Summary
Objectives
Neisseria meningitidis, together with the non-pathogenic Neisseria species (NPNs), are members of the complex microbiota of the human pharynx. This paper investigates the influence of NPNs on the epidemiology of meningococcal infection.
Methods
Neisseria isolates were collected during 18 surveys conducted in six countries in the African meningitis belt between 2010 and 2012 and characterized at the rplF locus to determine species and at the variable region of the fetA antigen gene. Prevalence and risk factors for carriage were analyzed.
Results
A total of 4694 isolates of Neisseria were obtained from 46,034 pharyngeal swabs, a carriage prevalence of 10.2% (95% CI, 9.8–10.5). Five Neisseria species were identified, the most prevalent NPN being Neisseria lactamica. Six hundred and thirty-six combinations of rplF/fetA_VR alleles were identified, each defined as a Neisseria strain type. There was an inverse relationship between carriage of N. meningitidis and of NPNs by age group, gender and season, whereas carriage of both N. meningitidis and NPNs was negatively associated with a recent history of meningococcal vaccination.
Conclusion
Variations in the prevalence of NPNs by time, place and genetic type may contribute to the particular epidemiology of meningococcal disease in the African meningitis belt.
Keywords: Non-meningococcal Neisseria, Pharyngeal carriage, African meningitis belt
Highlights
-
•
A prevalence of 10.2% of Neisseria infection was observed during the study.
-
•
Five Neisseria species were identified in nasopharyngeal samples.
-
•
High level of genetic diversity was observed in carried isolates.
-
•
Inverse relationship between carriage of Neisseria meningitidis and non-pathogenic Neisseria.
Introduction
The human pharynx hosts a complex microbiota, including bacteria belonging to the genus Neisseria. Most members of this genus are non-pathogenic commensals (non-pathogenic Neisseria, NPNs), which very rarely cause invasive disease, but Neisseria meningitidis (Nm), the meningococcus, is an exception.1 Despite advances in vaccine development, invasive meningococcal disease remains a public health challenge globally and especially in the African meningitis belt, where very large epidemics continue to occur,2, 3 despite the recent widespread deployment of a serogroup A meningococcal conjugate vaccine (PsA-TT, MenAfriVac®).4, 5, 6, 7, 8
Most pharyngeal carriage studies in the African meningitis belt have focused on the meningococcus,9, 10, 11, 12 with little attention paid to other Neisseria species apart from Neisseria lactamica (Nl). In northern Nigeria, pharyngeal carriage of Nl was common, especially in young children13 and genetic exchange among Nm, Nl and unspecified Neisseria species has been demonstrated in The Gambia.14 Molecular epidemiological studies of Nm and Nl conducted in Burkina Faso from 2009 to 2012 reported a high overall prevalence of Nl carriage (18.2%), a higher prevalence of Nl in males than in females, except in those aged 18–29 years, no change between dry and rainy season and no significant changes following vaccination with PsA-TT.10, 15, 16, 17 It has long been considered likely that carriage of NPNs influences host susceptibility to infection and invasion by Nm, a view supported by the fact that healthy subjects inoculated with Nl exhibit some protection against colonization with Nm.18 Until recently, Nl was considered to be the NPN genetically most similar to Nm. However, recent whole genome sequence (WGS) studies have shown that Neisseria polysaccharea (Np) and Neisseria bergeri (Nb) are more closely related to Nm than Nl.19 Consequently, these bacteria may also influence the epidemiology of Nm colonization and invasion. For this reason we have studied the prevalence of carriage with various Neisseria species in six countries of the African meningitis belt and investigated risk factors for their carriage.
Materials and methods
The methods employed in the MenAfriCar surveys, during which the isolates described in this paper were collected, have been described in detail previously11 and are summarized briefly here. Ethical approval for the surveys was obtained from the ethics committee of the London School of Hygiene & Tropical Medicine and from ethical committees in each partner country. The study was registered with ClinicalTrials.gov (NCT01119482).
Carriage surveys
Bacteria were isolated during 18 cross-sectional surveys conducted in Chad, Ethiopia, Ghana, Mali, Niger and Senegal during 2010–2012 (Fig. 1). Pre-vaccination surveys in Mali, Niger and Chad had a target of 5000 participants and post-vaccination surveys a target of 2000 participants in Mali and Niger and 6000 in Chad. The remaining surveys aimed to recruit 2000 participants. A representative sample of households was obtained either from an updated demographic surveillance system (DSS) or from a census conducted for the study. Within selected households, individuals in four different age groups (0–4 years, 5–14 years, 15–29 years and 30 years or more) were chosen randomly until the required sample size was reached, with a maximum of 5 individuals recruited per household. Once written consent had been obtained, standardized household and individual questionnaires inquiring about risk factors for meningococcal infection were administered. Pharyngeal swabs were obtained using a standardized technique that involved swabbing both the posterior-pharyngeal wall and the tonsils.20
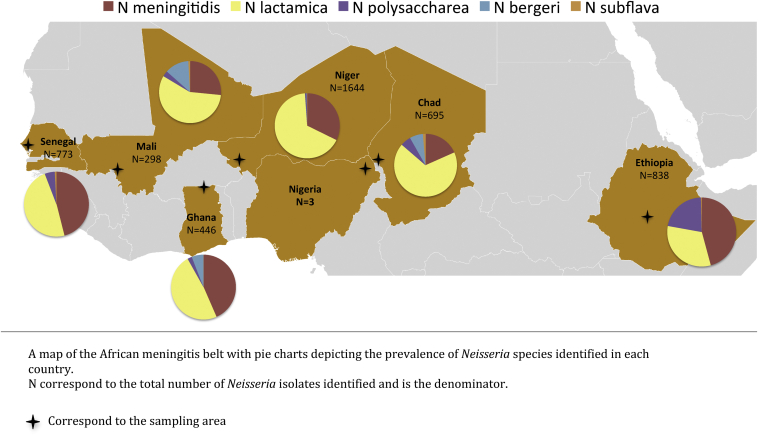
Figure 1.
Frequency distribution of Neisseria species in each country of the study.
Bacteriology
NPNs were isolated using the same conventional microbiology techniques that were employed for the detection of Nm described previously.11 Briefly, pharyngeal swabs were plated onto modified Thayer Martin agar plates and incubated 24–48 h at 37 °C in 5% CO2; oxidase and gram stain testing identified oxidase positive, Gram-negative diplococci. Further biochemical tests (ortho-nitrophenyl-β-galactoside, ץ-glutamyl transpeptidase and tributyrin) were used in each site to differentiate between putative Nl and Nm isolates and members of the genus Moraxella.
Molecular methods
A boiled cells suspension of each oxidase positive, Gram negative diplococci (OPGND) isolate was sent to the Department of Zoology at the University of Oxford for molecular typing, where Sanger sequencing was used to characterize gene targets as described in the Supplement. Sequences were assembled using SeqSphere (http://www.ridom.de/seqsphere/) and imported into the isolate's record previously created in a BIGSdb database.21
Neisseria speciation
Amplification and sequencing of a 413 bp fragment of the rplF gene was used to differentiate among Neisseria species as described previously.22 A phylogeny based on the f_rplF alleles from Bennett et al. (2014) and the unique alleles found in this study was reconstructed using the Neighbor-Joining algorithm23 and the Kimura 2-parameter substitution model24 in mega version 6.0.25 For isolates that did not yield results for the rplF assay, sequencing of the rrna gene, encoding 16S rRNA,26 was used to confirm the presence or absence of a bacterium and to determine its genus. Only rplF confirmed NPNs were included in this study with the exception of four Nm speciated on the basis of the 16S rRNA and the porA sequences. New alleles were investigated by a BLAST of obtained sequences against the 16S rRNA sequence data of the EzTaxon server (http://www.ezbiocloud.net/eztaxon;27).
Genetic diversity
Sequencing of the variable region of the fetA gene (feta_VR) was used to assess the genetic diversity of the Neisseria identified as described in the Supplementary methods.28 The sequences were assembled as for the other targets using SeqSphere (http://www.ridom.de/seqsphere/). The trace files of each new allele were manually curated in mega version 6.0,25 the quality of the sequences were assessed and the modified bases checked in both the forward and reverse trace file. The corresponding protein sequences were also aligned and compared with known sequences stored in the Neisseria sequence definition database of pubMLST21 before being entered into the allele database and the appropriate isolate record.
For isolates for which fetA_VR could not be amplified, an assay identifying the absence of the fetA gene (fetA null, fnl) was employed, using primers placed on genes on each side of the fetA gene: thdF and fetB as described in the Supplementary methods.29
Statistical methods
Analyzes were performed using Stata v12.0 (StataCorp, Texas). Survey design and potential household clustering were taken into account using the survey commands in Stata. Carriage prevalence of each of the different Neisseria species, together with 95% confidence intervals, was calculated for each country and each survey. Risk factors for carriage of Nm and NPNs were assessed simultaneously using multinomial logistic regression. Each risk factor was considered in turn using univariable, multinomial logistic regression. A multivariable model was then constructed including country, age group and sex a priori and any variable with a p-value <0.1 in the univariable analyses; only the variables with a p value < 0.05 in the multivariable analysis were kept in the final model. As a final check, dropped variables were re-entered into the model one at a time and the p-values re-examined; the variable was retained as significant if the p value was <0.05.
Results
Prevalence of carriage with Neisseria species
A total of 4694 of the 46034 pharyngeal swabs collected yielded a Neisseria species, giving a carriage prevalence of 10.2% [95% CI, 9.8–10.5%]: 696 Neisseria were identified out of the 946 OPGND samples received from Chad; 838 out of the 994 received from Ethiopia; 446 out of the 544 received from Ghana; 298 out of the 504 from Mali; 1644 out of 2321 from Niger and 773 out of the 971 received from Senegal. The most frequently isolated species was Nl with a 5.6% point prevalence [95% CI, 5.3–5.8%], followed by Nm at 3.6% [95% CI, 3.4–3.8%]), Np at 0.6% [95% CI, 0.5–0.7%], Nb at 0.2% [95% CI, 0.2–0.3%] and Neisseria subflava (Ns) at 0.05% [95% CI, 0.03–0.1%] (Supplementary Table 1). Twenty isolates from Chad were identified as belonging to the Neisseria genus but did not cluster with any known species on the Neighbor-Joining Tree (NJT; data not shown); they were designated Neisseria sp. and will be investigated further by whole genome sequencing methods.
Factors influencing the prevalence of carried Neisseria species
Country, season, age, gender, and a history of recent vaccination against Nm were associated with carriage of NPNs. Additionally, area of residence, household crowding and kitchen location were significant risk factors for carriage of Nm (Table 1).
Table 1.
Factors associated with carriage of Neisseria meningitidis and non-pathogenic Neisseria; results from a multinomial multivariable logistic regression.
| Factor | Number (carriers) | Adjusted RRR Neisseria meningitidis (95% CI) |
Adjusted RRR Non-pathogenic Neisseria (95% CI) |
|
|---|---|---|---|---|
| Age | <1 year | 2074 (207) | 0.40 (0.29, 0.55) | 3.11 (2.60, 3.73) |
| 1–4 years | 8291 (1355) | 0.70 (0.59, 0.82) | 5.90 (5.23, 6.65) | |
| 5–14 years | 12,563 (895) | 1.49 (1.31, 1.69) | 2.59 (2.29, 2.94) | |
| 15–29 years | 11,863 (4372) | 1.0 | 1.0 | |
| 30 + years | 11,243 (206) | 0.59 (0.50, 0.68) | 0.51 (0.43, 0.61) | |
| Sexa | Female | 26,619 (1701) | 1.0 | 1.0 |
| Male | 19,296 (1313) | 1.34 (1.21, 1.48) | 0.87 (0.80, 0.94) | |
| Season | Rainy (XS1 and XS2) | 30,522 (220) | 1.0 | 1.0 |
| Dry (XS3) | 15,512 (815) | 1.53 (1.35, 1.74) | 0.78 (0.70, 0.86) | |
| Country | Chad | 13,396 (584) | 1.0 | 1.0 |
| Ethiopia | 5970 (450) | 7.11 (5.43, 9.29) | 1.70 (1.44, 2.01) | |
| Ghana | 5209 (253) | 4.81 (3.64, 6.35) | 1.25 (1.03, 1.51) | |
| Mali | 8837 (219) | 1.45 (1.05, 1.99) | 0.48 (0.40, 0.58) | |
| Niger | 8213 (1112) | 11.39 (9.00, 14.43) | 3.58 (3.13, 4.01) | |
| Senegal | 4409 (427) | 10.85 (8.28, 14.23) | 2.39 (1.97, 2.89) | |
| Area | Urban | 19,462 (1398) | 1.0 | 1.0 |
| Rural | 26,572 (1637) | 1.44 (1.09, 1.60) | 0.97 (0.88, 1.06) | |
| Crowdingb | <2 people per room | 16,299 (889) | 1.0 | 1.0 |
| ≥2 people per room | 29,679 (2127) | 1.27 (1.12, 1.45) | 1.08 (0.98, 1.19) | |
| Kitchen location | Open air | 17,805 (1274) | 1.0 | 1.0 |
| Inside house | 12,741 (1027) | 1.32 (1.09, 1.60) | 0.90 (0.79, 1.02) | |
| Separate hut | 14927 (677) | 0.94 (0.76, 1.17) | 0.96 (0.84, 1.09) | |
| Missing information | 504 (38) | 1.06 (0.57, 1.98) | 0.77 (0.51, 1.17) | |
| Vaccinated recently with meningitis vaccine | No | 31,338 (2060) | 1.0 | 1.0 |
| Yes, <1 year ago | 9048 (545) | 0.71 (0.59, 0.84) | 0.82 (0.73, 0.92) | |
| Yes, 1–3 years ago | 4543 (356) | 0.51 (0.42, 0.63) | 0.94 (0.81, 1.10) | |
| Don't know/missing | 1020 (55) | 0.68 (0.47, 1.00) | 0.62 (0.46, 0.84) | |
The final multivariable logistic regression model included age group, sex, country, season area, crowding, kitchen location and recent vaccination.
Other risk factors used in the univariable model but not significant in the adjusted model: smoking, living in a house with smokers, cooking fuel, respiratory symptoms and attendance of social gatherings.
Sex not reported for 119 individuals.
Data not reported for 57 individuals.
Country: The prevalence of carriage of Neisseria species overall varied significantly among countries with Niger having the highest point prevalence (19.9% [95% CI, 18.9–20.9]), followed by Senegal (17.5% [95% CI, 16.1–18.8]), Ethiopia (13.8% [95% CI, 12.8–14.8]), Ghana (8.5%[95% CI, 7.6–9.4]), Chad (5.3%[95% CI, 4.9–5.7]) and Mali (3.4% [95% CI, 2.9–3.8]) (Supplementary Table 1). The distribution of the different species of Neisseria by country is shown in Fig. 1. The prevalence of Nm carriage varied significantly among countries, being highest in Senegal (8.0% [95% CI, 7.1–9.0]) and lowest in Mali (0.9% [95% CI, 0.7–1.1]). There were also major differences in the prevalence of carriage of NPNs by country and some NPNs were not identified in some countries, for example no Nb were isolated in Ethiopia and Senegal and no Ns in Ghana. Ethiopia was the only country where the prevalence of Nm carriage was higher than that of Nl.
Year and season: The prevalence of carriage of Neisseria species overall varied little over the three years of the study: 10.0% [95% CI, 9.4–10.5%] in the first survey, 11.1% [95% CI, 10.6–11.7%] in the second survey and 9.4% [95% CI, 8.9–9.9%] in the third survey (Table 2). The prevalence of carriage with both Nm and NPNs was also similar over time, with Nl being the most carried species, regardless of survey or season. There was, however, variation in the prevalence of carriage of NPNs over time at the country level: for example, there was an increase in the prevalence of Nl between surveys 1 and 2 in both Chad (from 1.1% to 5.9%) and Ghana (from 0.8% to 6.3%) and an increase in Nm prevalence between survey 2 and 3 in Senegal (from 3.1% to 19.8%4) (Supplementary Fig. 1). The relative risk of carriage of NPNs was significantly lower during the dry compared to the rainy season with an adjusted Relative Risk Ratio (aRRR) of 0.78, [95% CI, 0.70–0.86], whereas the opposite was true for Nm with an aRRR of 1.53, [95% CI, 1.35–1.74] in the dry season.
Table 2.
Number of isolates identified as a Neisseria species per survey.
| N. meningitidis | Prevalence [CI] | N. lactamica | Prevalence [CI] | N. polysaccharea | Prevalence [CI] | N. bergeri | Prevalence [CI] | N. subflava | Prevalence [CI] | Total | Prevalence [CI] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey 1 | 577 | 3.6 [3.3–3.9] | 896 | 5.7 [5.3–6.1] | 79 | 0.5 [0.4–0.6] | 17 | 0.1 [0.1–0.2] | 4 | 0.03 [0–0.05] | 1573 | 10.0 [9.4–10.5] |
| Survey 2 | 454 | 3.0 [2.7–3.3] | 1010 | 6.7 [6.3–7.2] | 117 | 0.8 [0.62–0.9] | 62 | 0.4 [0.3–0.5] | 17 | 0.1 [0.1–0.2] | 1660 | 11.1 [10.6–11.7] |
| Survey 3 | 648 | 4.2 [3.8–4.5] | 682 | 4.4 [4.0–4.7] | 94 | 0.6 [0.5–0.7] | 34 | 0.2 [0.1–0.3] | 3 | 0.02 [−0.002 to 0.04] | 1461 | 9.41 [8.9–9.9] |
| Total | 1679 | 3.6 [3.4–3.8] | 2588 | 5.6 [5.3–5.8] | 290 | 0.63 [0.5–0.7] | 113 | 0.2 [0.2–0.3] | 24 | 0.05 [0.03–0.1] | 4694 | 10.2 [9.8–10.5] |
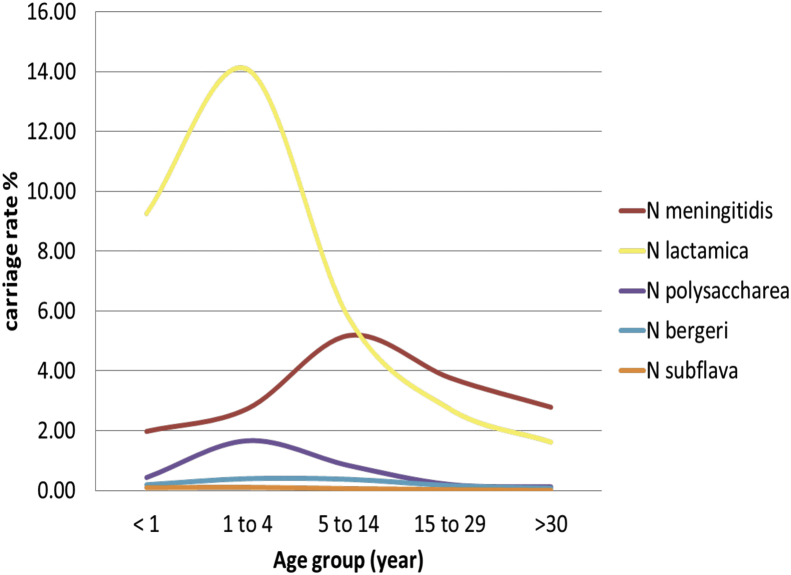
Age and gender: The relative risk of carrying NPNs overall was higher in children aged less than 15 years compared to the young adult age group (age 15–29 years) with an aRRR of 3.11 [95% CI, 2.60–3.73] for those <1 year, 5.90 [95% CI, 5.23–6.65] for the 1–4 year olds and 2.59 (95% CI, 2.29–2.94) for the 5–14 year olds. The oldest age group >30 years had a lower aRRR of 0.51 (95% CI, 0.43–0.61). The prevalence of carried Nl was highest in the 1–4 year old age group, reaching a peak of 14.1% [95% CI, 13.3–14.8%] in contrast to carriage of Nm, which reached a peak of 5.2% [95% CI, 4.8–5.6%] in the 5–14 year age group. Similarly to Nl, Np carriage also reached a peak prevalence of 1.7% [95% CI, 1.4–1.9%] in the 1–4 year old age group (Fig. 2). Carriage prevalence of Nb and Ns was too low to identify an overall trend in age group distribution. Males had a lower risk of carrying NPNs than females (aRRR of 0.87 [95% CI, 0.80–0.94]) but a higher risk of carrying Nm (aRRR of 1.34 [95% CI, 1.21–1.48].
Figure 2.
Prevalence of Neisseria species carried by age group.
Vaccination history: A history of vaccination within the past 12 months with any meningococcal vaccine was associated with a decrease in Nm carriage, as reported previously,8 but additionally with a decreased risk of carrying NPNs (aRRR of 0.82 [95% CI, 0.73–0.92]. In the three countries where MenAfriVac® was introduced during the course of the study (Chad, Mali, and Niger) a significant decrease in the prevalence of carriage of Nl from 6.4% to 4.9% was observed. An overall decrease in carriage of Nm was also observed in the three countries but this reduction was not consistent in all countries with Mali experiencing an increase from 0.6% to 1.2% (Supplementary Fig. 2).
Other risk factors: Area of residence (rural vs urban), crowding, cooking with cow dung or straw, kitchen location, and attendance at social gatherings in the past week all had a significant impact on the odds of carrying NPNs in the univariable regression model, but their effect was not significant in the multivariable model. Some of these risk factors were retained in the final model as, although they had no effect on NPN carriage, they were significant for Nm carriage.
Genetic diversity of identified Neisseria species
Forty-two different alleles were identified for the rplf fragment (f_rplf). Nl was the most diverse species with 17 f_rplf alleles, the most frequent of which was f_rplf 6 (1453 isolates, 56.1%). Eleven alleles were found for Nm the most frequent being f_rplf 2 (749 isolates, 44.6%) and f_rplf 1 (707 isolates, 42.1%); four f_rplf alleles were identified in Np, the most frequent of which was f_rplf 9 (207 isolates, 71.4%). Four alleles were also found among the Nb alleles with f_rplf 62 (57) and f_rplf 69 (46) representing 91.2% of these isolates. Finally, seven alleles were observed for Ns, with predominance of f_rplf 43 (15 isolates, 62.5%) (Supplementary Table 2). A Neighbor Joining tree was used to represent the phylogeny of the rplF fragment present in the Neisseria isolates of this study in relation to the original isolates used to create the f_rplF assay22 (Supplementary Fig. 3).
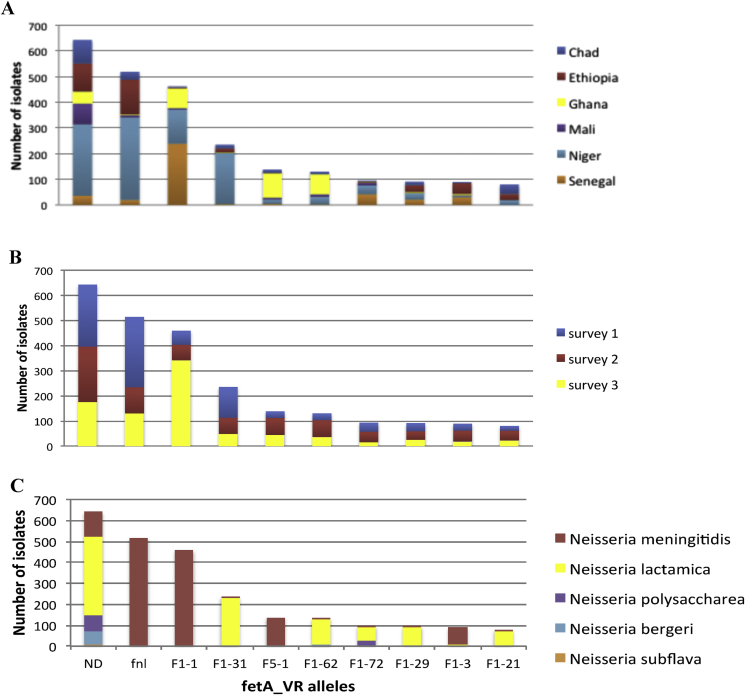
The diversity of fetA alleles varied by country (Fig. 3A; Supplementary Table 3); Niger had the highest number of fetA_vr alleles (111) and Ghana had the least (55). Some allele variability was seen between surveys, for example the proportion of the F1-1 allele increased from 61 (3.63%) in survey 2 to 342 (23.38%) in survey 3 and similar changes were observed for others alleles in survey 3 (Fig. 3B; Supplementary Table 4). A total of 234 different alleles were identified across all species: 75 of these were found in only one isolate; 184 in fewer than 20 isolates. A total of 194 alleles were observed for Nl, 80 for Nm; 35 for Np; 21 for Nb; and 16 for Ns. Most alleles were found predominantly in only one species, for example 99.61% of fnl alleles were found in Nm,29, 30 all F5-84 variants were only detected in Nl, all F5-1 were exclusive to Nm, all F11-4 were found only in Np and all F1-169 and all F1-193 variants were only observed in Ns. Some variants, however, were shared among different species, e.g. F1-72, F1-21, F2-24, F6-3 (Fig. 3C; Supplementary Table 5). Neither the fetA_VR nor the fnl fragment was successfully amplified in 643 isolates, which were designated Not Determined (ND). As defined by f_rplf and feta_VR alleles, there were 636 different Neisseria strain types identified, with almost half of these (297, 46.70%) observed only once. There was appreciable variation in the frequency of the strain types observed more than 20 times (Table 3).
Figure 3.
Frequency distribution of fetA_VR alleles. Frequency distribution of different alleles of the variable region of fetA. Only the 10 most common alleles are displayed; distribution by country (A), by survey (B) and by species (C).
Table 3.
Number of isolates for the most common commensal strain types per species.
| Commensal strain_type “f_ rplf:fetA” | Neisseria bergeri | Neisseria lactamica | Neisseria meningitidis | Neisseria polysaccharea | Total |
|---|---|---|---|---|---|
| 1:fnl | – | – | 470 | – | 470 |
| 2:F1-1 | – | – | 452 | – | 452 |
| 6:F1-31 | – | 205 | – | – | 205 |
| 2:F5-1 | – | – | 135 | – | 135 |
| 6:ND | – | 122 | – | – | 122 |
| 32:ND | – | 88 | – | – | 88 |
| 52:ND | – | 82 | 0 | – | 82 |
| 2:F1-3 | – | – | 74 | – | 74 |
| 1:ND | – | – | 68 | – | 68 |
| 9:ND | – | – | – | 66 | 66 |
| 6:F1-29 | – | 62 | – | – | 62 |
| 34:F1-62 | – | 56 | – | – | 56 |
| 6:F1-62 | – | 47 | – | – | 47 |
| 52:F3-60 | – | 46 | – | – | 46 |
| 6:F1-100 | – | 46 | – | – | 46 |
| 6:F1-72 | – | 46 | – | – | 46 |
| 1:F3-1 | – | – | 45 | – | 45 |
| 6:F5-133 | – | 45 | – | – | 45 |
| 6:F4-5 | – | 43 | – | – | 43 |
| 6:F4-17 | – | 42 | – | – | 42 |
| 9:F7-3 | – | – | – | 37 | 37 |
| 6:F5-84 | – | 36 | – | – | 36 |
| 33:F2-17 | – | 35 | – | – | 35 |
| 52:F1-21 | – | 35 | – | – | 35 |
| 62:ND | 35 | – | – | – | 35 |
| 33:ND | – | 34 | – | – | 34 |
| 88:fnl | – | – | 32 | – | 32 |
| 9:F2-24 | – | – | – | 32 | 32 |
| 1:F5-5 | – | – | 31 | – | 31 |
| 34:ND | – | 31 | – | – | 31 |
| 69:ND | 31 | – | – | – | 31 |
| 6:F4-6 | – | 29 | – | – | 29 |
| 6:F5-12 | – | 29 | – | – | 29 |
| 2:ND | – | – | 28 | – | 28 |
| 6:F5-18 | – | 26 | – | – | 26 |
| 2:F6-3 | – | – | 25 | – | 25 |
| 52:F1-29 | – | 25 | – | – | 25 |
| 6:F1-21 | – | 25 | – | – | 25 |
| 9:F11-4 | – | – | – | 25 | 25 |
| 4:F1-7 | – | – | 23 | – | 23 |
| 63:F1-72 | – | – | – | 23 | 23 |
| 6:F6-2 | – | 23 | – | – | 23 |
| 1:F4-23 | – | – | 22 | – | 22 |
| 33:F4-6 | – | 22 | – | – | 22 |
| 33:F5-34 | – | 22 | – | – | 22 |
| 6:F2-23 | – | 22 | – | – | 22 |
| 6:F1-120 | – | 21 | – | – | 21 |
| 6:F1-101 | – | 20 | – | – | 20 |
Discussion
Although the introduction of PsA-TT into the African meningitis belt has had a major impact on serogroup A epidemics,8, 31 the region will remain at risk of meningococcal disease until comprehensive vaccines targeting all serogroups are available.3 The reasons for the unique epidemiology of the African meningitis belt remain poorly understood,32 making it difficult to predict when and where epidemics caused by non-serogroup A meningococci might occur. Variations in the prevalence of NPN species, which potentially contribute cross immunity through sub-capsular antigens, could play a role in determining susceptibility to a potentially epidemic strain. A number of studies have indicated the movements of genes encoding various protein antigens from NPN to Nm14, 33 and variants of the FetA antigen have been previously shown to be shared amongst Neisseria species.14, 34 This antigen is known to generate protective responses in humans.35 Colonization with NPNs also affects colonization of humans in experimental studies.18
The novel sequence based techniques employed in this study enabled Nm and NPN to be identified and characterized rapidly and cost effectively from the very large numbers of samples obtained in the African centers. The rplF assay22 achieved reliable speciation, which would not have been possible with conventional methods such as 16S rRNA gene sequencing. An indication of diversity within species was achieved by sequencing the variable region of the gene encoding FetA (fetA_VR), an outer membrane protein (OMP) found in most Neisseria, and which is involved in iron metabolism and been shown to elicit protective immune responses.36 Further characterization of the meningococcal isolates has been previously published.12
Of the five known Neisseria species identified, with a possible novel species present in Chad, the most common were Nl and Nm. These are the species that have been observed mostly frequently in previous investigations in the African meningitis belt and they are known to have antagonistic interactions in colonization.18, 37 It is possible that the isolation of some of the other species was affected by the selective media used in this and other studies. Although growth of Np,38 Nl17, 39, 40, 41 and Ns42 on colistin containing agar (such as modified Thayer–Martin or New York city medium) has been reported, other species such as Neisseria perflava, Neisseria sicca, Neisseria mucosa, Neisseria cinerea may not have been identified using the culture technique employed in this study.43 The impact of these media on Nb is unknown. In future, the identification of NPNs could be enhanced by the use of molecular approaches, although these will have to have species-level resolution, such as the rplF assay.22 The Neisseria identified were highly diverse at both the strain and the species level and varied markedly over time and place. This is consistent with other carriage studies in the meningitis belt, but is different from the relatively stable carriage observed in countries that do not experience large-scale epidemic disease.44
The most common NPNs recovered, Np and Nl, were isolated predominantly from young children as opposed to Nm, which was isolated most frequently from older children. Nl is known to colonize infants and young children preferentially in many settings45 and this is consistent with carriage of NPNs having a role in the rapid acquisition of antibody against Nm among children in the meningitis belt, although given the age distribution of meningococcal disease these antibodies may not be protective.46
The risk factors for Nm and NPNs carriage were not the same and in some cases (eg. age, sex and season) were inversely related. Individuals were more likely to carry NPNs during the rainy season if they were a female and under 5 years old whereas they were more likely to carry Nm during the dry season, if they were male and between 5 and 29 years old.12 These results suggest that the physical presence of an NPN in the pharynx may prevent the colonization by Nm although this may not apply to the hyper-invasive meningococci since the incidence of meningococcal disease in the African meningitis belt is highest in under five year olds. Recent vaccination with a meningitis vaccine (primarily MenAfriVac®, which was used in Mali, Niger and Chad during the course of our study) appeared to be protective both against carriage of Nm and NPNs overall in the regression analysis. This effect could possibly be explained by disturbance of the microbiome following vaccination but could also be due to residual confounding or a reflection of the naturally dynamic patterns of carriage. In Mali, an increase in Nm was observed post-vaccination, due to an expansion of serogroup W.12 This seems unlikely to be an effect of MenAfriVac® but it is not known how the removal of one serogroup from the nasopharyngeal environment could affect the other serogroups. The influence of host factors on carriage of Nm and NPNs has not been investigated in this study but the IgA secretory status of the individuals sampled could also play a role as previously shown.47
Few published studies have characterized pharyngeal NPNs in the African meningitis belt. One recent study in Burkina Faso, which did not survey adults over the age of 30 years or identify species other than Nm and Nl,17 reported a number of differences compared to the MenAfriCar surveys described here: there was a higher overall prevalence of Nl carriage (18.2%); a higher prevalence in males, although it was significantly higher in women (9.1% vs 3.9%) for the 18–29 year age group and no significant changes between seasons or post vaccination. The reasons for these inconsistencies are unclear, although both studies indicated fluctuations in prevalence among surveys and these differences may reflect the highly dynamic nature of Neisseria carriage.
Some of the fetA_VR alleles found were shared among the different species, as reported previously.14, 34 Since the variable region of fetA plays an important role in its immunogenicity,28 the shared protein variants could create a cross-reactive immunity at the subscapular level. The inclusion of fetA_VR in the meningococcal typing system also increased discrimination between strains (supplemental Table 6). Although there was correlation between porA and fetA_VR alleles for some Nm strains (e.g. cnl:P1.18-11,42-1:fnl), in others the fetA_VR (e.g. W:P1.5,2:F1-1 and W:P1.5,2:F6-3) or both OMPs (e.g. W:P15-1,2-36:F5-1) sequences varied, suggesting that the serogroup W Nm strains are antigenically diverse. This has potential implications for using proteins such as porA or fetA in vaccine formulations.48 The Neighbor Joining phylogeny (Supplemental Fig. 3) shows the clustering of all of the isolates with the appropriate species except for one, which has the f_rplF allele 58 defined previously as Ns22 but clusters more closely, in this study, with the Nb species which was not discovered at the time of the previous study. This particular isolate may also be a Ns with a f_rplF allele similar to Nb. More sequence data from this isolate will clarify this issue.
This study has demonstrated the dynamic nature and high diversity of the genus Neisseria in pharyngeal carriage in countries of the African meningitis belt. The results are consistent with the idea that the carriage of NPNs may influence invasive meningococcal disease epidemiology in the African meningitis belt. Although more research is needed to elucidate such effects, understanding these organisms could potentially contribute to meningococcal disease control. This is of particular importance given the absence of comprehensive vaccines against all meningococcal serogroups and the continuing interest in the development of protein based vaccines.
Financial support
MenAfriCar was funded by the Wellcome Trust (086546/Z/08/Z) and the Bill and Melinda Gates Foundation (51251). Kanny Diallo holds a Wellcome Trust Training Fellowship in Public Health and Tropical Medicine. The funding sources had no role in the study design, collection, analysis and interpretation of the data, in the writing of the report or the decision to submit the paper for publication.
Conflict of interest
Caroline Trotter reports that she received a Consulting payment in 2013 for a critical review of health economic model of meningococcal ACWY vaccine by GlaxoSmithKline (GSK); Ray Borrow reports that he performed contract researches on behalf of Public Health England for Novartis Vaccines and Diagnostics, Baxter Biosciences, Sanofi Pasteur, Serum Institute of India and GSK. All other authors report no potential conflicts of interest.
Acknowledgments
We would like to extend our sincere gratitude to all the participants, fieldworkers, advisory board, LSHTM secretariat and other individuals that made the study possible. We also thank Professor Ian Feavers for his help in the curation of the fetA alleles.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2016.03.010.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Stephens D.S., Greenwood B., Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007 Jun 30;369(9580):2196–2210. doi: 10.1016/S0140-6736(07)61016-2. PubMed PMID: 17604802. [DOI] [PubMed] [Google Scholar]
- 2.Maurice J. Vaccine shortage threatens spread of meningitis in Niger. Lancet. 2015 Jun 6;385(9984):2241. doi: 10.1016/S0140-6736(15)61050-9. PubMed PMID: 26088485. [DOI] [PubMed] [Google Scholar]
- 3.WHO . 2015. Meningitis outbreak response in sub-Saharan Africa: WHO guidelines. [accessed 11.06.15] [PubMed] [Google Scholar]
- 4.Lee C.H., Kuo W.C., Beri S., Kapre S., Joshi J.S., Bouveret N. Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine. 2009 Jan 29;27(5):726–732. doi: 10.1016/j.vaccine.2008.11.065. PubMed PMID: 19063929. [DOI] [PubMed] [Google Scholar]
- 5.Frasch C.E., Preziosi M.P., LaForce F.M. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM) Hum Vaccin Immunother. 2012 Jun;8(6):715–724. doi: 10.4161/hv.19619. PubMed PMID: 22495119. [DOI] [PubMed] [Google Scholar]
- 6.Djingarey M.H., Barry R., Bonkoungou M., Tiendrebeogo S., Sebgo R., Kandolo D. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine. 2012 May 30;30(Suppl. 2):B40–B45. doi: 10.1016/j.vaccine.2011.12.073. PubMed PMID: 22607898. [DOI] [PubMed] [Google Scholar]
- 7.Caini S., Beck N.S., Yacouba H., Maiga I., Chaibou I., Hinsa I. From Agadez to Zinder: estimating coverage of the MenAfriVac conjugate vaccine against meningococcal serogroup A in Niger, September 2010–January 2012. Vaccine. 2013 Mar 15;31(12):1597–1603. doi: 10.1016/j.vaccine.2013.01.015. PubMed PMID: 23337027. [DOI] [PubMed] [Google Scholar]
- 8.Daugla D.M., Gami J.P., Gamougam K., Naibei N., Mbainadji L., Narbe M. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected] Lancet. 2014 Jan 4;383(9911):40–47. doi: 10.1016/S0140-6736(13)61612-8. PubMed PMID: 24035220. PMCID: 3898950. Epub 2013/09/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trotter C.L., Greenwood B.M. Meningococcal carriage in the African meningitis belt. Lancet Infect Dis. 2007 Dec;7(12):797–803. doi: 10.1016/S1473-3099(07)70288-8. PubMed PMID: 18045562. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen P.A., Diomande F., Wei S.C., Ouedraogo R., Sangare L., Sanou I. Baseline meningococcal carriage in Burkina Faso before the introduction of a meningococcal serogroup A conjugate vaccine. Clin Vaccin Immunol. 2011 Mar;18(3):435–443. doi: 10.1128/CVI.00479-10. PubMed PMID: 21228139. PMCID: 3067389. Epub 2011/01/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MenAfriCar Meningococcal carriage in the African meningitis belt. Trop Med Int Health. 2013;18(8):968–978. doi: 10.1111/tmi.12125. PubMed PMID: 23682910. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MenAfriCar c The diversity of meningococcal carriage across the African meningitis belt and the impact of vaccination with a group A meningococcal conjugate vaccine. J Infect Dis. 2015;212(8):1298–1307. doi: 10.1093/infdis/jiv211. PubMed PMID: 25858956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blakebrough I.S., Greenwood B.M., Whittle H.C., Bradley A.K., Gilles H.M. The epidemiology of infections due to Neisseria meningitidis and Neisseria lactamica in a northern Nigerian community. J Infect Dis. 1982 Nov;146(5):626–637. doi: 10.1093/infdis/146.5.626. PubMed PMID: 7130749. [DOI] [PubMed] [Google Scholar]
- 14.Linz B., Schenker M., Zhu P., Achtman M. Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol. 2000 Jun;36(5):1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. PubMed PMID: 10844690. [DOI] [PubMed] [Google Scholar]
- 15.Kristiansen P.A., Ba A., Ouedraogo A.S., Sanou I., Ouedraogo R., Sangare L. Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis. 2014 Dec 4;14(1):663. doi: 10.1186/s12879-014-0663-4. PubMed PMID: 25472422. PMCID: 4267149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen P.A., Diomande F., Ba A.K., Sanou I., Ouedraogo A.S., Ouedraogo R. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013 Feb;56(3):354–363. doi: 10.1093/cid/cis892. PubMed PMID: 23087396. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen P.A., Diomande F., Ouedraogo R., Sanou I., Sangare L., Ouedraogo A.S. Carriage of Neisseria lactamica in 1- to 29-year-old people in Burkina Faso: epidemiology and molecular characterization. J Clin Microbiol. 2012 Dec;50(12):4020–4027. doi: 10.1128/JCM.01717-12. PubMed PMID: 23035186. PMCID: 3503018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deasy A.M., Guccione E., Dale A.P., Andrews N., Evans C.M., Bennett J.S. Nasal inoculation of the commensal Neisseria lactamica inhibits carriage of Neisseria meningitidis by young adults: a controlled human infection study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015 May 15;60(10):1512–1520. doi: 10.1093/cid/civ098. PubMed PMID: 25814628. [DOI] [PubMed] [Google Scholar]
- 19.Bennett J.S., Jolley K.A., Earle S.G., Corton C., Bentley S.D., Parkhill J. A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria. Microbiology. 2012 Jun;158(Pt 6):1570–1580. doi: 10.1099/mic.0.056077-0. PubMed PMID: 22422752. PMCID: 3541776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basta N.E., Stuart J.M., Nascimento M.C., Manigart O., Trotter C., Hassan-King M. Methods for identifying Neisseria meningitidis carriers: a multi-center study in the African meningitis belt. PloS one. 2013;8(10):e78336. doi: 10.1371/journal.pone.0078336. PubMed PMID: 24194921. PMCID: 3806823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolley K.A., Maiden M.C. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. PubMed PMID: 21143983. PMCID: 3004885. Epub 2010/12/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett J.S., Watkins E.R., Jolley K.A., Harrison O.B., Maiden M.C. Identifying Neisseria species by use of the 50S ribosomal protein L6 (rplF) gene. J Clin Microbiol. 2014 May;52(5):1375–1381. doi: 10.1128/JCM.03529-13. PubMed PMID: 24523465. PMCID: 3993661. Epub 2014/02/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitou N., Nei M. The neighbor-joining method – a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. PubMed PMID: ISI: A1987J406700007. English. [DOI] [PubMed] [Google Scholar]
- 24.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. PubMed PMID: 7463489. eng. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013 Dec;30(12):2725–2729. doi: 10.1093/molbev/mst197. PubMed PMID: 24132122. PMCID: 3840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmsen D., Singer C., Rothganger J., Tonjum T., de Hoog G.S., Shah H. Diagnostics of neisseriaceae and moraxellaceae by ribosomal DNA sequencing: ribosomal differentiation of medical microorganisms. J Clin Microbiol. 2001 Mar;39(3):936–942. doi: 10.1128/JCM.39.3.936-942.2001. PubMed PMID: 11230407. PMCID: 87853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim O.S., Cho Y.J., Lee K., Yoon S.H., Kim M., Na H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012 Mar;62(Pt 3):716–721. doi: 10.1099/ijs.0.038075-0. PubMed PMID: 22140171. [DOI] [PubMed] [Google Scholar]
- 28.Thompson E.A.L. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003;149(7):1849–1858. doi: 10.1099/mic.0.26131-0. [DOI] [PubMed] [Google Scholar]
- 29.Claus H., Elias J., Meinhardt C., Frosch M., Vogel U. Deletion of the meningococcal fetA gene used for antigen sequence typing of invasive and commensal isolates from Germany: frequencies and mechanisms. J Clin Microbiol. 2007 Sep;45(9):2960–2964. doi: 10.1128/JCM.00696-07. PubMed PMID: 17626167. PMCID: 2045310. Epub 2007/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlow H., Vogel U., Mueller J.E., Curry A., Njanpop-Lafourcade B.M., Claus H. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J Infect Dis. 2007 Apr 1;195(7):1071–1077. doi: 10.1086/512084. PubMed PMID: 17330799. [DOI] [PubMed] [Google Scholar]
- 31.Gamougam K., Daugla D.M., Toralta J., Ngadoua C., Fermon F., Page A.L. Continuing effectiveness of serogroup A meningococcal conjugate vaccine, Chad, 2013. Emerg Infect Dis. 2015 Jan;21(1):115–118. doi: 10.3201/eid2101.140256. PubMed PMID: 25536336. PMCID: 4285275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999 Jul–Aug;93(4):341–353. doi: 10.1016/s0035-9203(99)90106-2. PubMed PMID: 10674069. [DOI] [PubMed] [Google Scholar]
- 33.Zhu P., van der Ende A., Falush D., Brieske N., Morelli G., Linz B. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. Proc Natl Acad Sci U. S. A. 2001 Apr 24;98(9):5234–5239. doi: 10.1073/pnas.061386098. PubMed PMID: 11287631. PMCID: 33193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett J.S., Thompson E.A., Kriz P., Jolley K.A., Maiden M.C. A common gene pool for the Neisseria FetA antigen. Int J Med Microbiol. 2009 Feb;299(2):133–139. doi: 10.1016/j.ijmm.2008.06.010. PubMed PMID: 18718812. PMCID: 3968273. Epub 2008/08/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsay L., Dold C., Green C.A., Rollier C.S., Norheim G., Sadarangani M. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: a phase I clinical trial. J Infect. 2015 Sep;71(3):326–337. doi: 10.1016/j.jinf.2015.05.006. PubMed PMID: 25982025. PMCID: 4535279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson E.A., Feavers I.M., Maiden M.C. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003 Jul;149(Pt 7):1849–1858. doi: 10.1099/mic.0.26131-0. PubMed PMID: 12855736. Epub 2003/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 37.Gagneux S.P., Hodgson A., Smith T.A., Wirth T., Ehrhard I., Morelli G. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002 Mar 1;185(5):618–626. doi: 10.1086/339010. PubMed PMID: 11865418. [DOI] [PubMed] [Google Scholar]
- 38.Boquete M.T., Marcos C., Saez-Nieto J.A. Characterization of Neisseria polysacchareae sp. nov. (Riou, 1983) in previously identified noncapsular strains of Neisseria meningitidis. J Clin Microbiol. 1986 May;23(5):973–975. doi: 10.1128/jcm.23.5.973-975.1986. PubMed PMID: 3086373. PMCID: 268766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saez-Nieto J.A., Dominguez J.R., Monton J.L., Cristobal P., Fenoll A., Vazquez J. Carriage of Neisseria meningitidis and Neisseria lactamica in a school population during an epidemic period in Spain. J Hyg (Lond) 1985 Jun;94(3):279–288. doi: 10.1017/s0022172400061507. PubMed PMID: 3924995. PMCID: 2129489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cartwright K.A., Stuart J.M., Jones D.M., Noah N.D. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987 Dec;99(3):591–601. doi: 10.1017/s0950268800066449. PubMed PMID: 3123263. PMCID: 2249239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kremastinou J., Tzanakaki G., Levidiotou S., Markou F., Themeli E., Voyiatzi A. Carriage of Neisseria meningitidis and Neisseria lactamica in northern Greece. FEMS Immunol Med Microbiol. 2003 Oct 24;39(1):23–29. doi: 10.1016/S0928-8244(03)00174-3. PubMed PMID: 14556992. [DOI] [PubMed] [Google Scholar]
- 42.Sheikhi R., Amin M., Rostami S., Shoja S., Ebrahimi N. Oropharyngeal colonization with Neisseria lactamica, other nonpathogenic Neisseria species and Moraxella catarrhalis among young healthy children in Ahvaz, Iran. Jundishapur J Microbiol. 2015 Mar;8(3):e14813. doi: 10.5812/jjm.14813. PubMed PMID: 25964847. PMCID: 4418171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saez Nieto J.A., Marcos C., Vindel A. Multicolonization of human nasopharynx due to Neisseria spp. Int Microbiol. 1998 Mar;1(1):59–63. PubMed PMID: 10943342. [PubMed] [Google Scholar]
- 44.Maiden M.C., Ibarz-Pavon A.B., Urwin R., Gray S.J., Andrews N.J., Clarke S.C. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008 Mar 1;197(5):737–743. doi: 10.1086/527401. PubMed PMID: 18271745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett J.S., Griffiths D.T., McCarthy N.D., Sleeman K.L., Jolley K.A., Crook D.W. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect Immun. 2005 Apr;73(4):2424–2432. doi: 10.1128/IAI.73.4.2424-2432.2005. PubMed PMID: 15784588. PMCID: 1087434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trotter C.L., Yaro S., Njanpop-Lafourcade B.-M., Drabo A., Kroman S.S., Idohou R.S. Seroprevalence of bactericidal, specific IgG antibodies and incidence of meningitis due to group A Neisseria meningitidis by age in Burkina Faso 2008. PloS one. 2013;8(2):e55486. doi: 10.1371/journal.pone.0055486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blackwell C.C., Weir D.M., James V.S., Todd W.T., Banatvala N., Chaudhuri A.K. Secretor status, smoking and carriage of Neisseria meningitidis. Epidemiol Infect. 1990 Apr;104(2):203–209. doi: 10.1017/s0950268800059367. PubMed PMID: 2323355. PMCID: PMC2271764. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koeberling O., Ispasanie E., Hauser J., Rossi O., Pluschke G., Caugant D.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA) Vaccine. 2014 May 13;32(23):2688–2695. doi: 10.1016/j.vaccine.2014.03.068. PubMed PMID: 24704334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.