. [DOI] [PubMed] [Google Scholar]
- Hui SL, Slemenda CW, Johnston CC Jr. The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int 1990; 1: 30–34. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. Peak bone mass and its regulation. In: Glorieux FH, Pettifor JM, Jüppner H (eds).Paediatric Bone 2nd edn Elsevier, Cambridge, MA, USA2012; pp 189–221. [Google Scholar]
- Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex 2009; 51(Suppl 1): S5–17. [DOI] [PubMed] [Google Scholar]
- Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 2012; 97: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012; 44: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EL, Danoy P, Kemp JP, Leo PJ, McCloskey E, Nicholson GC et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet 2011; 7: e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster L, Lorentzon M, Lehtimaki T, Eriksson J, Kahonen M, Raitakari O et al. Genetic determinants of trabecular and cortical volumetric bone mineral densities and bone microstructure. PLoS Genet 2013; 9: e1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington NM, Kemp JP, Tilling K, Tobias JH, Evans DM. Genetic variants in adult bone mineral density and fracture risk genes are associated with the rate of bone mineral density acquisition in adolescence. Hum Mol Genet 2015; 24: 4158–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JP, Medina-Gomez C, Estrada K St, Pourcain B, Heppe DH, Warrington NM et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet 2014; 10: e1004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Kemp JP, Estrada K, Eriksson J, Liu J, Reppe S et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 Locus. PLoS Genet 2012; 8: e1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemenda CW, Turner CH, Peacock M, Christian JC, Sorbel J, Hui SL et al. The genetics of proximal femur geometry, distribution of bone mass and bone mineral density. Osteoporos Int 1996; 6: 178–182. [DOI] [PubMed] [Google Scholar]
- Smith DM, Nance WE, Kang KW, Christian JC, Johnston CC Jr. Genetic factors in determining bone mass. J Clin Invest 1973; 52: 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden NK, Baker J, Hogg C, Baan K, Spetor TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: A study of postmenopausal twins. J Bone Miner Res 1996; 11: 530–534. [DOI] [PubMed] [Google Scholar]
- Moayyeri A, Hammond CJ, Hart DJ, Spector TD. Effects of age on genetic influence on bone loss over 17 years in women: the Healthy Ageing Twin Study (HATS). J Bone Miner Res 2012; 27: 2170–2178. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011; 88: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson NJ, Tobias JH, Richards JB, Soranzo N, Duncan EL, Sims AM et al. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet 2009; 18: 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med 2008; 358: 2355–2365. [DOI] [PubMed] [Google Scholar]
- Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 2015; 526: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 2009; 41: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int 2006; 17: 337–347. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Rizzoli R, Slosman D, Bonjour JP. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab 1998; 83: 358–361. [DOI] [PubMed] [Google Scholar]
- Albagha OM. Genetics of Paget's disease of bone. Bonekey Rep 2015; 4: 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Chesi A, Heppe DH, Zemel BS, Yin JL, Kalkwarf HJ et al. BMD loci contribute to ethnic and developmental differences in skeletal fragility across populations: assessment of evolutionary selection pressures. Mol Biol Evol 2015; 32: 2961–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Chesi A, Elci O, McCormack SE, Roy SM, Kalkwarf HJ et al. Genetic risk scores implicated in adult bone fragility associate with paediatric bone density. J Bone Miner Res. 2016; 31: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Chesi A, Elci O, McCormack SE, Kalkwarf HJ, Lappe JM et al. Genetics of bone mass in childhood and adolescence: effects of sex and maturation interactions. J Bone Miner Res 2015; 30: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee DJ. Head and face. In: Hart C (ed.).Orthopedic Physical Assessment 6th edn Elsevier: Canada, 2014; pp 84. [Google Scholar]
- Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest 1999; 104: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradney M, Karlsson MK, Duan Y, Stuckey S, Bass S, Seeman E. Heterogeneity in the growth of the axial and appendicular skeleton in boys: implications for the pathogenesis of bone fragility in men. J Bone Miner Res 2000; 15: 1871–1878. [DOI] [PubMed] [Google Scholar]
- Morgan EF, Barnes GL, Einhorn TA. The Bone Organ System: Form and Function. In: Marcus R, Feldam D, Dempster DW, Luckey M, Cauley JA (eds). 4th edn Academic Press, Cambridge, MA, USA2013;.
- Gilbert SF. Osteogenesis: the development of bones. In: Sunderland MA (ed.).Developmental Biology 6th edn Sinauer Associates2000;. [Google Scholar]
- Patsch JM, Burghardt AJ, Kazakia G, Majumdar S. Noninvasive imaging of bone microarchitecture. Ann NY Acad Sci 2011; 1240: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354: 2250–2261. [DOI] [PubMed] [Google Scholar]
- Wilhelm G, Felsenberg D, Bogusch G, Willnecker J, Thaten J, Gummert P. Biomechanical examinations for validation of the bone strength strain index SSI, calculated by peripheral quantitative computed tomography. In: Lyritis GP (ed.).Clinical Applications of Musculoskeletal Interactions Holonome Editions: Athens, Greece, 1999; pp 105–108. [Google Scholar]
- Havill LM, Mahaney MC, T LB, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res 2007; 22: 737–746. [DOI] [PubMed] [Google Scholar]
- Paternoster L, Lorentzon M, Vandenput L, Karlsson MK, Ljunggren O, Kindmark A et al. Genome-wide association meta-analysis of cortical bone mineral density unravels allelic heterogeneity at the RANKL locus and potential pleiotropic effects on bone. PLoS Genet 2010; 6: e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HF, Tobias JH, Duncan E, Evans DM, Eriksson J, Paternoster L et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet 2012; 8: e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 2012; 13: 576–588. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, O'Brien CA, Almeida M. The role of oestrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013; 9: 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A. Mechanisms of osteoclast-dependent bone formation. Bonekey Rep 2013; 2: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkensammer G, Griesmacher A et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab 2007; 92: 443–449. [DOI] [PubMed] [Google Scholar]
- Kemp JP, Sayers A, Paternoster L, Evans DM, Deere K, St Pourcain B et al. Does bone resorption stimulate periosteal expansion? A cross-sectional analysis of beta-C-telopeptides of type I collagen (CTX), genetic markers of the RANKL pathway, and periosteal circumference as measured by pQCT. J Bone Miner Res 2014; 29: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. The periosteum--a surface for all seasons. Osteoporos Int 2007; 18: 123–128. [DOI] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Borjesson AE et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med 2014; 20: 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Wu J, Henning P, Gustafsson KL, Sjogren K, Windahl SH et al. The bone-sparing effects of oestrogen and WNT16 are independent of each other. Proc Natl Acad Sci USA 2015; 112: 14972–14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergedal JE, Kesavan C, Brommage R, Das S, Mohan S. Role of WNT16 in the regulation of periosteal bone formation in female mice. Endocrinology 2015; 156: 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanseau P, Agarwal P, Barnes MR, Pastinen T, Richards JB, Cardon LR et al. Use of genome-wide association studies for drug repositioning. Nat Biotechnol 2012; 30: 317–320. [DOI] [PubMed] [Google Scholar]
- Brommage R. Genetic approaches to identifying novel osteoporosis drug targets. J Cell Biochem 2015; 116: 2139–2145. [DOI] [PubMed] [Google Scholar]
- Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AA, Lee SH et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat Genet 2015; 47: 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Kemp JP, Chesi A, Kreiner-Møller E, Ahluwalia T, Mook D et al. GWAS meta-analysis for total body BMD unveils 14 new BMD loci and variants exerting age-specific effects. In: Compston JE, (ed.). 37th Annual Meeting of the American Society for Bone and Mineral Research; October 9–12; Seattle, Washington, USA,2015; pp S489.
- Ferreira MA, Purcell SM. A multivariate test of association. Bioinformatics 2009; 25: 132–133. [DOI] [PubMed] [Google Scholar]
- O'Reilly PF, Hoggart CJ, Pomyen Y, Calboli FC, Elliott P, Jarvelin MR et al. MultiPhen: joint model of multiple phenotypes can increase discovery in GWAS. PLoS ONE 2012; 7: e34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JP, Medina-Gomez C, Chesi A, Wang C, Eriksson J, Warrington NM et al. Genome-wide association study of bone mineral density, content and area measured at the axial and appendicular skeleton identifies four novel loci and suggests a possible reason why genetic loci are associated with bone mineral density at some sites but not others. In: Compston JE (ed.). 37th Annual Meeting of the American Society for Bone and Mineral Research; October 9–12; Seattle, Washington, USA,2015; pp S159.
- Medina-Gomez C, Kemp JP, Kreiner-Møller E, Chesi A, Heppe DH, Zemel BS et al. Variants in regulatory regions of SREBF1, a Lamin A interaction factor exert pleiotropic effects on BMD and lean mass in children. In: Compston JE (ed.). 37th Annual Meeting of the American Society for Bone and Mineral Research; October 9–12; Seattle, Washington, USA2015; pp S489.
- Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 2002; 87: 3057–3067. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE et al. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res 2008; 23: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandrayen K, Norris SA, Pettifor JM. Fracture rates in urban South African children of different ethnic origins: the Birth to Twenty cohort. Osteoporos Int 2009; 20: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren TA, Shepherd JA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S et al. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr 2012; 161: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 2011; 96: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhudhikanok GS, Wang MC, Eckert K, Matkin C, Marcus R, Bachrach LK. Differences in bone mineral in young Asian and Caucasian Americans may reflect differences in bone size. J Bone Miner Res 1996; 11: 1545–1556. [DOI] [PubMed] [Google Scholar]
- Chesi A, Mitchell JA, Kalkwarf HJ, Bradfield JP, Lappe JM, McCormack SE et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing paediatric aBMD and BMC at the distal radius. Hum Mol Genet 2015; 24: 5053–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]