Abstract
Angiogenesis is the formation of new capillaries from pre-existing vasculature, which plays a critical role in the pathogenesis of several inflammatory autoimmune diseases such as rheumatoid arthritis (RA), spondyloarthropathies, psoriasis, systemic lupus erythematosus, systemic sclerosis and atherosclerosis. In RA, excessive migration of circulating leukocytes into the inflamed joint necessitates formation of new blood vessels to provide nutrients and oxygen to the hypertrophic joint. The dominance of the pro-angiogenic factors over the endogenous angiostatic mediators triggers angiogenesis. In this review article, we highlight the underlying mechanisms by which cells present in the RA synovial tissue are modulated to secrete pro-angiogenic factors. We focus on the significance of pro-angiogenic factors such as growth factors, hypoxia inducible factors, cytokines, chemokines, matrix metalloproteinase and adhesion molecules on RA pathogenesis. As pro-angiogenic factors are primarily produced from RA synovial tissue macrophages and fibroblasts, we emphasize the key role of RA synovial tissue lining layer in maintaining synovitis through neovascularization. Lastly, we summarize the specific approaches utilized to target angiogenesis. We conclude that the formation of new blood vessels plays an indispensable role in RA progression. However since the function of several pro-angiogenic mediators is cross regulated, discovering novel approaches to target multiple cascades or selecting an upstream cascade that impairs the activity of a number of pro-angiogenic factors may provide a promising strategy for RA therapy.
Keywords: RA, angiogenesis, growth factors, cytokines, chemokines, matrix metalloproteinase and adhesion molecules
INTRODUCTION
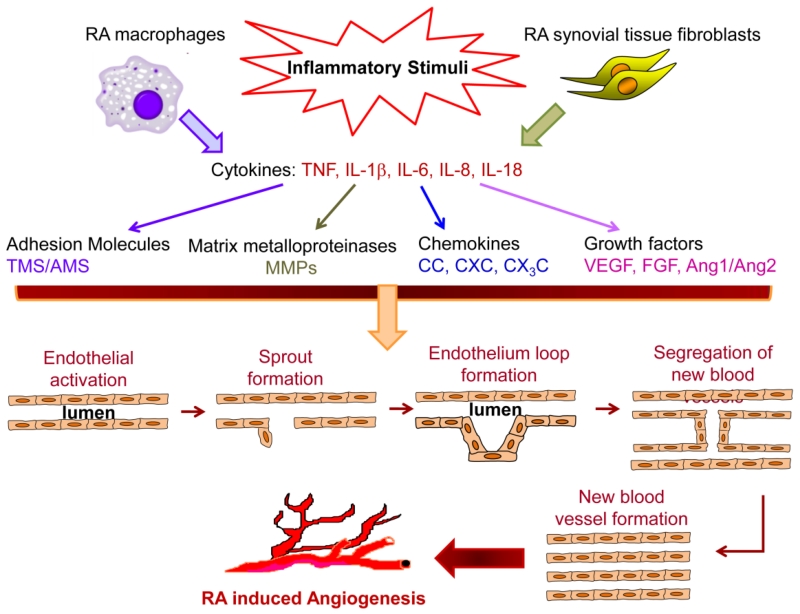
Formation of new capillaries from the pre-existing vessels is defined as angiogenesis [1-4]. Rheumatoid arthritis (RA) is a chronic systemic disorder in which angiogenesis can foster the infiltration of inflammatory cells into the joints leading to synovial hyperplasia and progressive bone destruction [1,5,6,4]. Angiogenesis is involved in several physiological events including embryonic organ development, reproduction, tissue repair and wound healing. However, uncontrolled neovascularization can contribute to angiogenic disorders including RA, psoriasis, atherosclerosis and tumor formation [5,7-12]. Angiogenesis involves several steps and each step is modulated by specific factors. The process starts with growth factors such as VEGF and FGF binding to their cognate receptors on endothelial cells and activation of these cells to produce proteolytic enzymes. Subsequently, the basement membrane is degraded by matrix metalloproteinases (MMP)s which results in migration and further endothelial proliferation to vascular tubules that are in part developed by adhesion molecules such as integrins. Lastly, blood vessels are stabilized by pro-angiogenic factors such as Ang1, followed by incorporation of pericytes into the newly formed basement membrane to facilitate the blood flow process [5,13,9,3,14] (Fig. 1).
Fig. 1. RA angiogenesis is driven by pro-inflammatory cytokines released from the cells in the synovial tissue-lining layer.
In response to inflammatory stimuli, RA synovial tissue macrophages and fibroblasts produce pro-inflammatory cytokines that can modulate expression of adhesion molecules, MMPs, chemokines and growth factors which are all important in different stages of angiogenesis. There are several steps involved in angiogenesis; which consist of endothelial cells migration, endothelial cell proliferation into vascular tubules, separation of the newly formed blood vessels that mature and become interconnected to the circulatory system.
In RA, the excessive pro-angiogenic factors counteract the angiogenic inhibitors to support the elevated transendothelial leukocyte infiltration that fosters synovial inflammation as well as the bone and cartilage destruction. Conversely, inhibition of joint neovascularization can alleviate synovitis and pannus formation [15,14,16].
Previous studies document that synovial macrophages and fibroblasts exert a predominant role in RA angiogenesis. Consistent with this notion, the number of synovial tissue macrophages is the most reliable marker for assessing disease severity and response to therapy as the number of myeloid cells correlates with RA synovial inflammation, joint pain and bone destruction [17-20]. The cell-to-cell contact between RA fibroblasts and macrophages in the lining layer amplifies the inflammatory signaling cascades since the mere contact of these cells provokes IL-6 and IL-8 production [21]. In this review, we categorize pro-angiogenic factors based on their mechanism of function and cell origination. Since the vast majority of these angiogenic factors are secreted from RA synovial tissue macrophages and fibroblasts, we have therefore highlighted the importance of these cell types in RA angiogenesis. In addition, we discuss factors that are highly pathogenic due to their multifunctional effect on leukocyte migration and neovascularization. Finally, we conclude that the anti-angiogenic agents may offer a promising therapy for RA and different types of cancer.
Pro-angiogenic factors released from RA synovial tissue myeloid cells and fibroblasts
RA Macrophages
Tissue-resident macrophages are long-living phagocytic cells that persist in many tissues and are characterized by high surface expression of CD64, MerTK, and CD14. Tissue-resident macrophages originate primarily from embryonic progenitors and to less extent from circulating monocyte intermediates, additionally many of them are capable of self-renewal [22-25]. In contrast, other tissues have mobile, short-living populations of mononuclear phagocytes that patrol different tissues and are previously classified as monocyte derived macrophages. These cells are characterized by the high surface expression of Ly6C, CCR2, CD11b, and low/absence of tissue-resident macrophages markers CD64, MerTK, and CD14 [25-27,24,28,29]. The recruited population of monocyte derived macrophages significantly increase during inflammatory conditions such as RA.
Circulating monocytes infiltrate from blood into the inflamed RA joint where they differentiate into macrophages. Macrophages are classified into two groups namely M1 and M2 macrophages. M1 macrophages are the classically activated cells that produce pro-inflammatory cytokines mediating resistance to pathogens and tissue destruction, whereas M2 macrophages are the alternatively activated cells that produce anti-inflammatory cytokines which promote tissue repair [30,31]. M1 macrophages express cell surface markers such as CCR7, CD215, CD80 and CD86 and secrete TNF, IL-6 and IL-1β CCL2, IL-8, IL-12 and IL-23 upon activation with interferon (IFN)γ, lipopolysaccharides (LPS) or granulocyte macrophage colony stimulating factor (GM-CSF) [32-34]. However M2 macrophages are distinguished by CD206, CD209 and Dectin1 surface markers and are capable of producing TGF-β and IL-10 upon IL-4, IL-13, glucocorticoid, IL-10 and macrophage colony stimulating factor (M-CSF) stimulation [32].
RA synovial tissue fibroblasts
RA synovial tissue fibroblasts reside in the most superficial part of the lining layer where they are in direct contact with macrophages [35,36]. RA synovial tissue fibroblasts that become exposed to TLR endogenous ligands and pro-inflammatory cytokines produced from their neighboring macrophages, transform into “tumor like” cells [37]. During active disease, these transformed RA synovial tissue fibroblasts are triggered by inflammatory mediators or hypoxia to produce pro-angiogenic growth factors, cytokines, chemokines, MMPs and adhesion molecules [38,39,35,36] (Fig. 1). As discussed below a tightly regulated network of pro-angiogenic factors come together to pathologically alter the endothelial cell function and foster joint angiogenesis in order to exacerbate RA severity.
Growth Factors
VEGF and HIF connection
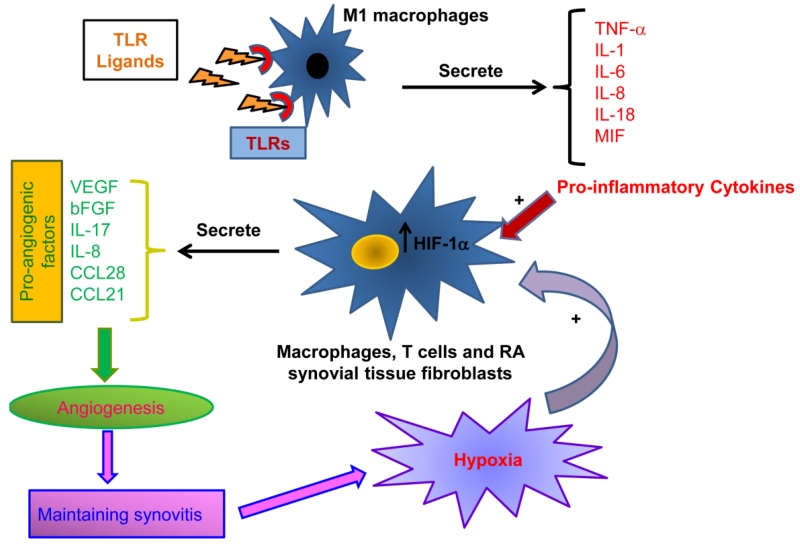
TNF, IL-1, IL-6 and IL-18 secreted from TLR driven M1 macrophages combined with the local hypoxic conditions activate RA macrophages and synovial tissue fibroblasts to secrete growth factors such as vascular endothelial growth factor (VEGF) and/or basic fibroblast growth factor (bFGF) [40-43,34,44] (Fig. 2). VEGF and bFGF are the key regulators of angiogenesis since they are associated with proliferation, migration and vascular tube formation as well as the prevention of endothelial cell apoptosis [44]. Hypoxia, induced by the metabolic demand of the increasing number of leukocytes recruited into the RA joint, leads to the accumulation of hypoxia inducible factor-1α (HIF-1α) in the cytoplasm which translocates to the nucleus where it associates with HIF-β and other co-activators and finally induces the expression and secretion of VEGF by macrophages and RA synovial tissue fibroblasts (Fig. 2) [34,44]. Interestingly, during hypoxia, angiogenesis can be triggered by the positive feedback regulation detected between HIF-1α and VEGF pathways [45].
Fig. 2. Ligation of TLRs fosters angiogenesis indirectly through induction of pro-inflammatory cytokines and pro-angiogenic factors.
Potentially, TLR endogenous ligands can bind to the RA joint macrophages and activate the production of pro-inflammatory factors such as TNF, IL-1β, IL-6, IL-8, IL-18 and MIF. Excessive leukocyte migration in the RA joint increases the oxygen demand resulting in hypoxia and the subsequent accumulation of the hypoxia inducible factor-1α (HIF-1α). The pro-inflammatory cytokines released into the joint space together with the increased intracellular levels of the HIF-1α can then activate the production of pro-angiogenic factors from the RA synovial tissue macrophages, T cells and fibroblasts. The pro-angiogenic factors increase the joint neovascularization process (Angiogenesis) in order to maintain synovitis.
In experimental arthritis models, preventative treatment of anti-VEGF antibody delayed the collagen induced arthritis (CIA) onset, joint swelling and vascularization. In contrast, post onset treatment with anti-VEGF antibodies did not affect severity or progression of the arthritis, suggesting that angiogenesis mediated by VEGF plays a crucial role in the early stage of arthritis development [46]. In vitro studies have shown that HIF-1α deletion impaired angiogenesis through a VEGF dependent mechanism. However, conditional knockout of HIF-1α in myeloid cells attenuated experimental arthritis in mice by decreasing myeloid cell homing and activation, independent of VEGF, suggesting that HIF-1α can modulate inflammation and arthritis, independent of its angiogenic effect [47].
Ang1, Ang2 and Tie2
We and others have shown that angiopoietin (Ang)1 and its receptor tyrosine kinase receptor (Tie)2 are highly elevated in RA compared to normal synovial tissue lining, sublining macrophages and endothelial cells [48-50]. Previous studies have shown that Ang1 expression is differentially regulated in RA synovial tissue fibroblasts compared to endothelial cells, as TNF could strongly increase the concentration of Ang1 in RA fibroblasts while the levels of endothelial Ang1 was unaffected by the stimulation [50]. It was found that Ang1 acts at a later stage of angiogenesis compared to VEGF, in order to form and increase blood vessel stability [51]. Furthermore, the endogenous antagonist to the Tie2, Ang2 can destabilize VEGF mediated angiogenesis [52] suggesting that there is a crosstalk between Ang and VEGF cascades. The depletion of Tie2, by adenoviral expression of a soluble Tie2 receptor, ameliorated CIA joint vascularization, swelling and bone destruction indicating that inhibition of neovascularization can prevent ankle edema and osteoclastic bone erosion [53].
Other growth factors
IL-17 and TNF synergize in producing growth factors such as keratinocyte growth factor (KGF), hepatocyte growth factor (HGF) and heparin-binding endothelial growth factor (HB-EGF) from RA synovial tissue fibroblasts [54]. Two of the other growth factors expressed in RA joints are platelet-derived growth factor (PDGF) and transforming growth factor (TGF)β that can together with TNF increase the hypertrophic architecture of the RA synovial tissue lining layer. These growth factors elevate the RA synovial fibroblast expression of matrix metalloproteinase-3 (MMP3), Cadherin-11, and PI3Kδ, demonstrating that multiple factors contribute to the synovial tissue lining hyperplasia in RA [55]. Potent pro-angiogenic factors such as PDGF and VEGF promote angiogenesis through activation of receptor tyrosine kinases (RTKs) [44,55]. Imatinib mesylate (Imatinib, an inhibitor for tyrosine kinases such as PDGFR) was primarily developed to treat chronic myelogenous leukemia (CML) and other cancers [56]. Additionally, Imatinib was capable of attenuating the clinical and histological signs of collagen antibody induced arthritis (CAIA) as well as reducing the joint VEGF levels [56], suggesting that ligation of PDGF to PDGFR plays an essential role both in early and late stages of RA.
Pro-inflammatory Cytokines
Pro-inflammatory cytokines produced from M1 macrophages include TNF, IL-1, IL-6, IL-8, IL-18 and macrophage migration inhibitory factor (MIF) play a central role in RA angiogenesis both through their direct effect on endothelial cells as well as their indirect effect on different cell types in RA synovium to produce pro-angiogenic factors (Fig. 2).
TNF
Levels of TNF and its receptor, sTNFRII, are markedly elevated prior to RA onset [57], and TNF directly affects endothelial migration and proliferation as well as the formation of new blood vessels [58]. Moreover, angiogenesis can be indirectly promoted by TNF through its synergic effect with IL-1β and IL-17 in producing VEGF from RA synovial tissue fibroblasts [54]. Previous studies document that in RA patients who respond to TNF blockers, serum VEGF levels are markedly reduced by treatment, indicating that TNF may play a role in VEGF mediated neovascularization [59]. TNF is also involved in regulation of Ang1/Tie2 network [60]. As such, anti-TNF therapy has shown to reduce RA joint vascularization by suppressing the expression levels of Ang1/Tie2 and survivin through Ang2 stimulation [60]. TNF together with IL-1, IL-6 and IL-23 promote TH-17 cell differentiation [61] and IL-17 has been shown to be important for RA angiogenesis [62,63]. Consistently, RA patients who responded to anti-TNF therapy, had reduction in the number of circulating TH-17 cells as well as lower serum concentrations of IL-17, IL-6, IL-21 and IL-23 [64]. In contrast, TNF non-responders had a markedly elevated number of circulating TH-17 cells and serum IL-17 levels suggesting that TNF is important for TH-17 cells differentiation in RA [64]. Others have shown that TNF can impact RA angiogenesis by modulating endothelial cell secretion of pro-angiogenic adhesion molecules (E-selectin, ICAM1 and VCAM1) and chemokines (CXCL1, CXCL5, CXCL8, CCL2 and CCL5) [65,63].
IL-1β
In contrast to TNF, IL-1β has no direct effect on RA angiogenesis. However, IL-1β can induce expression of Ang1, Tie2 and VEGF from RA synovial tissue fibroblasts [66] in addition to its synergistic effect with IL-17 on the production of VEGF from RA fibroblasts [54]. IL-1β can also upregulate RA fibroblast expression of CCL21 which can bind to its corresponding receptor, CCR7 on endothelial cells and facilitate cell migration, capillary tube formation and in vivo blood vessel formation [67]. Furthermore, endothelial CXCR6 is elevated by IL-1β and ligation of CXCR6 to CXCL16 fosters RA angiogenesis [68]. These finding suggest that IL-1β can indirectly contribute to angiogenesis by enhancing the expression levels of growth factors, chemokines or chemokine receptors from the cells present in the RA synovium. Studies have shown that IL-1β driven arthritis was in part due to IL-17 function; since the spontaneous development of arthritis detected in IL-1R antagonist knockout mice was impaired in IL-17 deficient mice [69]. This may also indicate that IL-1β mediated RA angiogenesis can in part be due to IL-17 produced from TH-17 cells.
IL-6
IL-6 is mainly secreted from pro-inflammatory M1 macrophages and RA synovial tissue fibroblasts [70,71] and the cell-to-cell contact of myeloid cells with RA fibroblasts facilitates this process [72]. TNF and IL-1 can synergize with IL-6 in stimulating VEGF production from RA fibroblasts [73]. As such, serum VEGF levels are elevated in RA patients and anti-IL-6R antibody therapy can normalize the VEGF concentration in the sera [73]. Furthermore, treatment of RA synovial tissue fibroblasts with anti-IL-6R antibody impairs the synergistic effect of IL-6, IL-1β, and TNF on VEGF production, while the blockade of IL-1β or TNF has no effect on this function [73].
In RA fibroblasts and endothelial cells co-culture system, IL-6 was capable of inducing VEGF and Ang2 protein levels [74]. In endothelial cells, IL-6 potentiates TNF mediated angiogenesis through induction of NF-κB and IL-8 [75]. Furthermore, the pro-angiogenic chemokine, CCL28 and its corresponding receptor CCR10, are modulated by IL-6 in RA macrophages [76]. Together with IL-1β, IL-6 plays an indispensable role for TH-17 cell differentiation, which is important in RA angiogenesis [62,63]. The use of Tocilizumab (TCZ), an anti-IL-6 antibody, both as monotherapy and in combination with Methotrexate or other disease modifying anti-rheumatic drugs (DMARDs), can effectively reduce joint inflammation and radiological disease progression [77], suggesting that IL-6 contributes to angiogenesis and RA pathogenesis.
IL-8/CXCL8
IL-8 is predominantly secreted from RA macrophages and fibroblasts [78]. Macrophages produce IL-8 in response to CCL19 and CCL21 stimulation [67] and ligation of TLR2 and TLR4 [78], whereas, IL-17, IL-1β, and TNF induce the production of IL-8 from RA synovial tissue fibroblasts [79,80]. Results from earlier studies show that IL-8, like TNF, can directly trigger RA angiogenesis by binding to its corresponding receptors, CXCR1 and CXCR2 on endothelial cells and neutralization of IL-8 reduced the angiogenic activity of synovial tissue macrophage conditioned media [81].
IL-18
IL-18 is a member of IL-1 superfamily [82] that is produced from RA synovial lining macrophages and fibroblasts as well as from endothelial cells and synovial fluid neutrophils [83-85]. RA synovial tissue fibroblasts release biologically active IL-18 in response to TNF stimulation [86]. Interestingly, a number of factors are secreted from RA fibroblasts in response to IL-18 stimulation which consist of adhesion molecules (VCAM1, ICAM1), neutrophil chemoattractants (CXCL1, CXCL5, CXCL12), monocyte chemoattractants (CCL2, CXCL20) and proangiogenic factors (VEGF, IL-8), suggesting that IL-18 can contribute to RA pathogenesis through different mechanisms [87]. Early in disease, IL-18 promotes neutrophil migration [88] and during active disease, myeloid cells are recruited into the joints [89] and inflammation progresses by triggering angiogenesis [90,91]. In addition to IL-18 indirect effects on monocyte and endothelial cell migration [92-94], data obtained from our group of investigators, reveal that IL-18 present in RA synovial fluid can directly attract myeloid and endothelial cells into the inflamed joint [89,91].
Macrophage migration inhibitory factor (MIF)
MIF is highly expressed on RA synovial tissue macrophages, fibroblasts and endothelial cells in addition to RA sera and synovial fluid [95,96]. Human macrophages produce MIF following TLR4 ligation whereas in mouse macrophages, MIF is modulated by TNF stimulation [97]. Macrophages stimulated with MIF secrete M1 associated cytokines such as TNF, IL-1β, IL-8 and IL-6 [98,99]. RA synovial tissue fibroblasts activated with MIF produce IL-1β as well as MMP-3, 9, and 13 which play an important role in cartilage degradation [100,101]. MIF can directly promote in vitro endothelial cell migration and tube formation as well as developing blood vessel in vivo, in the matrigel plugs corneal bioassay, with an effect as potent as bFGF [102]. Consistent with this notion, immuno-neutralization of MIF abrogates tumor induced endothelial proliferation and tumor angiogenesis [103,104]. In preclinical arthritis models, blockade of MIF function relieves arthritis and reduces joint recruitment of T and myeloid cells [105-107].
Chemokines
Chemokines are chemotactic cytokines that have been classified into the CXC, CC, C and CX3C families [108].
CXC Chemokines
The pro-angiogenic function of CXC chemokines involves three amino acid residues (Glu-Leu-Arg), the ELR amino acid motif, at the N terminus of the first cysteine residue of these chemokines [2]. The ELR-containing CXCL1, CXCL5, CXCL8 and CXCL16 are mainly produced by RA synovial tissue macrophages or fibroblasts in response to pro-inflammatory factors such as TNF, IL-1, IL-6 and IL-17 [109,41]. However, CXC chemokines that lack the ELR motif such as CXCL4, CXCL9, and CXCL10 inhibit RA neovascularization with the exception of CXCL12 which promotes angiogenesis in RA and cancer [110-115,2]. CXCR4, the CXCL12 receptor, is widely expressed on hemopoietic stem cells, monocytes and lymphocytes [115]. In RA synovial tissue, CXCR4 is highly expressed on synovial tissue lining and endothelial cells [116]. Although several CC and CXC chemokine receptors are expressed on endothelial cells, CXCR4 is the most abundant chemokine receptor on the endothelium and hence CXCR4 deficiency results in impaired neovascularization [117]. Elevated levels of CXCL12 are detected in RA synovial fluid [118]. Interestingly, CXCL12 expression in endothelial cells is induced by lymphotoxin alpha-1 beta-2 and LIGHT; however CXCR4 levels are modulated by pro-angiogenic factors such as VEGF and bFGF [119,120]. Similarly, ligation of CXCL12 to endothelial CXCR4 can also contribute to production of VEGF and bFGF, suggesting that activation of CXCL12/CXCR4 cascade can indirectly induce angiogenesis as well [121]. Others reported that the RA synovial fluid mediated blood vessel formation was inhibited by CXCL12 neutralization in vivo [116]. Consistently, earlier studies show that ligation of CXCL12 to CXCR4 is involved in angiogenesis observed in human glioblastoma [122] and pancreas cancer [123]. Blockade of CXCR4 by a non-peptide antagonist, ameliorated CIA by impairing the migration of CXCR4+MAC1+ myeloid cells, however; the effect of the treatment on joint neovascularization was not investigated [124].
In addition to being pro-angiogenic, CXCL1, CXCL5 and CXCL8/IL-8 can strongly attract neutrophils into the RA joint [7,41]. In RA synovial tissue fibroblasts and macrophages as well as experimental arthritis models, expression of CXCL1 and CXCL5 was shown to be associated with IL-17 mediated pathology [63]. Interestingly, while inhibition of CXCL1 function had no effect on IL-17 induced angiogenesis, blockade of CXCL5 could resolve IL-17 driven arthritis in part by reducing neovascularization through an IL-17 independent mechanism [63]. Moreover, it has been shown that CXCL16 can exert its pathogenic effect by attracting circulating monocytes and endothelial cells into the RA synovial tissue implanted into SCID mouse chimera model [125,68]. Corroborating with these findings, joint myeloid cell homing and neovascularization were impaired in CXCR6 (CXCL16 receptor) deficient mice provoked with K/BxN induced arthritis compared to wild type controls [68]. Taken together these results suggest that chemokines utilize multiple mechanisms to foster RA pathology.
CC chemokines
Similar to CXC chemokines, CC chemokines are predominately secreted from RA synovial tissue macrophages or fibroblasts that are stimulated by pro-inflammatory factors including TNF, IL-1β, IL-6, IL-8 and IL-17 [109,41]. The CC chemokines are chemotactic for monocytes and lymphocytes. CCL2, CCL3 and CCL5 are highly elevated in RA synovial tissue and can strongly attract monocytes and the blockade of their function alleviates experimental arthritis [126,127,2,128-130]. We have recently identified two CC chemokines in RA synovial tissues, namely CCL21 (binds to CCR7) (59, 112) and CCL28 (binds to CCR10) [76], that are integral for RA angiogenesis.
Notably the expression of CCR7 ligands, CCL19 and CCL21, is markedly increased in RA compared to normal synovial tissue endothelial cells as well as lining fibroblasts and macrophages [67]. Both CCL19 and CCL21 can bind to CCR7, however, only CCL21 can induce endothelial cell migration and capillary tube formation [67,131]. In RA peripheral blood differentiated macrophages and synovial tissue fibroblasts, CCL19 expression levels were upregulated by similar pro-inflammatory factors, whereas CCL21 concentrations were differentially modulated in these cell types [67]. Conversely, endothelial expression of CCR7 and CCL21 was similarly modulated by IL-17 and RA synovial fluid [67,131]. Despite, CCL19’s lack of direct effect on RA angiogenesis, like CCL21, it is capable of promoting angiogenesis indirectly through VEGF, IL-8 and Ang-1 secretion from RA synovial tissue fibroblasts or macrophages [67].
CCL28 and CCR10 expression levels are accentuated in synovial tissue/fluid of RA patients compared to normal controls and this chemokine and its receptor were predominately co-expressed in RA myeloid and endothelial cells [76]. We found that protein expression of CCL28 and CCR10 is modulated by TNF and TLR4 ligation in RA peripheral blood monocytes and endothelial cells, and by IL-6 stimulation in RA peripheral blood in vitro differentiated macrophages [76]. Antibody neutralization of CCL28 in RA synovial fluid or the use of anti-CCR10 antibody in human endothelial progenitor cells (EPC)s significantly reduced synovial fluid induced endothelial cell migration and capillary tube formation, demonstrating that ligation of CCL28 to CCR10+ endothelial cells participates in RA angiogenesis [76]. We conclude that while CCL2, CCL3, and CCL5 are important for joint monocyte infiltration, CCL21 and CCL28 can highly impact RA angiogenesis.
CX3C Chemokines
Fractalkine (CX3CL1) is expressed in RA peripheral blood monocytes and RA synovial tissue macrophages, fibroblasts and endothelial cells [132]. TNF, IL-1β, IFN-γ and LPS stimulation can enhance fractalkine expression in endothelial cells [133,134]. Levels of fractalkine are highly elevated in RA compared to osteoarthritis (OA) synovial fluid, and neutralization of fractalkine results in compromised monocyte and endothelial cell migration [132,135].
Matrix metalloproteinase and adhesion molecules
MMPs
MMPs are a family of zinc containing, calcium-dependent proteinases, that break down the basement membrane and the extracellular matrix components [136]. The collagenases (MMP-1, 8, 13), the gelatinases A and B (MMP-2 and 9), the stromelysins (MMP-3, 10, 11), the matrilysins (MMP-7, 26), and the MT-MMPs (membrane-type MMPs) are expressed at low levels in normal joint tissue; however their expression is highly elevated in arthritic joints [137-139]. MMP-2 is constitutively expressed in RA joints, however expression levels of MMP-1, MMP-3, MMP-9, MMP-8, and MMP-13 are accentuated by IL-1β, TNF or hypoxia [140-142]. MMP-2 and MMP-9 are expressed from RA synovial tissue myeloid cells, fibroblasts and endothelial cells, and together with MMP-1 and MMP-13 participate in RA angiogenesis [143]. Synovial fluid levels of MMP-9 and MMP-13, but not MMP-1 or MMP-2, correlated with those of VEGF suggesting that certain class of MMPs may play an important role in RA angiogenesis [144]. In animal models of RA, MMP-2, MMP-9 and MMP-13 deficient mice demonstrated compromised joint vascularization or bone growth; however mice deficient in MMP-3 and MMP-7 had no altered phenotype, further highlighting the importance of specific MMPs in RA pathogenesis [145].
Adhesion molecules
JAMs
Junctional adhesion molecule (JAM)-C is highly expressed in RA synovial fibroblasts [146]. JAM-C is also cleaved from the surface of endothelial cells by ADAM10 and ADAM17 or can be released from endothelial cells in response to IL-1β, IL-17, LPS, MIF, TNF, or PMA stimulation [147]. JAM-C is shown to promote adhesion of myeloid cells to the endothelium as well as facilitating myeloid cell retention and angiogenesis in RA [146,147]. In acute models of preclinical arthritis, treatment with anti-JAM-C antibody ameliorated antigen induced arthritis (AIA) by reducing synovial neutrophil migration and delayed the onset of K/BxN serum induced arthritis [148]. However in the AIA model, blockade of JAM-C had no significant impact on myeloid or endothelial cell migration [148]. Conversely, in JAM-C deficient mice, tumor micro-vessel formation was impaired compared to wild type mice [149], suggesting that chronic arthritis models may be more appropriate for assessing the impact of inflammatory factors on joint neovascularization.
CAMs
Soluble intracellular adhesion molecule (sICAM)1, sICAM3 and soluble vascular cell adhesion molecule (sVCAM)1 are primarily expressed by RA synovial tissue macrophages and/or fibroblasts and their expression is modulated by TNF stimulation [150,151]. Elevated levels of sICAM1, sICAM3 and sVCAM1 were also detected in RA synovial fluids [152-154]. In streptococcal cell wall induced arthritis and CIA models, treatment with anti-ICAM1 antibody reduced ankle edema and neutrophil infiltration without affecting joint vascularization [155,156]. In contrast, RA joint angiogenesis was markedly reduced by neutralization of sVCAM1 in RA synovial fluid [154]. Despite the lack of direct effect of ICAM1 on RA angiogenesis, earlier studies document that serum concentrations of sICAM1 and sVCAM1 closely correlate with serum levels of VEGF, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and the number of swollen joints [157], suggesting an indirect role for ICAM1 on neovascularization.
T cells
TH-17 cells
The frequency of TH-17 cells is significantly higher in RA synovial fluid compared to RA and normal peripheral blood [158]. Our group recently uncovered that differentiation of TH-17 cells is fostered by ligation of TLR5 in RA peripheral blood mononuclear cells. Further, we demonstrate that TLR5 mediated angiogenesis is in part due to TH-17 cell development and IL-17 production [159].
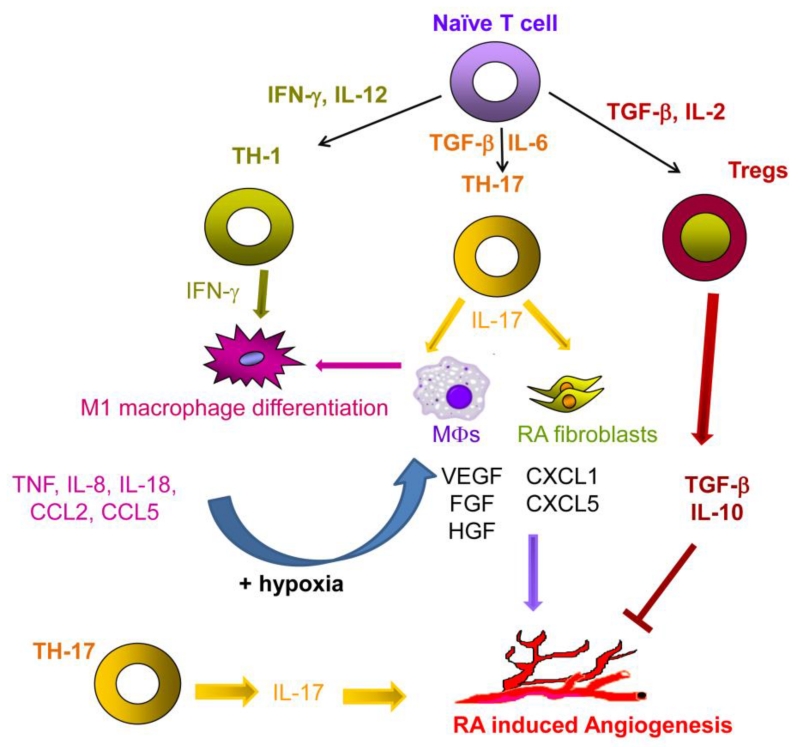
We documented that the in vitro RA synovial fluid mediated endothelial migration was reduced by neutralization of IL-17 or by blockade of IL-17RC on endothelial cells [62]. Moreover, IL-17 contributes to matrigel plugs blood vessel formation and joint vascularization in preclinical arthritis models [62,63]. IL-17 driven joint angiogenesis can be impaired in CIA by IL-27 administration at early onset [160]. However, others determined that IL-17 can indirectly induce angiogenesis, by promoting production of pro-angiogenic factors such as VEGF, bFGF and HGF from RA synovial tissue fibroblasts [54]. We demonstrate that in addition to the direct effect of IL-17/IL-17R on angiogenesis, joint IL-17 mediated CXCL5, but not CXCL1, plays a key role in IL-17 induced arthritis and vascularization [63] (Fig. 3).
Fig. 3. Differentiation of TH-1 and TH-17 cells can foster RA angiogenesis.
In RA joint, IFN-γ and IL-12 drive the polarization of naïve T cells into TH-1 cells that promote angiogenesis indirectly through inducing the production of TNF, IL-8, IL-18, CCL2 and CCL5 by M1 macrophages. Whereas, TGF-β and IL-6 differentiate naïve T cells into TH-17 cells. TH-17 cells can provoke angiogenesis both directly through the production of IL-17 and indirectly by inducing the secretion of pro-angiogenic factors from RA macrophages and fibroblasts. In contrast, inducible T regulatory cells (iTregs) are differentiated from naïve T cells through the effect of TGF-β and IL-2 and release TGF-β and IL-10 that has an inhibitory effect on RA angiogenesis.
Endothelial Cells
Selectins
Members of selectin family include soluble E-, L- and P-Selectins. While sE- and sP- Selectins are produced from endothelial cells, sL-Selectin is mainly secreted from circulating leukocytes [150]. Elevated levels of sE-Selectins and sP-Selectins have been also detected in RA sera and synovial fluid [150]. Within few hours of TNF, IL-1β and LPS stimulation expression of sE-Selectins is highly accentuated on vascular endothelial cells [161-163]. sE-Selectin is the only member of the family that is involved in RA angiogenesis as it can contribute to a dose dependent endothelial migration while its depletion significantly reduces RA synovial fluid mediated endothelial migration [154]. Whereas, previous studies have shown that sP-Selectins is responsible for adhesion of neutrophils and monocytes to endothelial cells [164,150].
Future directions for anti-angiogenic therapy in RA
RA therapeutics targeting angiogenesis include the use of anti-cancer therapies-as well as blockers of angiogenic pro-inflammatory factors of which a few are FDA approved and others are under development [165]. Current cancer treatments which have been studied in RA preclinical models include VEGF inhibitors, Taxol, CPT, Vitaxin and FTY720 [165]. Approved RA treatment strategies that may function in part through inhibiting angiogenesis include TNF, IL-1β, and IL-6 inhibitors, Thalidomide, and Cox-2 inhibitors. Pro-inflammatory mediators that promote RA angiogenesis and can be evaluated as future therapeutic targets include cytokines (IL-17, IL-18 and MIF), chemokines (CXCL12), growth factors (Ang1 and Ang2), proteases (MMPs) and adhesion molecules (ICAM1 and VCAM1) (Table 1).
Table 1.
Angiogenic pro-inflammatory mediators in RA that may be future therapeutic targets.
| Target | Drug | Nature/Mode of action |
Status | References |
|---|---|---|---|---|
| Cytokines | ||||
| IL-17 | Secukinum ab |
Humanized monoclonal neutralizing anti-IL-17 |
In phase III clinical trials. |
167 |
| Ixekizumab | Humanized monoclonal neutralizing anti-IL-17 |
In phase III clinical trials. |
168 | |
| Brodaluma b |
Humanized monoclonal neutralizing anti-IL-17 receptor |
In phase III clinical trials. |
169 | |
| IL-18 | GSK10708 06 |
Humanized monoclonal neutralizing antibody to IL-18 |
In phase I clinical trials. | 171 |
| rhIL-18bp | Recombinant human IL-18 binding protein |
In phase I clinical trials. | 172 | |
| MIF | anti-MIF | Anti-MIF neutralizing antibody |
In phase I clinical trials. | (clinicaltrials.gov ) |
| Milatuzuma b |
Anti-CD74 (part of MIF receptor) monoclonal antibody |
In phase I clinical trials. | (clinicaltrials.gov ) |
|
| Chemokines | ||||
| CXCL12 | Plerixafor | CXCR4 (CXCL12 receptor) antagonist |
FDA approved for treatment of certain cancers. In clinical trials for other disorders. |
176 |
| BMS- 936564 |
Fully human anti- CXCR4 antibody |
In phase I clinical trials. | 177 | |
| Growth Factors | ||||
| Ang1 and Ang2 |
Trebananib, | Neutralizing peptibody to both Ang1 and Ang2 |
In phase I, II and III clinical trials. |
178 |
| Double anti- angiogenic protein (DAAP) |
A dimeric decoy receptor with strong binding affinity to Ang1, Ang2 and VEGF |
In preclinical trails. | 179 | |
|
Adhesion
Molecules |
||||
| VCAM-1 | Natalizuma b |
Fully human anti-α4 integrin (VCAM-1 receptor) antibody |
FDA approved for treatment of MS. In phase II clinical trials for RA. |
189 |
Post onset treatment of CIA mice with a neutralizing anti-IL-17 antibody markedly reduced the disease severity and serum IL-6 levels as well as IL-1β and RANKL positive cells [166]. Ixekizumab and Secukinumab, are both monoclonal neutralizing anti-IL-17 antibodies, that can significantly reduce RA serum CRP levels and DAS28 scores and are currently being evaluated in phase II and III clinical trials [167,168]. Additionally, Brodalumab, an anti-IL-17 RA monoclonal antibody, was examined in phase II clinical trials and psoriatic arthritis patients that received treatment for 24 weeks had markedly improved response rates compared to the placebo group [169]. However, the effect of Ixekizumab, Secukinumab and Brodalumab has not been assessed on RA synovial angiogenesis.
IL-18 can impact RA disease activity through both direct and indirect effects on inflammation and angiogenesis. Adenovirally expressed IL-18bp/IL-4 fusion protein reduces the secretion of pro-angiogenic mediators such as TNF, IL-6, IL-8 and IL-18 from RA synovial tissue fibroblasts [170]. In a more recent phase I clinical trial, up to 10 mg/kg i.v. injection of the humanized neutralizing antibody to IL-18 (GSK1070806) was well tolerated in healthy subjects [171]. Consistently, the use of recombinant human IL-18bp (rhIL-18bp) in a Phase I clinical trial demonstrated a dose dependent pharmacokinetics with no adverse effect in moderate to severe RA patients [172]. However, the impact of GSK1070806 and rhIL-18bp therapies was not determined on human angiogenesis.
Neutralization of MIF relieves joint swelling in experimental arthritis and suppresses vascularization in tumors and arthritic mice [173,105]. MIF can be targeted through numerous approaches which include anti-MIF neutralizing antibodies, anti-MIF receptor blocking antibodies or soluble small molecule antagonists of MIF [174,175]. An anti-MIF antibody is in phase I clinical trials for the treatment of solid tumors (clinicaltrails.gov). Additionally, Milatuzumab (anti-CD74 monoclonal antibody) which targets CD74, a part of the MIF receptor, is currently in phase I clinical trials for the treatment of SLE (clinicaltrails.gov). Yet, the anti-angiogenic properties of these therapeutic approaches have not been tested in the clinical trials.
In CIA, treatment with an antagonist that targets CXCL12 ligation to CXCR4 (Plerixafor; AMD3100) relieves joint inflammation, although the significance of the therapy was not examined on synovial vascularization [124]. Plerixafor is currently FDA approved for the treatment of non-Hodgkins lymphoma and multiple myeloma and is in several clinical trials for the treatment of other cancers [176]. A fully human anti-CXCR4 antibody, BMS-936564, that impairs CXCL12-induced cell migration by disrupting its binding to CXCR4, is currently in phase I clinical trials for the treatment of multiple myeloma [177]. Despite the use of Plerixafor and MBS-936564 in cancer therapy, their effect on human angiogenesis remains to be determined.
Trebananib, a neutralizing peptibody to Ang1 and Ang2, has a strong binding affinity to Ang1 and Ang2 and as a result can prevent their ligation to Tie2 [178,179]. Hence, Trebananib is currently being tested in clinical trials for the cancer treatment [179]. Double anti-angiogenic protein (DAAP), a dimeric decoy receptor with strong binding affinity to Ang1, Ang2 and VEGF [180], could markedly reduce CIA joint vascularization. However, the effect of Trebananib and DAAP on RA patients remains undetermined.
MMPs have been the target of many clinical trials for different diseases with little success. Two clinical trials studying MMP inhibitors, Apratastat and Cipemastat Trocade, in RA were discontinued after reaching phase II [181,182].
Future treatments targeting adhesion molecules will need to advance from the previous attempts to target CD11a, a ligand of ICAM-1 [183,184]. An anti-CD11a monoclonal antibody, Efalizumab, was FDA approved for the treatment of psoriasis until it was later removed from the market for adverse reactions [183,184]. Another anti-CD11/CD18 monoclonal antibody, Rovelizumab, was earlier shown to not be efficacious in the treatment of several disease states including multiple sclerosis (MS) [185]. An anti-ICAM1 monoclonal antibody was in clinical trials for the treatment of RA, unfortunately it was determined that repeated treatments had reduced efficacy [186-188]. More recently, a monoclonal antibody targeting the VCAM1 receptor α4 integrin, Natalizumab, has been FDA approved for the treatment of MS and has been in clinical trials for RA [189]. Interestingly, studies performed in vitro and in animal models of cancer have shown that Natalizumab has an impact on VEGF expression and angiogenesis [190]. Another anti-α4 integrin monoclonal antibody, Vedolizumab, which specifically targets α4β7 integrin, is approved for the treatment of Crohn’s disease and ulcerative colitis. Vedolizumab has not been studied in models of arthritis however it blocks lymphocyte α4β7, an integrin important for RA pathogenesis [191].
Overall there are many therapeutic approaches that have successfully resolved arthritic joint vascularization in RA preclinical models. However, it is less likely that targeting one specific cascade will have a critical effect in ameliorating RA angiogenesis. Hence, discovering novel strategies to target multiple pathways or selecting an upstream cascade that modulates numerous pro-angiogenic factors may be utilized in future RA therapies.
CONCLUSION
In this review we have discussed the potential impact of growth factors, hypoxia induced factors, cytokines, chemokines, matrix metalloproteinase and adhesion molecules on RA angiogenesis. The majority of these pro-angiogenic factors are released from cells in the lining layer to foster pannus formation and leukocyte infiltration. To relieve disease progression and RA bone destruction, many of these pro-angiogenic regulators have been evaluated in RA preclinical studies. In many cases because of the complexity and heterogeneity of the human disease, promising results obtained from the experimental arthritis models could not be translated into successful treatment strategies in RA. This could in part be due to activation of multiple pro-angiogenic factors that are in crosstalk with each other; hence targeting a single player may not impact disease severity or bone destruction. Therefore the recent innovative approaches to target several pro-inflammatory factors through bispecific antibodies may be effective in blocking RA angiogenesis. Regardless of the approach, emerging evidence based on ultrasonographic vascular imaging and expression of pro-angiogenic biomarkers strongly support the indispensable role of neovascularization in RA pathology, thus implicating that inhibition of synovial angiogenesis may provide a promising therapeutic strategy.
Acknowledgements
This work was supported in part by awards from the National Institutes of Health AR056099 and AR065778, funding provided by Department of Defense PR093477 and Arthritis Foundation Innovative Research Grant. We apologize to colleagues whose studies were not cited because of space limitation.
Footnotes
Compliance with Ethical Standards:
1-DISCLOSURE OF POTENTIAL CONFLICTS OF INTETREST:
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10(5):224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3(11):635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 4.Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3(8):434–442. doi: 10.1038/ncprheum0559. [DOI] [PubMed] [Google Scholar]
- 5.Koch AE. Angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41(6):951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Veale DJ, Fearon U. Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol. 2006;20(5):941–947. doi: 10.1016/j.berh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Szekanecz Z, Koch AE. Chemokines and angiogenesis. Curr Opin Rheumatol. 2001;13(3):202–208. doi: 10.1097/00002281-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach W, Auerbach R. Angiogenesis inhibition: a review. Pharmacol Ther. 1994;63(3):265–311. doi: 10.1016/0163-7258(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 9.Koch AE, Distler O. Vasculopathy and disordered angiogenesis in selected rheumatic diseases: rheumatoid arthritis and systemic sclerosis. Arthritis Res Ther. 2007;9(Suppl 2):S3. doi: 10.1186/ar2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua RA, Arbiser JL. The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity. 2009;42(7):574–579. doi: 10.1080/08916930903002461. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano R, Felice F, Balbarini A. Angiogenesis as risk factor for plaque vulnerability. Curr Pharm Des. 2009;15(10):1095–1106. doi: 10.2174/138161209787846892. [DOI] [PubMed] [Google Scholar]
- 12.Kushner EJ, Bautch VL. Building blood vessels in development and disease. Curr Opin Hematol. 2013;20(3):231–236. doi: 10.1097/MOH.0b013e328360614b. [DOI] [PubMed] [Google Scholar]
- 13.Szekanecz Z, Koch AE. Endothelial cells in inflammation and angiogenesis. Current drug targets Inflammation and allergy. 2005;4(3):319–323. doi: 10.2174/1568010054022187. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J, Brem H. Angiogenesis and Inflammation. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Second edn. Raven Press; New York: 1992. pp. 821–839. [Google Scholar]
- 15.Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 17.Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, McInnes IB, Bresnihan B, Tak PP. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(6):834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tak PP, Breedveld FC. Analysis of serial synovial biopsies as a screening method for predicting the effects of therapeutic interventions. J Clin Rheumatol. 1997;3(4):186. doi: 10.1097/00124743-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43(12):2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 21.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 22.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42(12):3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 25.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 26.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6(3):498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jojic V, Shay T, Sylvia K, Zuk O, Sun X, Kang J, Regev A, Koller D, Best AJ, Knell J, Goldrath A, Joic V, Cohen N, Brennan P, Brenner M, Kim F, Rao TN, Wagers A, Heng T, Ericson J, Rothamel K, Ortiz-Lopez A, Mathis D, Benoist C, Bezman NA, Sun JC, Min-Oo G, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Blair DA, Dustin ML, Shinton SA, Hardy RR, Laidlaw D, Collins J, Gazit R, Rossi DJ, Malhotra N, Kreslavsky T, Fletcher A, Elpek K, Bellemarte-Pelletier A, Malhotra D, Turley S. Identification of transcriptional regulators in the mouse immune system. Nat Immunol. 2013;14(6):633–643. doi: 10.1038/ni.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills CD. Molecular basis of “suppressor” macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991;146(8):2719–2723. [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 34.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8(3):153–162. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 35.Neumann E, Lefevre S, Zimmermann B, Gay S, Muller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med. 2010;16(10):458–468. doi: 10.1016/j.molmed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pap T, Meinecke I, Muller-Ladner U, Gay S. Are fibroblasts involved in joint destruction? Ann Rheum Dis. 2005;64(Suppl 4):iv52–54. doi: 10.1136/ard.2005.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45(6):669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- 39.Niedermeier M, Pap T, Korb A. Therapeutic opportunities in fibroblasts in inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24(4):527–540. doi: 10.1016/j.berh.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 42.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152(8):4149–4156. [PubMed] [Google Scholar]
- 43.Brouwer E, Gouw AS, Posthumus MD, van Leeuwen MA, Boerboom AL, Bijzet J, Bos R, Limburg PC, Kallenberg CG, Westra J. Hypoxia inducible factor-1-alpha (HIF-1alpha) is related to both angiogenesis and inflammation in rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(6):945–951. [PubMed] [Google Scholar]
- 44.Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011;10(10):595–598. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X, Shao W, Li G, Li M, Su Y, Cohen PL, Qiu X, Li Z. Hypoxia and hypoxia-inducible factor-1alpha provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis. 2014;73(5):928–936. doi: 10.1136/annrheumdis-2012-202444. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164(11):5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]
- 47.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahrara S, Volin MV, Connors MA, Haines GK, Koch AE. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 2002;4(3):201–208. doi: 10.1186/ar407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearon U, Griosios K, Fraser A, Reece R, Emery P, Jones PF, Veale DJ. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol. 2003;30(2):260–268. [PubMed] [Google Scholar]
- 50.Gravallese EM, Pettit AR, Lee R, Madore R, Manning C, Tsay A, Gaspar J, Goldring MB, Goldring SR, Oettgen P. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis. 2003;62(2):100–107. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clavel G, Bessis N, Boissier MC. Recent data on the role for angiogenesis in rheumatoid arthritis. Joint Bone Spine. 2003;70(5):321–326. doi: 10.1016/s1297-319x(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 52.Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73(1):108–118. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52(5):1585–1594. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 54.Honorati MC, Neri S, Cattini L, Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14(4):345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Shibuya H, Yoshitomi H, Murata K, Kobayashi S, Furu M, Ishikawa M, Fujii T, Ito H, Matsuda S. TNFalpha, PDGF, and TGFbeta synergistically induce synovial lining hyperplasia via inducible PI3Kdelta. Mod Rheumatol. 2015;25(1):72–78. doi: 10.3109/14397595.2014.900847. [DOI] [PubMed] [Google Scholar]
- 56.Koyama K, Hatsushika K, Ando T, Sakuma M, Wako M, Kato R, Haro H, Sugiyama H, Hamada Y, Ogawa H, Nakao A. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod Rheumatol. 2007;17(4):306–310. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- 57.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62(2):383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 58.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 59.Strunk J, Bundke E, Lange U. Anti-TNF-alpha antibody Infliximab and glucocorticoids reduce serum vascular endothelial growth factor levels in patients with rheumatoid arthritis: a pilot study. Rheumatol Int. 2006;26(3):252–256. doi: 10.1007/s00296-005-0619-5. [DOI] [PubMed] [Google Scholar]
- 60.Markham T, Mullan R, Golden-Mason L, Rogers S, Bresnihan B, Fitzgerald O, Fearon U, Veale DJ. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy. J Am Acad Dermatol. 2006;54(6):1003–1012. doi: 10.1016/j.jaad.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 61.Lubberts E. Th17 cytokines and arthritis. Seminars in immunopathology. 2010;32(1):43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pickens SR, Volin MV, Mandelin AM, 2nd, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184(6):3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickens SR, Chamberlain ND, Volin MV, Gonzalez M, Pope RM, Mandelin AM, 2nd, Kolls JK, Shahrara S. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogen. 2011;14(4):443–455. doi: 10.1007/s10456-011-9227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther. 2011;13(4):R126. doi: 10.1186/ar3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shu Q, Amin MA, Ruth JH, Campbell PL, Koch AE. Suppression of endothelial cell activity by inhibition of TNFalpha. Arthritis Res Ther. 2012;14(2):R88. doi: 10.1186/ar3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choe JY, Lee SJ, Park SH, Kim SK. Tacrolimus (FK506) inhibits interleukin-1beta-induced angiopoietin-1, Tie-2 receptor, and vascular endothelial growth factor through down-regulation of JNK and p38 pathway in human rheumatoid fibroblast-like synoviocytes. Joint Bone Spine. 2012;79(2):137–143. doi: 10.1016/j.jbspin.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 67.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM, 2nd, Shahrara S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011;63(4):914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isozaki T, Arbab AS, Haas CS, Amin MA, Arendt MD, Koch AE, Ruth JH. Evidence that CXCL16 is a potent mediator of angiogenesis and is involved in endothelial progenitor cell chemotaxis: studies in mice with K/BxN serum-induced arthritis. Arthritis Rheum. 2013;65(7):1736–1746. doi: 10.1002/art.37981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badolato R, Oppenheim JJ. Role of cytokines, acute-phase proteins, and chemokines in the progression of rheumatoid arthritis. Semin Arthritis Rheum. 1996;26(2):526–538. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 71.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blue ML, Conrad P, Webb DL, Sarr T, Macaro M. Interacting monocytes and synoviocytes induce adhesion molecules by a cytokine-regulated process. Lymphokine Cytokine Res. 1993;12(4):213–218. [PubMed] [Google Scholar]
- 73.Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 74.Kayakabe K, Kuroiwa T, Sakurai N, Ikeuchi H, Kadiombo AT, Sakairi T, Matsumoto T, Maeshima A, Hiromura K, Nojima Y. Interleukin-6 promotes destabilized angiogenesis by modulating angiopoietin expression in rheumatoid arthritis. Rheumatology. 2012;51(9):1571–1579. doi: 10.1093/rheumatology/kes093. [DOI] [PubMed] [Google Scholar]
- 75.Ogami K, Yamaguchi R, Imoto S, Tamada Y, Araki H, Print C, Miyano S. Computational gene network analysis reveals TNF-induced angiogenesis. BMC systems biology. 2012;6(Suppl 2):S12. doi: 10.1186/1752-0509-6-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z, Kim SJ, Essani AB, Volin MV, Vila OM, Swedler W, Arami S, Volkov S, Sardin LV, Sweiss N, Shahrara S. Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, Sawaqed R, Perkins D, Shahrara S, Sweiss NJ. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug design, development and therapy. 2014;8:349–364. doi: 10.2147/DDDT.S41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 79.Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147(7):2187–2195. [PubMed] [Google Scholar]
- 80.Cho ML, Ju JH, Kim HR, Oh HJ, Kang CM, Jhun JY, Lee SY, Park MK, Min JK, Park SH, Lee SH, Kim HY. Toll-like receptor 2 ligand mediates the upregulation of angiogenic factor, vascular endothelial growth factor and interleukin-8/CXCL8 in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;108(2):121–128. doi: 10.1016/j.imlet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 82.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276(6):3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 83.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104(10):1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moller B, Kessler U, Rehart S, Kalina U, Ottmann OG, Kaltwasser JP, Hoelzer D, Kukoc-Zivojnov N. Expression of interleukin-18 receptor in fibroblast-like synoviocytes. Arthritis Res. 2002;4:139–144. doi: 10.1186/ar390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verri WA, Jr., Cunha TM, Ferreira SH, Wei X, Leung BP, Fraser A, McInnes IB, Liew FY, Cunha FQ. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. 2007;37(12):3373–3380. doi: 10.1002/eji.200737488. [DOI] [PubMed] [Google Scholar]
- 86.Marotte H, Ahmed S, Ruth JH, Koch AE. Blocking ERK-1/2 reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity in rheumatoid arthritis synovial fibroblasts by induction of interleukin-18 binding protein A. Arthritis Rheum. 2010;62(3):722–731. doi: 10.1002/art.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Volin MV, Koch AE. Interleukin-18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res. 2011;31(10):745–751. doi: 10.1089/jir.2011.0050. [DOI] [PubMed] [Google Scholar]
- 88.Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171(2):1009–1015. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- 89.Ruth JH, Park CC, Amin MA, Lesch C, Marotte H, Shahrara S, Koch AE. Interleukin-18 as an in vivo mediator of monocyte recruitment in rodent models of rheumatoid arthritis. Arthritis Res Ther. 2010;12(3):R118. doi: 10.1186/ar3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amin MA, Rabquer BJ, Mansfield PJ, Ruth JH, Marotte H, Haas CS, Reamer EN, Koch AE. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann Rheum Dis. 2010;69(12):2204–2212. doi: 10.1136/ard.2009.127241. [DOI] [PubMed] [Google Scholar]
- 91.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167(3):1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 92.Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, Koch AE. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum. 2007;56(6):1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- 93.Morel JC, Park CC, Kumar P, Koch AE. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001;81(10):1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- 94.Yoo JK, Kwon H, Khil LY, Zhang L, Jun HS, Yoon JW. IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J Immunol. 2005;175(12):8280–8286. doi: 10.4049/jimmunol.175.12.8280. [DOI] [PubMed] [Google Scholar]
- 95.Onodera S, Tanji H, Suzuki K, Kaneda K, Mizue Y, Sagawa A, Nishihira J. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine. 1999;11(2):163–167. doi: 10.1006/cyto.1998.0402. [DOI] [PubMed] [Google Scholar]
- 96.Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, Weedon H, Holdsworth SR, Bucala R, Morand EF. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999;42(8):1601–1608. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 97.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 99.Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3(3):320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 100.Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000;275(1):444–450. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 101.Onodera S, Nishihira J, Koyama Y, Majima T, Aoki Y, Ichiyama H, Ishibashi T, Minami A. Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 2004;50(5):1437–1447. doi: 10.1002/art.20190. [DOI] [PubMed] [Google Scholar]
- 102.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93(4):321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 103.Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor (MIF) in human vascular endothelial cells and its induction by lipopolysaccharide. Cytokine. 1998;10(3):199–205. doi: 10.1006/cyto.1997.0276. [DOI] [PubMed] [Google Scholar]
- 104.Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T, Todo S. An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine. 2000;12(4):309–314. doi: 10.1006/cyto.1999.0562. [DOI] [PubMed] [Google Scholar]
- 105.Leech M, Metz C, Santos L, Peng T, Holdsworth SR, Bucala R, Morand EF. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 1998;41(5):910–917. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 106.Santos LL, Dacumos A, Yamana J, Sharma L, Morand EF. Reduced arthritis in MIF deficient mice is associated with reduced T cell activation: down-regulation of ERK MAP kinase phosphorylation. Clin Exp Immunol. 2008;152(2):372–380. doi: 10.1111/j.1365-2249.2008.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh A, Leng L, Fan J, Gajda M, Brauer R, Fingerle-Rowson G, Bucala R, Illges H. Macrophage-derived, macrophage migration inhibitory factor (MIF) is necessary to induce disease in the K/BxN serum-induced model of arthritis. Rheumatol Int. 2013;33(9):2301–2308. doi: 10.1007/s00296-013-2713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szekanecz Z, Vegvari A, Szabo Z, Koch AE. Chemokines and chemokine receptors in arthritis. Front Biosci (Schol Ed) 2010;2:153–167. doi: 10.2741/s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Macrophages in rheumatoid arthritis. Histol Histopathol. 2007;22(5):581–586. doi: 10.14670/HH-22.581. [DOI] [PubMed] [Google Scholar]
- 110.Asquith DL, Bryce SA, Nibbs RJ. Targeting cell migration in rheumatoid arthritis. Curr Opin Rheumatol. 2015;27(2):204–211. doi: 10.1097/BOR.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 111.Park YJ, Kim JY, Park J, Choi JJ, Kim WU, Cho CS. Bone erosion is associated with reduction of circulating endothelial progenitor cells and endothelial dysfunction in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(6):1450–1460. doi: 10.1002/art.38352. [DOI] [PubMed] [Google Scholar]
- 112.Wu PF, Lu ZP, Cai BB, Tian L, Zou C, Jiang KR, Miao Y. Role of CXCL12/CXCR4 signaling axis in pancreatic cancer. Chinese medical journal. 2013;126(17):3371–3374. [PubMed] [Google Scholar]
- 113.Wang H, Liu W, Wei D, Hu K, Wu X, Yao Y. Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor proliferation, migration and invasion of ovarian cancer cell lines. Oncology letters. 2014;7(5):1581–1585. doi: 10.3892/ol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. American journal of translational research. 2014;6(4):340–352. [PMC free article] [PubMed] [Google Scholar]
- 115.Villalvilla A, Gomez R, Roman-Blas JA, Largo R, Herrero-Beaumont G. SDF-1 signaling: a promising target in rheumatic diseases. Expert Opin Ther Targets. 2014;18(9):1077–1087. doi: 10.1517/14728222.2014.930440. [DOI] [PubMed] [Google Scholar]
- 116.Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, Garcia-Lazaro FJ. Synoviocyte-Derived CXCL12 Is Displayed on Endothelium and Induces Angiogenesis in Rheumatoid Arthritis. J Immunol. 2003;170(4):2147–2152. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- 117.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 118.Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, Pitzalis C. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis Rheum. 2002;46(3):824–836. doi: 10.1002/art.10102. [DOI] [PubMed] [Google Scholar]
- 119.Madge LA, Kluger MS, Orange JS, May MJ. Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells. J Immunol. 2008;180(5):3467–3477. doi: 10.4049/jimmunol.180.5.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noort AR, van Zoest KP, Weijers EM, Koolwijk P, Maracle CX, Novack DV, Siemerink MJ, Schlingemann RO, Tak PP, Tas SW. NF-kappaB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J Pathol. 2014;234(3):375–385. doi: 10.1002/path.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154(4):1125–1135. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6(1):102–111. [PubMed] [Google Scholar]
- 123.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6(9):3530–3535. [PubMed] [Google Scholar]
- 124.Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, De Clercq E, Billiau A, Schols D. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001;167(8):4686–4692. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- 125.Ruth JH, Haas CS, Park CC, Amin MA, Martinez RJ, Haines GK, 3rd, Shahrara S, Campbell PL, Koch AE. CXCL16-mediated cell recruitment to rheumatoid arthritis synovial tissue and murine lymph nodes is dependent upon the MAPK pathway. Arthritis Rheum. 2006;54(3):765–778. doi: 10.1002/art.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katschke KJ, Jr., Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, Ponath P, Park CC, Pope RM, Koch AE. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44(5):1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 127.Ruth JH, Rottman JB, Katschke KJ, Jr., Qin S, Wu L, LaRosa G, Ponath P, Pope RM, Koch AE. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44(12):2750–2760. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 128.Shahrara S, Amin MA, Woods JM, Haines GK, Koch AE. Chemokine receptor expression and in vivo signaling pathways in the joints of rats with adjuvant-induced arthritis. Arthritis Rheum. 2003;48(12):3568–3583. doi: 10.1002/art.11344. [DOI] [PubMed] [Google Scholar]
- 129.Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC, Haas CS, Pope RM, Haines GK, Zha YY, Koch AE. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52(6):1907–1919. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180(5):3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 131.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Role of the CCL21 and CCR7 pathways in rheumatoid arthritis angiogenesis. Arthritis Rheum. 2012;64(8):2471–2481. doi: 10.1002/art.34452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ruth JH, Volin MV, Haines GK, 3rd, Woodruff DC, Katschke KJ, Jr., Woods JM, Park CC, Morel JC, Koch AE. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44(7):1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 133.Garcia GE, Xia Y, Chen S, Wang Y, Ye RD, Harrison JK, Bacon KB, Zerwes HG, Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol. 2000;67(4):577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- 134.Imaizumi T, Matsumiya T, Fujimoto K, Okamoto K, Cui X, Ohtaki U, Yoshida Hidemi, Satoh K. Interferon-gamma stimulates the expression of CX3CL1/fractalkine in cultured human endothelial cells. The Tohoku journal of experimental medicine. 2000;192(2):127–139. doi: 10.1620/tjem.192.127. [DOI] [PubMed] [Google Scholar]
- 135.Volin MV, Woods JM, Amin MA, Connors MA, Harlow LA, Koch AE. Fractalkine: a novel angiogenic chemokine in rheumatoid arthritis. Am J Pathol. 2001;159(4):1521–1530. doi: 10.1016/S0002-9440(10)62537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M, Stracke J, Clark I. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4(Suppl 3):S39–49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, Billingham M, Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43(6):1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 138.Ahrens D, Koch AE, Pope RM, Stein-Picarella M, Niedbala MJ. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 1996;39(9):1576–1587. doi: 10.1002/art.1780390919. [DOI] [PubMed] [Google Scholar]
- 139.Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56(9):542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 142.Akhavani MA, Madden L, Buysschaert I, Sivakumar B, Kang N, Paleolog EM. Hypoxia upregulates angiogenesis and synovial cell migration in rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):R64. doi: 10.1186/ar2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 144.Kim KS, Choi HM, Lee YA, Choi IA, Lee SH, Hong SJ, Yang HI, Yoo MC. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol Int. 2011;31(4):543–547. doi: 10.1007/s00296-010-1592-1. [DOI] [PubMed] [Google Scholar]
- 145.Mengshol JA, Mix KS, Brinckerhoff CE. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull’s-eye or missing the mark? Arthritis Rheum. 2002;46(1):13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 146.Rabquer BJ, Pakozdi A, Michel JE, Gujar BS, Haines GK, 3rd, Imhof BA, Koch AE. Junctional adhesion molecule C mediates leukocyte adhesion to rheumatoid arthritis synovium. Arthritis Rheum. 2008;58(10):3020–3029. doi: 10.1002/art.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rabquer BJ, Amin MA, Teegala N, Shaheen MK, Tsou PS, Ruth JH, Lesch CA, Imhof BA, Koch AE. Junctional adhesion molecule-C is a soluble mediator of angiogenesis. J Immunol. 2010;185(3):1777–1785. doi: 10.4049/jimmunol.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Palmer G, Busso N, Aurrand-Lions M, Talabot-Ayer D, Chobaz-Peclat V, Zimmerli C, Hammel P, Imhof BA, Gabay C. Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res Ther. 2007;9(4):R65. doi: 10.1186/ar2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leinster DA, Colom B, Whiteford JR, Ennis DP, Lockley M, McNeish IA, Aurrand-Lions M, Chavakis T, Imhof BA, Balkwill FR, Nourshargh S. Endothelial cell junctional adhesion molecule C plays a key role in the development of tumors in a murine model of ovarian cancer. Faseb J. 2013;27(10):4244–4253. doi: 10.1096/fj.13-230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mojcik CF, Shevach EM. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997;40(6):991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- 151.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25(4):215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 152.Hosaka S, Shah MR, Pope RM, Koch AE. Soluble forms of P-selectin and intercellular adhesion molecule-3 in synovial fluids. Clin Immunol Immunopathol. 1996;78(3):276–282. doi: 10.1006/clin.1996.0039. [DOI] [PubMed] [Google Scholar]
- 153.Koch AE, Shah MR, Harlow LA, Lovis RM, Pope RM. Soluble intercellular adhesion molecule-1 in arthritis. Clin Immunol Immunopathol. 1994;71:208–215. doi: 10.1006/clin.1994.1074. [DOI] [PubMed] [Google Scholar]
- 154.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature. 1995;376(6540):517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 155.Schimmer RC, Schrier DJ, Flory CM, Dykens J, Tung DK, Jacobson PB, Friedl HP, Conroy MC, Schimmer BB, Ward PA. Streptococcal Cell Wall-Induced Arthritis: Requirements for Neutrophils, P-Selectin, Intercellular Adhesion Molecule-1, and Macrophage-Inflammatory Protein-2. J Immunol. 1997;159:4103–4108. [PubMed] [Google Scholar]
- 156.Kakimoto K, Nakamura T, Ishii K, Takashi T, Iigou H, Yagita H, Okumura K, Onoue K. The effect of anti-adhesion molecule antibody on the development of collagen-induced arthritis. Cell Immunol. 1992;142(2):326–337. doi: 10.1016/0008-8749(92)90294-y. [DOI] [PubMed] [Google Scholar]
- 157.Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J, Chwiecko J. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann Rheum Dis. 2002;61(9):804–809. doi: 10.1136/ard.61.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim SJ, Chen Z, Chamberlain ND, Volin MV, Swedler W, Volkov S, Sweiss N, Shahrara S. Angiogenesis in Rheumatoid Arthritis Is Fostered Directly by Toll-like Receptor 5 Ligation and Indirectly Through Interleukin-17 Induction. Arthritis Rheum. 2013;65(8):2024–2036. doi: 10.1002/art.37992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63(8):2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 162.Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 163.Bevilacqua MP, Stengelin S, Gimbrone MA, Jr., Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 164.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25(4):215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 165.Maruotti N, Cantatore FP, Ribatti D. Putative effects of potentially anti-angiogenic drugs in rheumatic diseases. Eur J Clin Pharmacol. 2014;70(2):135–140. doi: 10.1007/s00228-013-1605-6. [DOI] [PubMed] [Google Scholar]
- 166.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 167.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, Aelion JA, Lee SH, Codding CE, Kellner H, Ikawa T, Hugot S, Mpofu S. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72(6):863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 168.Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, Benichou O, Xie L, Braun D, Berclaz PY, Banerjee S. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2014;66(7):1693–1704. doi: 10.1002/art.38617. [DOI] [PubMed] [Google Scholar]
- 169.Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, Newmark R, Feng J, Erondu N, Nirula A. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 170.Yao HP, Qian Y, Shao XT, Xu ZR, Cheng LF, Feng L, Wu NP, Yang YM. Construction of a recombinant adenovirus vector expressing IL-18BP/IL-4 fusion gene and the anti-inflammatory effect induced by this gene on lipopolysaccharide-stimulated synovial fibroblasts. Inflamm Res. 2010;59(2):97–104. doi: 10.1007/s00011-009-0072-0. [DOI] [PubMed] [Google Scholar]
- 171.Mistry P, Reid J, Pouliquen I, McHugh S, Abberley L, DeWall S, Taylor A, Tong X, Rocha Del Cura M, McKie E. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin-18 mAb GSK1070806 in healthy and obese subjects. International journal of clinical pharmacology and therapeutics. 2014;52(10):867–879. doi: 10.5414/CP202087. [DOI] [PubMed] [Google Scholar]
- 172.Tak PP, Bacchi M, Bertolino M. Pharmacokinetics of IL-18 binding protein in healthy volunteers and subjects with rheumatoid arthritis or plaque psoriasis. European journal of drug metabolism and pharmacokinetics. 2006;31(2):109–116. doi: 10.1007/BF03191127. [DOI] [PubMed] [Google Scholar]
- 173.Ogawa H, Nishihira J, Sato Y, Kondo M, Takahashi N, Oshima T, Todo S. An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour-associated angiogenesis. Cytokine. 2000;12(4):309–314. doi: 10.1006/cyto.1999.0562. [DOI] [PubMed] [Google Scholar]
- 174.Greven D, Leng L, Bucala R. Autoimmune diseases: MIF as a therapeutic target. Expert Opin Ther Targets. 2010;14(3):253–264. doi: 10.1517/14728220903551304. [DOI] [PubMed] [Google Scholar]
- 175.Cournia Z, Leng L, Gandavadi S, Du X, Bucala R, Jorgensen WL. Discovery of human macrophage migration inhibitory factor (MIF)-CD74 antagonists via virtual screening. J Med Chem. 2009;52(2):416–424. doi: 10.1021/jm801100v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fruehauf S. Current clinical indications for plerixafor. Transfusion medicine and hemotherapy: offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2013;40(4):246–250. doi: 10.1159/000354229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, Cardarelli PM. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19(2):357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 178.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, Lee TA, Sun JR, Estrada J, Bolon B, McCabe J, Wang L, Rex K, Caenepeel S, Hughes P, Cordover D, Kim H, Han SJ, Michaels ML, Hsu E, Shimamoto G, Cattley R, Hurh E, Nguyen L, Wang SX, Ndifor A, Hayward IJ, Falcon BL, McDonald DM, Li L, Boone T, Kendall R, Radinsky R, Oliner JD. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Molecular cancer therapeutics. 2010;9(10):2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weeraratne DK, Lofgren J, Dinnogen S, Swanson SJ, Zhong ZD. Development of a biosensor-based immunogenicity assay capable of blocking soluble drug target interference. J Immunol Methods. 2013;396(1-2):44–55. doi: 10.1016/j.jim.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 180.Hah YS, Koh YJ, Lim HS, Kim HO, Cheon YH, Noh HS, Jang KY, Lee SY, Lee GM, Koh GY, Lee SI. Double-antiangiogenic protein DAAP targeting vascular endothelial growth factor A and angiopoietins attenuates collagen-induced arthritis. Arthritis Res Ther. 2013;15(4):R85. doi: 10.1186/ar4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dorman G, Cseh S, Hajdu I, Barna L, Konya D, Kupai K, Kovacs L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010;70(8):949–964. doi: 10.2165/11318390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 182.Thabet MM, Huizinga TW. Drug evaluation: apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs. 2006;7(11):1014–1019. [PubMed] [Google Scholar]
- 183.Gordon KB, Papp KA, Hamilton TK, Walicke PA, Dummer W, Li N, Bresnahan BW, Menter A. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290(23):3073–3080. doi: 10.1001/jama.290.23.3073. [DOI] [PubMed] [Google Scholar]
- 184.Prater EF, Day A, Patel M, Menter A. A retrospective analysis of 72 patients on prior efalizumab subsequent to the time of voluntary market withdrawal in 2009. Journal of drugs in dermatology: JDD. 2014;13(6):712–718. [PubMed] [Google Scholar]
- 185.Jones R. Rovelizumab (ICOS Corp) IDrugs: the investigational drugs journal. 2000;3(4):442–446. [PubMed] [Google Scholar]
- 186.Kavanaugh AF, Davis LS, Nichols LA, Norris SH, Rothlein R, Scharschmidt LA, Lipsky PE. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994;37(7):992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]
- 187.Kavanaugh AF, Davis LS, Jain RI, Nichols LA, Norris SH, Lipsky PE. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J Rheumatol. 1996;23(8):1338–1344. [PubMed] [Google Scholar]
- 188.Kavanaugh AF, Schulze-Koops H, Davis LS, Lipsky PE. Repeat treatment of rheumatoid arthritis patients with a murine anti-intercellular adhesion molecule 1 monoclonal antibody. Arthritis Rheum. 1997;40(5):849–853. doi: 10.1002/art.1780400511. [DOI] [PubMed] [Google Scholar]
- 189.Pucci E, Giuliani G, Solari A, Simi S, Minozzi S, Di Pietrantonj C, Galea I. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev. 2011;(10):CD007621. doi: 10.1002/14651858.CD007621.pub2. [DOI] [PubMed] [Google Scholar]
- 190.Podar K, Zimmerhackl A, Fulciniti M, Tonon G, Hainz U, Tai YT, Vallet S, Halama N, Jager D, Olson DL, Sattler M, Chauhan D, Anderson KC. The selective adhesion molecule inhibitor Natalizumab decreases multiple myeloma cell growth in the bone marrow microenvironment: therapeutic implications. Br J Haematol. 2011;155(4):438–448. doi: 10.1111/j.1365-2141.2011.08864.x. [DOI] [PubMed] [Google Scholar]
- 191.Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]