Abstract
Aims
Triglycerides droplets are massively stored in muscle in Lipid Storage Myopathies (LSM). We studied in muscle regulators of lipophagy, the expression of the transcription factor-EB (TFEB) (a master regulator of lysosomal biogenesis), and markers of autophagy which are induced by starvation and exert a transcriptional control on lipid catabolism.
Methods
We investigated the factors that regulate lipophagy in muscle biopsies from 6 patients with different types of LSM: 2 cases of riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency (MADD), 1 case of primary carnitine deficiency (CD), 2 cases of neutral lipid storage myopathy (NLSD-M), 1 case of carnitine–palmitoyl-transferase-II (CPT) deficiency.
Results
Conventional morphology and electron microscopy documented the lipid accumulation and its dramatic resolution after treatment. Muscle immunofluorescence showed that while in MADD and NLSD-M there was a co-localized expression of TFEB and p62-SQSTM1 (marker of protein aggregates) in some atrophic fibers, in CD and CPT-II deficiency the reaction was almost normal. In regenerating fibers, TFEB localized in the cytoplasm (inactive form), whereas in atrophic fibers it localized in the nuclei (active form). Lipid-accumulated/atrophic fibers did not display p62-positive protein aggregates, indicating, together with the LC3-II (marker of autophagosomes) and p62-SQSTM1 analysis, that the autophagic flux is often preserved and lipophagy occurs.
Conclusion
In atrophic and regenerating fibers of patients with NLSD-M we observed TFEB over-expression; in other conditions autophagy markers are increased, suggesting lipophagy active role on human lipid metabolism.
Abbreviations: ATGL, adipose triglyceride lipase; ATP-ase, adenosine tri-phosphatase; CD, carnitine deficiency; CK, creatine kinase; COX, cytochrome oxidase; CPT, carnitine-palmitoyl-transferase; DAPI, 4′,6-diamidin-2-phenylindole; EMG, electromyography; ETF, electron transfer flavoprotein; FOXO, fork head box protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSL, hormone-sensitive lipase; LC3, microtubule-associated proteins light chain-3; LSM, lipid storage myopathy; MADD, multiple acyl-CoA dehydrogenase deficiency; MCT, medium chain triglyceride; NADH-TR, nicotinamide adenine dinucleotide dehydrogenase tetrazolium reductase; NLSD-M, neutral lipid storage disease with myopathy; OCTN2, carnitine organic cation transporter-2; p62-SQSTM1, p62-sequestosome-1; PAS, perjodic acid Schiff; PPARα, peroxisome proliferator-activated receptor-γ-coactivator-1α; SDH, succinate dehydrogenase; TFEB, transcription factor-EB
Keywords: Lipid storage myopathy, MADD, NLSD-M, Carnitine deficiency, TFEB
Highlights
-
•
Fatty acid disorders are due to defective oxidation or transport in mitochondria.
-
•
Lipophagy mobilizes fatty acids from lipid droplets within muscle fibers.
-
•
Lipophagy has a role in utilization of triglycerides from lipid droplets.
-
•
LC3-II and p62-SQSTM1 accumulate in atrophic fibers in lipid storage myopathies.
-
•
TFEB, a regulator of lipophagy, is increased when lipophagy or oxidation is blocked.
1. Introduction
Lipid Storage Myopathies (LSM) are a group of clinically, biochemically and genetically heterogeneous disorders characterized by accumulation of lipid droplets in muscle often apposed to mitochondria, with prevalent limb and neck muscle weakness, sometimes associated with multisystem dysfunction.
Multiple acyl-CoA dehydrogenase deficiency (MADD, MIM#231680) is caused by defects in electron transfer flavoprotein (ETF), or ETF-dehydrogenase (ETF-DH) [1], [2], two mitochondrial enzymes. The clinical phenotype in late-onset patients consists in proximal myopathy, high creatine kinase (CK) levels, lethargy, vomiting, hypoglycemia, metabolic acidosis, hepatomegaly, glutaric aciduria during crises, and lipid storage in muscle. The diagnosis is suggested by the acyl-carnitine profile and urinary organic acids, revealing low serum free carnitine but elevated acyl-carnitines. The disease is responsive to treatment with riboflavin as well as carnitine supplementation.
Neutral lipid storage disease with myopathy (NLSD-M, MIM# 610717) is characterized by slowly progressive proximal muscle weakness affecting the upper and lower limbs, with increased CK levels, cardiomyopathy, diabetes mellitus, hepatic steatosis, and hypertriglyceridemia. Leukocytes show a characteristic accumulation in cytoplasm of triglycerides called “Jordan's anomaly”. The disease is due to PNPLA2 gene mutations [3], causing a defect in the adipose triglyceride lipase (ATGL), which catalyzes the initial step in the breakdown of intracellular triglycerides (lipolysis), and as a treatment only medium chain triglycerides appear promising.
Carnitine deficiency (CD, MIM#212140) is a potentially lethal but very treatable disorder which is due to a defect in the carnitine organic cation transporter (OCTN2), which results in impaired fatty acid oxidation in striated muscle. CD is clinically characterized by carnitine-responsive dilated cardiomyopathy, episodes of hypoglycemic, hypoketotic coma, or acute hepatic failure such as Reye's syndrome, with very low plasma carnitine content and severe renal leak of carnitine. Primary CD has to be differentiated by secondary carnitine deficiency status that occurs in MADD.
The deficiency of carnitine–palmitoyl-transferase-II (CPT-II), an enzyme involved in the transport of fatty acids into the mitochondrial matrix, is clinically characterized by recurrent myoglobinuric attacks (MIM#255110) triggered by risk factors such as exercise, fasting, fever, and infection, which may be complicated by acute renal failure and respiratory failure.
While triglycerides are massively stored in MADD and NLSD-M, lipid droplets are almost absent in CPT-II deficiency. The reason for this morphological difference is unknown.
Autophagy, a lysosomal-dependent catabolic process, is generally activated during starvation and plays a central role in lipid metabolism, i.e. lipophagy, because it shuttles lipid droplets to the lysosome or to mitochondria, where they are degraded or oxidized. The Transcription Factor EB (TFEB), a master regulator of lysosomal biogenesis and autophagy [4], is induced by starvation through an auto-regulatory feedback loop and exerts a transcriptional control of genes involved in lipid catabolism and indirectly in the transport of fatty acid chains across the membrane, and the beta-oxidation of fatty acids in mitochondria, via nuclear peroxisome proliferator-activated receptor-γ-coactivator-1α (PPARα) and of its co-activator PGC1α [5]. Inactive TFEB resides in the cytoplasm when it is phosphorylated by mTORC1, and after de-phosphorylation it translocates to the nucleus during starvation [6], [7], where it induces its expression as active form and initiates the metabolic shift of the catabolism of lipids. Therefore, TFEB over-expression is known to promote lysosome expansion and autophagy [8], [4].
Recent advances have been made using PPARγ agonist treatment (i.e. bezafibrate), with benefit in fibroblasts of CPT-II deficiency but without consistent effects at clinical levels [9], [10], [11].
We investigate the factors that regulate fatty acid degradation and lipophagy in muscle from patients with LSM of different etiology. The autophagic flux and its possible impairment was monitored by the expression of LC3 and p62-SQSTM1-containing protein aggregates.
2. Materials and methods
2.1. Patients' selection criteria
For this study we selected 6 patients (4 females, 2 males), including 5 cases with LSM of different etiology (2 MADD, 1 CD, 2 NLSD-M) and 1 case with CPT-II deficiency (Table 1).
Table 1.
Clinical data, laboratory findings, and treatment.
| Patient, gender | Diagnosis | Age at biopsy (years) | Age at onset (years) | Main clinical features | Causes of metabolic stress | Muscle carnitine level⁎ | Mitochondrial respiratory chain enzyme activity | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1, F | MADD | 41 | 38 | Respiratory insufficiency, proximal weakness, unable to walk, acute quadriplegia | Poor nutrition | 3.88 (17%) | Complex I (65%), complex II + III (30%), complex II (46%), complex IV (61%) | Riboflavin (200 mg/day), carnitine (4 g/day), MCT oil |
| 2, F | MADD | 35 | 35 | Waddling gait, hypotonia, epilepsy, dysphagia, psychosis | Poor nutrition, alcoholism, lactacidemia | 2.27 (11%) | Complex IV (16%), complex II + III (25%) | Riboflavin (200 mg/day), carnitine (4 g/day), carbamazepine |
| 3, F | CD | 36 | 7 | Muscle weakness, difficulty walking, weight loss | Poor nutrition | 0.81 (5%) | Complex IV (25%) | Carnitine (4 g/day), MCT oil |
| 4, F | NLSD-M | 39 | 18 | Muscle weakness and atrophy, difficulty walking, hepatomegaly | Diabetes | 8.5 (54%) | N.d. | Dietary, MCT oil |
| 5, M | NLSD-M | 71 | 66 | Muscle weakness, dropped head syndrome | Diabetes | 11.3 (73%) | Normal activity | Dietary, MCT oil |
| 6, M | CPT-II deficiency | 36 | 17 | Recurrent exertional myoglobinuria, acute renal failure | Muscle exercise | N.d. | N.d. | Dietary, MCT oil, avoiding triggering factors |
N.d.: not determined.
Values are expressed as nanomoles/mg protein. Normal values: 10.5–29.5 nmol/mg protein. In brackets is indicated the percentage of control mean.
2.2. Muscle morphology and biochemical analyses
Muscle biopsies, which were performed following written consent (according to ethical standards approved by University of Padova) was obtained as part of the diagnostic process, were frozen in liquid nitrogen and stored at − 80°. Cryostat-cut sections 10 μm thick were used to perform a panel of routine stains, including hematoxylin–eosin, Gomori trichrome, Oil-Red-O, PAS, NADH-TR, COX, SDH, ATP-ases, acid phosphatase. Muscle morphology was evaluated by conventional optic examination.
For electron microscopy analysis, fresh muscle tissues were routinely processed and examined with a Philips 400T transmission electron microscope. Two biopsies of Patient 1 were examined, before and after treatment.
Muscle homogenates were used to determine the content of total carnitine and acyl-carnitines, and the activity of mitochondrial enzymes, using conventional radiochemical or spectrophotometric assays, respectively.
2.3. Immunohistochemical analysis
To investigate the expression of TFEB and p62-SQSTM1 (marker of protein aggregates) in muscle fibers, serial muscle sections (6–8 μm thick) were fixed with 4% paraformaldehyde, treated with 0.1% Triton, blocked in 0.5% albumin + 10% horse serum, and incubated overnight at 4 °C with the following primary antibodies: p62-SQSTM1 (GP62-C, Progen Biotechnik, Heidelberg, Germany), TFEB (MBS120432, MyBioSource, San Diego, CA), and developmental myosin heavy chain (marker of regeneration) (MONX10806, Monosan, Uden, The Netherlands) [12]. Appropriate secondary fluorescent antibodies (Alexa-Fluor, Invitrogen, Paisley, UK) were used. Slides were mounted using Vectashield medium with DAPI stain (Vector, Burlingame, CA) and examined by fluorescence microscopy.
2.4. Immunoblot analysis
To investigate the expression of p62-SQSTM1 and LC3 (marker of autophagosome proliferation) by semi-quantitative immunoblot analysis, muscle sections were lysed in Laemmli buffer, proteins were resolved by polyacrylamide gel electrophoresis, and immunoblotted using the following primary antibodies: LC3 (L7543, Sigma-Aldrich, St. Louis, MO), p62-SQSTM1 (GP62-C, Progen), TFEB (4240, Cell Signaling, Milan, Italy), GAPDH-glyceraldehyde 3-phosphate dehydrogenase (8245, Abcam, Cambridge, UK) [12]. Only using LC3 immunoblotting, the LC3-II band (corresponding to LC3 linked to membranes and therefore used as marker of autophagosomes) can be separated and measured from that corresponding to LC3-I (the cytosolic component not linked to autophagosomes). Immunolabeling was visualized using the chemiluminescent substrate (GE Healthcare, Milan, Italy). The expression levels of p62-SQSTM1 and LC3-II bands were determined by densitometric quantification using the ImageJ software (US National Institutes of Health) and normalized to GAPDH band.
3. Results
3.1. Case reports (Table 1)
3.1.1. Patient 1 (MADD)
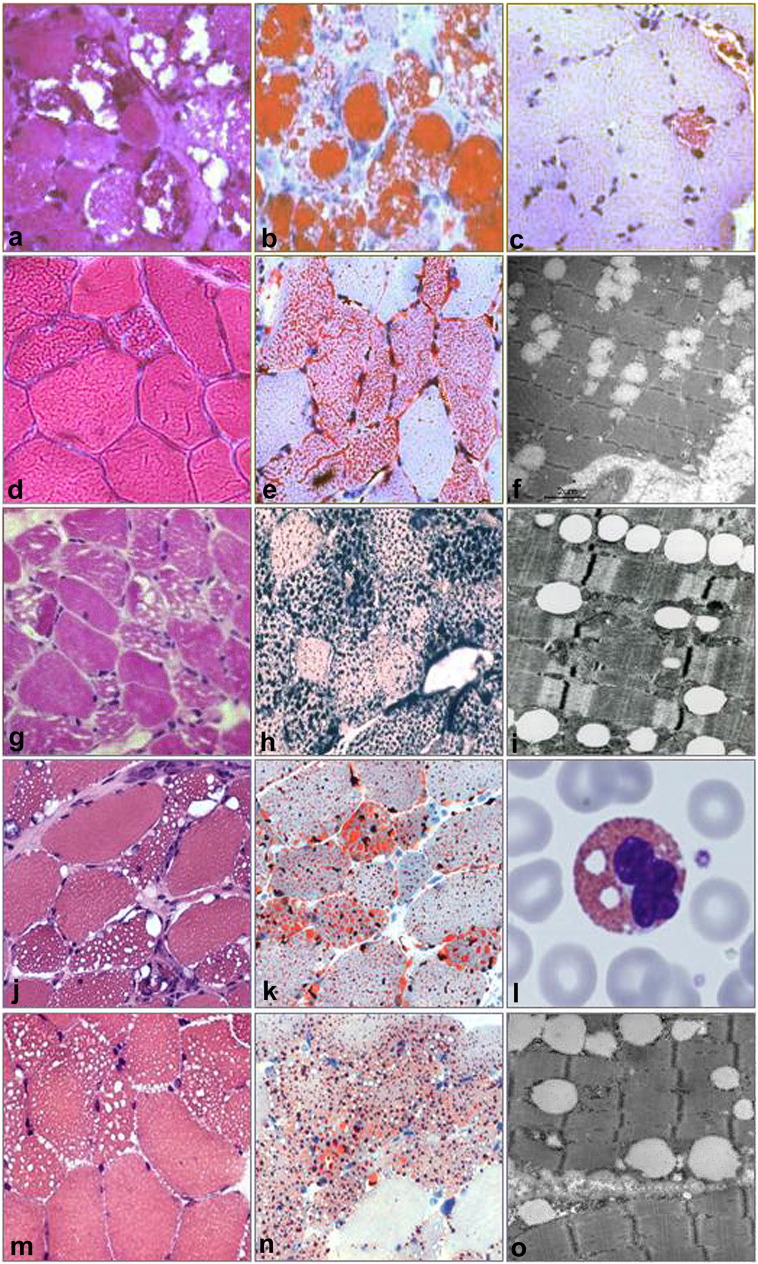
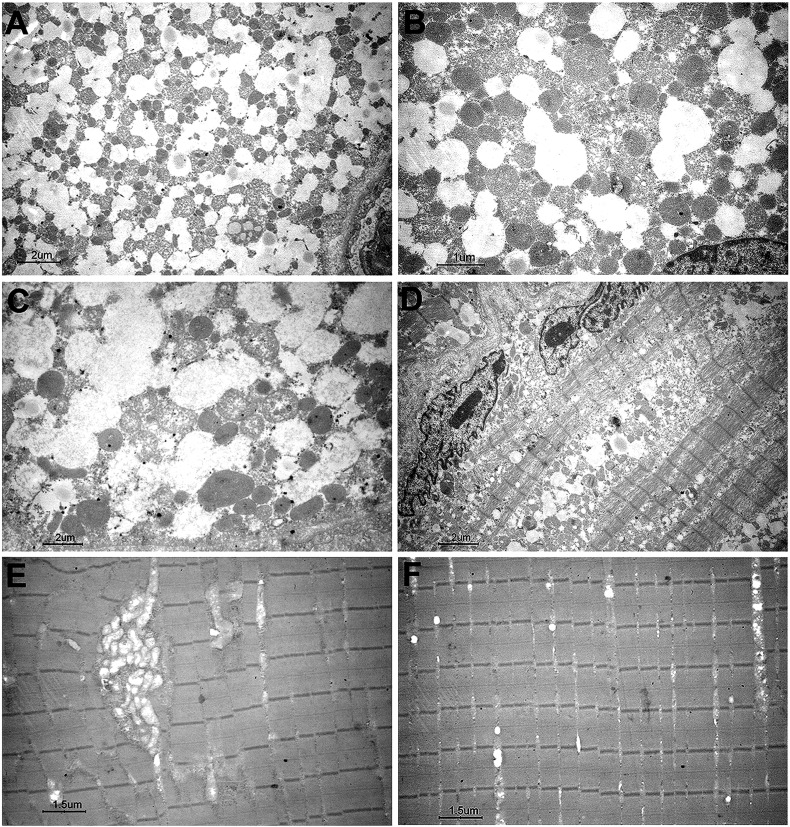
At 38 years of age, this woman complained of acute weakness of upper girdle muscle, neck flexors and respiratory muscles, and she became virtually quadriplegic and respirator-dependent. CK was 2277 U/L, EMG was myopathic. She was hospitalized in an intensive care unit for acute respiratory infection, where she was tracheotomised and remained for over 3 weeks. A first quadriceps femoris muscle biopsy showed massive lipid storage (Fig. 1) and, on electron microscopy, a number of swollen and abnormal mitochondria (Fig. 2). Biochemically, a secondary carnitine-deficiency consistent with MADD was found: elevated levels of medium and long chain acyl-carnitines in plasma (Table 1); mitochondrial enzymes were variably reduced including citrate synthase (Table 1). Treatment with low-fat and high-protein diet directed to prevent autophagy, medium chain triglycerides (MCT) oil (which enters directly into mitochondria) and 4 g of carnitine produced a fast improvement and normalization of carnitine in plasma and in muscle of a second biopsy on deltoid muscle. We observed a dramatic recovery of myofiber structure with decreased lipid droplets. Riboflavin supplementation (200 mg/day) also produced subsequent marked improvement, preventing further metabolic crises.
Fig. 1.
Muscle morphology and electron microscopy of patient 1 (a–c), patient 2 (d–f), patient 3 (g–i), patient 4 (j,k), patient 5 (m–o). Muscle sections stained with hematoxylin–eosin (a,d,g,j,m) showed vacuolar myopathy involving many fibers, with pronounced cytoplasmic accumulation of neutral lipids, as documented by Oil-Red-O (b,c,e,k,n) or Sudan Black B (h) stains. Note that in patient 1, the massive lipid deposition observed in the first biopsy (a,b) was significantly reduced after treatment in a second biopsy (c). Ultrastructural examination of muscle (f,i,o) showed lipid droplet deposition without mitochondrial disruption. Giemsa stain of leukocytes from patient 5 (l) showed lipid vacuoles corresponding to Jordan's anomaly.
Fig. 2.
Muscle electron microscopy of patient 1 before (A–D) and after (E, F) treatment. Note that in the first biopsy there are a number of lipid droplets surrounded by a number of swollen mitochondria (A–C) and diffuse lysis of myofibrils (D). In the second biopsy there is a complete recovery of the muscle structure, where only few abnormal mitochondria (E) and few lipids are present (F).
3.1.2. Patient 2 (MADD)
At 36 years of age this woman was hospitalized for psychosis, alcoholism and poor nutrition, she had muscle pain in upper limbs, weakness in lower limbs, difficulty walking and raising from a chair, was unable to raise from the floor and climb stairs. CK was 868 U/L and EMG was myopathic. She had increased plasma acyl-carnitines and glutaric aciduria was diagnosed. Quadriceps femoris muscle biopsy revealed LSM (Fig. 1), low muscle carnitine content with increased acyl-carnitines, and reduced activity of mitochondrial OXPHOS complex enzymes (Table 1). Treatment with low-fat, high-protein diet and 4 g/day carnitine produced some improvement; however, only riboflavin supplements (200 mg/day) produced marked clinical improvement. At age 45 years she presented pneumonia ab ingestis with septic shock, metabolic acidosis, dysphagia and respiratory insufficiency which required tracheostomy and assisted ventilation. She slowly improved with riboflavin, and carnitine treatment, which she had previously abandoned.
3.1.3. Patient 3 (CD)
At 7 years of age, this girl had difficulty climbing stairs, waddling gait and elevated CK levels. She was hospitalized at age 10 for apparently unexplained anorexia, weight loss and increased weakness. She was not unusually thin or frail but had Gowers sign, weakness of neck muscles, triceps, scapular rotator, ileopsoas, thigh abductors, and was unable to raise arms. EMG was myogenic. CK was normal. Quadriceps femoris muscle biopsy showed lipid droplet accumulation (Fig. 1) which was responsive to carnitine supplementation. She had low muscle carnitine levels (Table 1). Measurement of respiratory chain enzymes activity showed reduced complex IV activity (Table 1). The patient responded to 3 g/day carnitine and MCT oil supplementation [13].
3.1.4. Patient 4 (NLSD-M)
This 44-year-old woman of Iranian origin was born from consanguineous parents. She complained of early fatigability since late teens with increasing difficulties in running and walking. After age 30, she developed a progressive weakness in both girdles. CK was 1200 U/L, EMG was myopathic. She had hepatomegaly and marked fatty liver on ultrasound exam. Laboratory tests revealed increased plasma levels of glucose, total triglycerides and VLDL. No ichthyosis or cataracts was reported. There was no clinical or instrumental signs of cardiac involvement. She was found to be homozygous carrier for apoE2 allele and developed type 2 diabetes. Biceps brachii muscle biopsy showed LSM (Fig. 1) with reduced muscle carnitine content (Table 1). She had a homozygous frame-shift mutation (c.695delT, p.L255X) in the PNPLA2 gene [14].
3.1.5. Patient 5 (NLSD-M)
This 79-year-old man had progressive muscle weakness in the arms and dropped head since the age of 66. At 70 years of age, he complained of cervical pain and received radiation therapy to the upper limbs. CK level was 330 U/L. EMG was neurogenic. Quadriceps femoris muscle biopsy showed LSM (Fig. 1) with only slightly reduced muscle carnitine content and normal mitochondrial enzymes (Table 1). Jordan's anomaly was found in leukocytes (Fig. 1). At age 75, he presented increased weakness in biceps and finger extensors, had anterocollis, kyphosis, bilateral pes cavus, and atrophy of the pectoral muscles, and was unable to lift arms over the head. At age 77, he developed diabetes mellitus. No ichthyosis or cataracts was reported. There was no clinical or instrumental signs of cardiac involvement. He has a homozygous missense mutation in the PNPLA2 gene (c.570A > C, p.S191R) [15].
3.1.6. Patient 6 (CPT-II)
This man was born from consanguineous parents and has a brother similarly affected. At age 17 years, after intense exercise, the patient had severe asthenia in lower limbs, painful cramps in calf muscles and myoglobinuria. CK was 997 U/L. In the following years he presented recurrent myoglobinuric episodes with renal insufficiency, triggered by muscle exercise. At 36 years a neurological examination was normal. Quadriceps femoris muscle biopsy showed myopathic changes with few and thin lipid droplets accumulation, and deficient CPT activity. He was homozygous for the common missense mutation in the CPT2 gene (c.338C > T, p.S113L).
3.2. Muscle morphology, immunohistochemistry and western blotting
Muscle biopsies showed pronounced lipid storage in 5 of the 6 chosen patients (Table 1, Fig. 1), whereas in the CPT-II deficient patient there where only thin and sparse lipid droplets.
In two consecutive biopsies of Patient 1 before and after treatment we documented disappearance of lipid storage (Fig. 2).
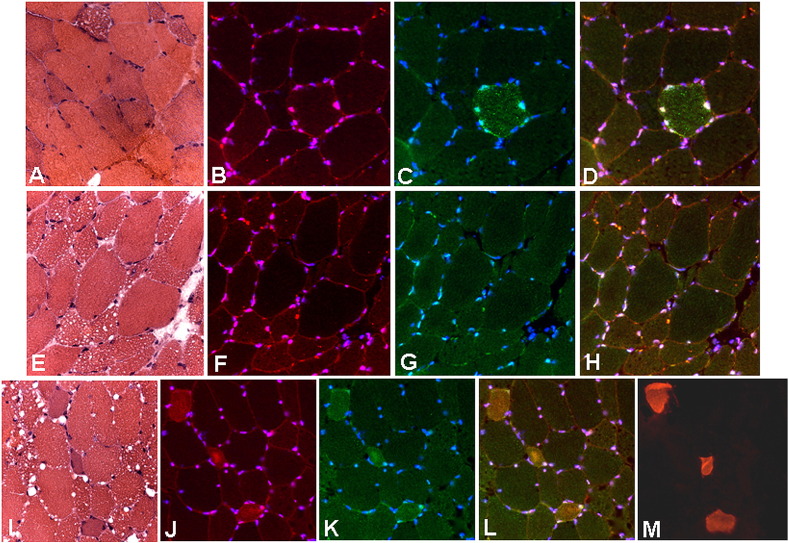
Immunofluorescence study revealed in several cases the presence of atrophic and lipid-accumulated fibers which also displayed increased nuclear TFEB localization and accumulation of p62-SQSTM1-positive inclusions. While in patients 2 (MADD), 4 and 5 (NLSD-M) there was an evident co-localized over-expression of p62-SQSTM1 and TFEB in some atrophic fibers (Fig. 3), in patients 3 (CD) and 6 (CPT-II deficiency) the reaction appeared normal (Supplemental Fig. 1, Supplemental Fig. 2, Supplemental Fig. 3). Interestingly, in regenerating fibers, TFEB localized in the cytoplasm, whereas in atrophic and lipid-accumulated fibers it localized in the nuclei (Fig. 3). Most of these latter fibers did not show p62-positive protein aggregates, indicating that the autophagic flux was still preserved.
Fig. 3.
Immunohistochemical analysis. Serial sections from muscle biopsy of Patient 2 (A–D), Patient 5 (E–H) and Patient 4 (L–M) stained with hematoxylin–eosin (A,E,L), and immunolabeled for TFEB (B,F,J, in red), p62-SQSTM1 (C,G,K, in green), TFEB/p62-SQSTM1 (D,H,L, merged), developmental myosin heavy chain (M). Nuclei are counterstained with DAPI (blue). Note that in some atrophic-vacuolated fibers displayed increased TFEB localization in the nuclei and cytoplasmic accumulation of p62-SQSTM1-positive inclusions (B–D), whereas there is an evident co-localized over-expression of p62-SQSTM1 and TFEB in the cytoplasm of atrophic fibers undergoing regeneration (J–M).
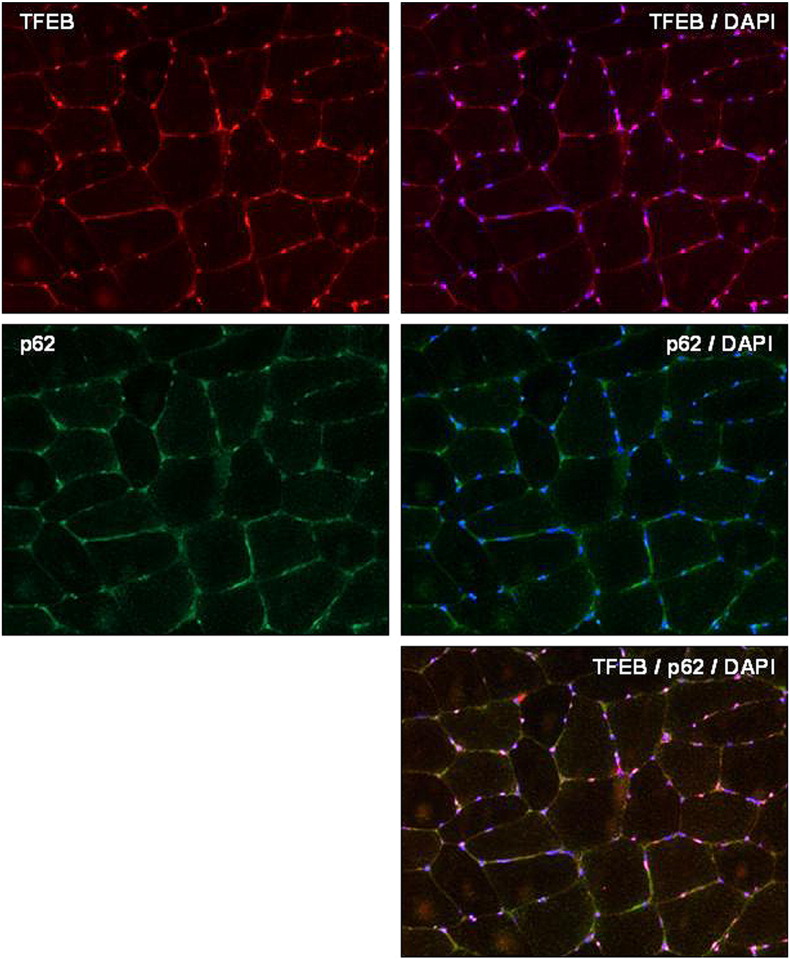
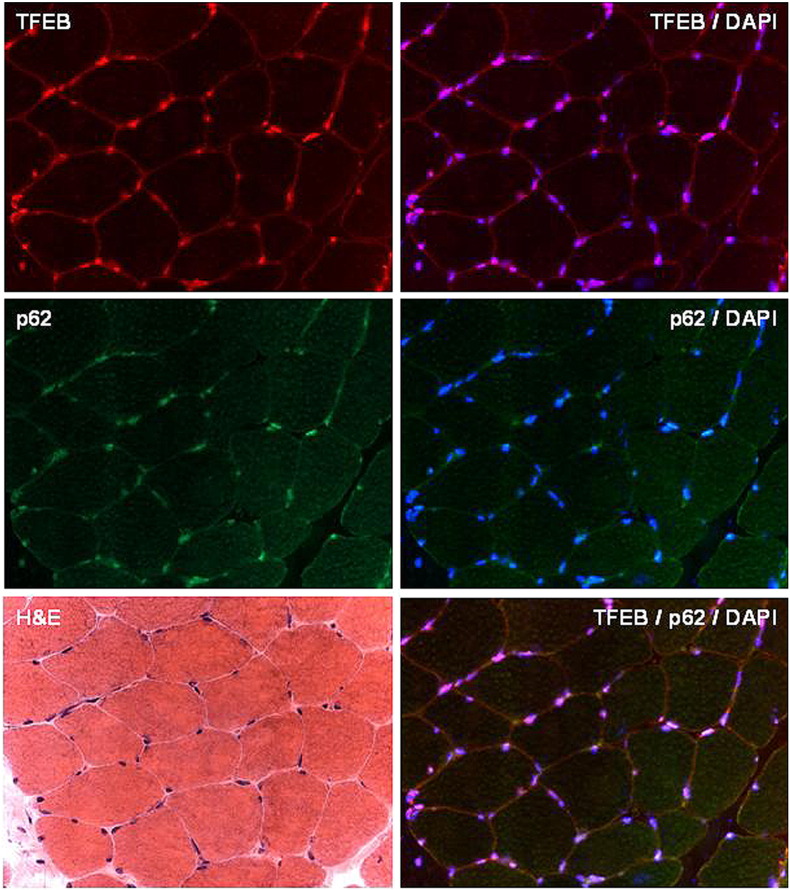
Supplemental Fig. 1.
Immunohistochemical analysis. Serial sections from muscle biopsy of a control patient immunolabeled for TFEB, TFEB/DAPI (merge), p62-SQSTM1, p62/DAPI (merge), TFEB/p62/DAPI (merge).
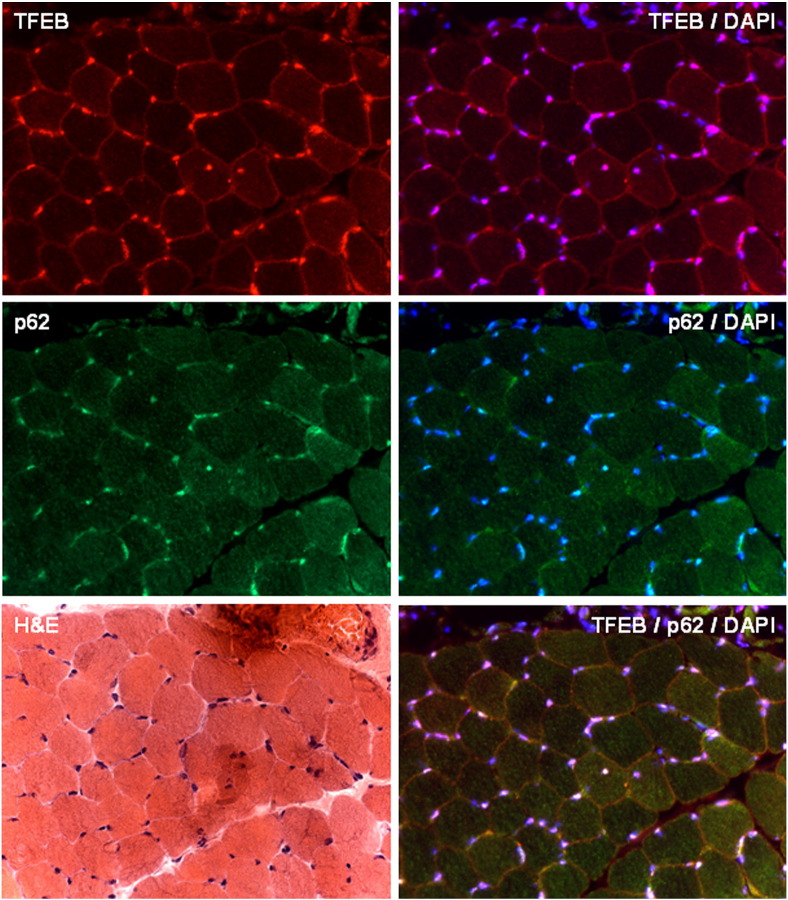
Supplemental Fig. 2.
Immunohistochemical analysis. Serial sections from muscle biopsy of Patient 6 stained with hematoxylin–eosin (H&E) and immunolabeled for TFEB, TFEB/DAPI (merge), p62-SQSTM1, p62/DAPI (merge), TFEB/p62/DAPI (merge). TFEB reaction was almost normal and p62 showed no protein aggregates.
Supplemental Fig. 3.
Immunohistochemical analysis. Serial sections from muscle biopsy of Patient 3 stained with hematoxylin–eosin (H&E) and immunolabeled for TFEB, TFEB/DAPI (merge), p62-SQSTM1, p62/DAPI (merge), TFEB/p62/DAPI (merge). TFEB reaction was almost normal and p62 showed no protein aggregates.
The autophagic activity, which was clearly active using LC3 and p62-SQSTM1 in at least 4 of our patients, is likely to be due to the fact that the availability of lipids can offer nutrients through two pathways: lipophagy and lipolysis. Evidence of autophagic activity was found by both immunohistochemistry and western blotting in NLSD-M and MADD but not in patients with CD or CPT-II deficiency (Table 2).
Table 2.
Differential molecular and biochemical pathways regulation in various LMS.
| Normal condition | MADD | NLSD-M | Carnitine deficiency | CPT deficiency |
|---|---|---|---|---|
| Lipolysis and lipophagy are active only during starvation | Lipolysis and lipophagy are increased | Due to ATGL deficiency, lipolysis is impaired, lipophagy is active | Normal lipolysis and lipophagy | Normal lipolysis and lipophagy |
| Normal carnitine | Low carnitine | Normal carnitine | Low carnitine | Normal carnitine |
| Low acyl-carnitines | Increased acyl-carnitines | Low acyl-carnitines | Low acyl-carnitines | Increased acyl-carnitines |
| No glutaric aciduria | Gutaric aciduria | No glutaric aciduria | No glutaric aciduria | No glutaric aciduria |
| Normal mitochondria | Decreased mitochondria (palmitate oxidation) | Decreased mitochondria | Normal mitochondria | Normal mitochondria |
| Free diet | Treatment with riboflavin and carnitine | Free diet | Carnitine | Bezafibrate (?) |
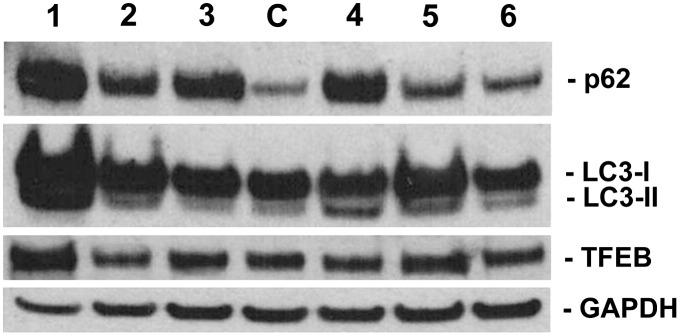
On LC3 and p62 immunoblotting (Fig. 4), patients 1,2,4,5 (MADD or NLSD-M) showed a markedly increased LC3-II band (marker of autophagosome proliferation), which was associated with an increased p62-SQSTM1 band in patients 1 and 4, suggesting a block in the autophagic flux. TFEB immunoblotting showed variable levels, with higher expression in patients 1 and 5 (Fig. 4).
Fig. 4.
Immunoblot analysis of p62-SQSTM1, LC3, TFEB, and the loading control protein GAPDH in one control muscle (C) and the 6 LSM patients (1–6). Note that patients 1,4,5,2 (MADD and NLSD-M) displayed increased LC3-II band (marker of autophagosome proliferation), and that some of them showed also an increased p62 band, suggesting a block in the autophagic flux.
4. Discussion
Here we report the first ultrastructural and morphological evidence of lipid droplet decrease in two patients with riboflavin-responsive MADD. We think that both lipophagy, TFEB and autophagosomes are linked in a critical regulatory role of lipid droplets in MADD. Until recently, autophagy regulation was thought to occur by accumulation of LC3-II in response to autophagic stimulus. We observed that in human biopsies, TFEB activation was present both in cytoplasm and in nucleus and that LC3-II increase was seen both associated to p62 accumulation. Therefore, the accumulation of lipid droplets in human muscle provokes adaptative lipophagic reaction.
The combustion of lipid droplets for energy begins with the action of lipases on the lipid droplets monolayer. Recently, however, lipophagy has been implicated in neutral lipid utilization from lipid droplets. The mechanism by which lipophagy is regulated requires TFEB activation.
Patients 1 and 5 had important lipophagy impairment (increased LC3-II), and they showed also the higher levels of TFEB in muscle. Therefore, lipophagy impairment and TFEB increase corresponds to p62-positive inclusions and in the most severe muscle impairment at clinical level and consequent muscle atrophy. Lipophagy impairment results in p62-aggregates in myofibers and may reflect the impairment of the last chance to mobilize fatty acids from lipid droplets to give energy to mitochondria.
The phenotypic consequences of TFEB over-expression in muscle fibers are striking and consist in reduced size, and presence of p62-positive protein aggregates. It is well known that the inhibition of autophagy in muscle was proposed to cause fiber atrophy [16]. Our data agree with such experimental data suggesting that autophagy might exert a positive role in lipid metabolism to decrease lipid accumulation.
In NLSD-M the accumulated lipid droplets are hydrolyzed by three different lipases: the lipid droplet-associated adipose triglyceride lipase (ATGL), which is primarily defective, and the cytosolic hormone-sensitive lipase (HSL) and the monoacyl-glycerol lipase, which are present but not adequate to perform lipolysis. In our NLSD-M cases we observed LC3-II over-expression, which was consistent with activation of autophagy, and was also associated in some cases with p62 over-expression (block of lipophagy). In muscle biopsy from two NLSD-M patients we observed TFEB over-expression, which might have a role in autophagy induction. In 4 LSM cases we documented lipophagy activation, which was probably consequent to lipid droplets increase.
To sustain cell metabolism, lipid droplets have to be degraded to release fatty acids, and this may be triggered by nutrient deprivation. Several molecules facilitate the cellular import of fatty acids including the plasma membrane OCTN2 translocase. Once in the cytosol, fatty acids are esterified to CoA and then conjugated to carnitine by CPT-I and imported by CPT-II.
The expression and activity of enzymes involved in lipid catabolism are tightly regulated by transcriptional and post-transcriptional mechanisms, the main of which involve the activities of PPARα and PGC1α induced during fasting.
Increasing evidence supports the role by the nutrient-sensitive TFEB and other members of the FOXO family in regulating lipophagy during fasting; PPARα and PGC1α might link lipophagy to other factors involved in lipid catabolism.
The way in which lipophagy is variably regulated in MADD versus NLSD-M is intriguing, and it is puzzling to understand how TFEB and p62 are activated in these two conditions, since the efficacy of lipolysis might differ. On the contrary, in CPT-II deficiency and CD we did not observe activation of either pathways, and we found neither autophagosomes nor p62-protein aggregates.
ATGL, which is the key enzyme in the first step of lipid degradation in muscle, provides a fatty acid moiety that can be used for regulating transcriptional response and mitochondrio-genesis. On the contrary, in CD and CPT-II deficiency there must be a way to circumvent the metabolic derangement without recurring to lipophagy. In fact, in CPT-II deficient muscle there is only slight atrophy, and occasional regenerating fibers only after rhabdomyolysis.
We might speculate that TFEB activation might be a new therapeutic target to avoid deterioration in LSM, but we know from animal models that TFEB up-regulation could be extremely toxic [17]. Our study in MADD patients confirmed the sensitivity of beta-oxidation pathway. Lipophagy occurs in-vivo during exercise or starvation, since autophagosomes sequester portions of lipid droplets and target them to lysosomes for degradation. It has been suggested that intracellular accumulation of lipid droplets may promote autophagy, because lipids provide precursors for the nascent autophagosome membrane. In ATGL deficiency, which function is to mobilize triglycerides to form di-acyl-glycerol, triglycerides could be used to build the phospholipids necessary for membrane formation. In NLSD-M ATGL is defective, and the lysosomal protein aggregates might cause atrophy.
Increasing evidence suggests that TFEB and p62 regulate lipophagy during several physiological conditions, including exercise and fasting [18]. PPARα and PGC1α link lipophagy to other pathways involved in lipid catabolism (i.e. lipolysis) [19]; therefore, a number of factors coordinate lipid degradation pathways in muscle. Autophagy pathways rely on the activity of various organelles: autophagosomes, endoplasmic reticulum and lysosomes. The process by which lipid catabolic pathways link to other lipid oxidative pathways are still under investigation; however, lipophagy and lipolysis must be co-regulated to optimize lipid use and prevent excessive accumulation of lipids, and avoid damage to myofibers. Four of our patients, two with ATGL deficiency, displayed increased levels of LC3-II and also p62-positive protein aggregation, possibly suggesting that autophagy might have been activated and subsequently inhibited [20], [12]. Therefore, the physio-pathological role of such signaling mechanism would contribute to the development of these pathological conditions (Table 2).
It is likely that the role of lipolytic process is directed to avoid the toxicity of fatty acid accumulation on mitochondrial function. Most of our patients were studied during a metabolic crisis provoked by periods of fasting; one MADD patient was initially in deep distress and then recovered, and the other MADD patient had multiple causes of metabolic stress (including malnutrition and alcoholism). The two NLSD-M patients had progressive weakness, diabetes, and abnormal hydrolysis of triglyceride storage that requires indeed the ATGL lipase.
Given that during lipolysis intracellular lipid droplets are degraded to fatty acids, one could wonder how fatty acids are continuously delivered to mitochondria. In humans, it is clear that fatty acid trafficking depends from lipid droplets lipolysis but lipophagy is also needed [21]. We consider that in the patients we studied, both of these two different processes are at play: lipophagy, which is due to autophagic digestion of lipid droplets is mainly required in catabolic states; lipolysis, where cytoplasmic lipases directly hydrolyze triacylglycerol from lipid droplets, subsequently providing energy. The ATGL mechanism is finely regulated, and the fatty acids move directly from lipid droplets into mitochondria. In our MADD patients, an increased autophagic activity was suggested by western blotting results, while in NLSD-M patients, fatty acids flux into mitochondria seemed to be impaired as well as endogenous lipid droplet lipolysis, although some evidence of lipophagy was also seen.
It is worth noting that these metabolic conditions can be reversible, since the elimination of lipid droplets can be obtained either by riboflavin treatment, or by using medium-chain triglycerides, that directly go to mitochondria as a fuel. In all our patients, risk factors, such as poor nutrition, precipitated their catabolic state. Avoiding such risk factors is an important part of clinical management.
5. Conclusions
This is a pilot study on three different disorders of lipid metabolism, which were studied on muscle with markers of lipophagy. In two conditions (MADD and NLSD-M) there was an activation of p62/SQSTM1 and LC3-II.
Further studies are required to understand whether ATGL-mediated fat catabolism might regulate mitochondrial complexes and energy production.
The following are the supplementary data related to this article.
Transparency document
Transparency document.
Acknowledgments
This work was supported by grants from the Association Française contre le Myopathies-AFM (#14199 to A.N.), the Comitato Telethon Fondazione Onlus #GGP14066) and Eurobiobank network (#QLRT2001-027769 to C.A).
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Colombo I., Finocchiaro G., Garavaglia B., Garbuglio N., Yamaguchi S., Frerman F.E., Berra B., DiDonato S. Mutations and polymorphisms of the gene encoding the beta-subunit of the electron transfer flavoprotein in three patients with glutaric acidemia type II. Hum. Mol. Genet. 1994;3:429–435. doi: 10.1093/hmg/3.3.429. [DOI] [PubMed] [Google Scholar]
- 2.Dusheiko G., Kew M.C., Joffe B.I., Lewin J.R., Mantagos S., Tanaka K. Recurrent hypoglycemia associated with glutaric aciduria type II in an adult. N. Eng. J. Med. 1979;301(1979):1405–1409. doi: 10.1056/NEJM197912273012601. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J., Lefevre C., Morava E., Mussini J.M., Laforêt P., Negre-Salvayre A., Lathrop M., Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 4.Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S.U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D.C., Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011;17:1076–1086. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Settembre C., Zoncu R., Medina D.L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M.C., Facchinetti V., Sabatini D.M., Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peña-Llopis S., Vega-Rubin-de-Celis S., Schwartz J.C., Wolff N.C., Tran T.A., Zou L., Xie X.J., Corey D.R., Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardiello M., Palmieri M., Di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S., Banfi S., Parenti G., Cattaneo E., Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 9.Van de Weijer T., Havekes B., Bilet L., Hoeks J., Sparks L., Bosma M. Effects of bezafibrate treatment in a patient and a carrier with mutations in the PNPLA2 gene, causing neutral lipid storage disease with myopathy. Circ. Res. 2013;112:e51–e54. doi: 10.1161/CIRCRESAHA.113.300944. [DOI] [PubMed] [Google Scholar]
- 10.Sun A., Merritt J.L. Orphan drugs in development for long-chain fatty acid oxidation disorders: challenges and progress. Orphan Drugs Res. Rev. 2015;5:33–41. [Google Scholar]
- 11.Orngreen M.C., Vissing J., Laforet P. No effect of bezafibrate in patients with CPTII and VLCAD deficiencies. J. Inherit. Metab. Dis. 2015;38:373–374. doi: 10.1007/s10545-014-9779-3. [DOI] [PubMed] [Google Scholar]
- 12.Nascimbeni A.C., Fanin M., Masiero E., Angelini C., Sandri M. Impaired autophagy contributes to muscle atrophy in glycogen storage disease type II (GSDII) patients. Autophagy. 2012;8:1697–1700. doi: 10.4161/auto.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelini C., Lucke S., Cantarutti F. Carnitine deficiency of skeletal muscle: report of a treated case. Neurology. 1975;26:633–637. doi: 10.1212/wnl.26.7.633. [DOI] [PubMed] [Google Scholar]
- 14.Campagna F., Nanni L., Quagliarini F., Pennisi E., Michailidis C., Pierelli F., Bruno C., Casali C., DiMauro S., Arca M. Novel mutations in the adipose triglyceride lipase gene causing neutral lipid storage disease with myopathy. Biochem. Biophys. Res. Commun. 2008;377:843–846. doi: 10.1016/j.bbrc.2008.10.081. [DOI] [PubMed] [Google Scholar]
- 15.Tavian D., Missaglia S., DiMauro S., Bruno C., Pegoraro E., Cenacchi G., Coviello D., Angelini C. A late-onset case of neutral lipid storage disease with myopathy, dropped head syndrome, and peripheral nerve involvement. J. Genet. Syndr. Gene Ther. 2013;4:1–4. 1000198. [Google Scholar]
- 16.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settembre C., Ballabio A. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 2014;24:743–750. doi: 10.1016/j.tcb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Settembre C., De Cegli R., Mansueto G., Saha P.K., Vetrini F., Visvikis O. TFEB controls cellular lipid metabolism through a strarvation-induced autoregulatory loop. Nat. Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh A., Jana M., Modi K., Gonzalez F.J., Sims K.B., Berry-Kravis E., Pahan K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells. J. Biol. Chem. 2015;290:10309–10324. doi: 10.1074/jbc.M114.610659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambold A.S., Cohen S., Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy and mitochondrial fusion dynamics. Dev. Cell. 2015;32:678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.