Abstract
Rationale
Anandamide and Δ9-tetrahydrocannabinol (Δ9-THC) sometimes produce different discriminative stimulus effects and, therefore, appear to differ in their mechanism of action. In order to understand the widespread use of cannabis and the therapeutic potential of cannabinoids, mechanisms responsible for behavioral effects need to be identified.
Objective
Drug discrimination was used to compare the mechanism of action of Δ9-THC, anandamide, and two structural analogs of anandamide in rhesus monkeys.
Materials and methods
Monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle. Δ9-THC, anandamide, methanandamide, and arachidonylcyclopropylamide (ACPA) were administered i.v. alone and in combination with at least one dose of rimonabant. Schild analysis and single-dose apparent affinity estimates were used to estimate the potency of rimonabant as an antagonist of each cannabinoid; these values were compared to examine whether the same receptors mediated discriminative stimulus effects.
Results
Δ9-THC, ACPA, methanandamide, and anandamide produced greater than 96% of responses on the Δ9-THC lever. The ED50 values were 0.024 mg/kg for Δ9-THC, 0.14 mg/kg for ACPA, 0.28 mg/kg for methanandamide, and 1.7 mg/kg for anandamide. The duration of action of Δ9-THC was 4–6 h and longer than the duration of action ACPA, methanandamide, and anandamide (i.e., each less than 50 min). Rimonabant surmountably antagonized the discriminative stimulus effects of each agonist, and the apparent affinity estimates (pA2 and pKB values) were 6.24–6.83.
Conclusions
Rimonabant can produce surmountable antagonism of the behavioral effects of not only Δ9-THC but also anandamide, methanandamide, and ACPA, and the interactions appear simple, competitive, and reversible. These cannabinoid agonists act at the same receptors to produce discriminative stimulus effects.
Keywords: Anandamide, Arachidonylcyclopropylamide, Cannabinoid, Δ9-tetrahydrocannabinol, Drug discrimination, Methanandamide, Rhesus monkey, Rimonabant, Schild analysis, SR 141716A
Introduction
The prototypic cannabinoid agonist Δ9-tetrahydrocannabinol (Δ9-THC) is responsible for many of the behavioral effects resulting from the use of Cannabis sativa (i.e., marijuana smoking; Wachtel et al. 2002). Δ9-THC is an agonist at two cannabinoid receptor subtypes (designated CB1 and CB2) that are coupled to inhibitory G proteins (Howlett et al. 2002). The CB1 antagonist rimonabant blocks many of the behavioral effects of Δ9-THC, including its antiemetic (Darmani 2001), antinociceptive (Compton et al. 1996; Vivian et al. 1998), memory impairing (Lichtman and Martin 1996), discriminative stimulus (Järbe et al. 2001, 2006; McMahon 2006), and positive reinforcing effects (Tanda et al. 2000). The antinociceptive and some other effects of Δ9-THC are reportedly absent in CB1 knockout mice (Ledent et al. 1999; Zimmer et al. 1999). Thus, CB1 receptors appear to mediate those effects responsible for the widespread use of marijuana.
The endogenous CB1 and CB2 agonist anandamide (Devane et al. 1992) shares some behavioral effects with Δ9-THC, including antiemetic (Sharkey et al. 2007), antinociceptive (Adams et al. 1998), and positive reinforcing effects (Justinova et al. 2005). However, rimonabant does not always block the behavioral effects of anandamide (Adams et al. 1998), providing evidence for actions at non-CB1 receptors. In previous drug discrimination studies, anandamide did not always substitute for Δ9-THC in rats and rhesus monkeys (Wiley et al. 1995, 1997; Burkey and Nation 1997; Järbe et al. 2001), suggesting that anandamide and Δ9-THC differ in their mechanism of action. These differences might result from metabolism of anandamide to metabolites (i.e., ethanolamine and arachidonic acid; Deutsch and Chin 1993) that act at non-CB1 receptors to produce behavioral effects (Wiley et al. 2006). Strategies available for decreasing the metabolism of anandamide include modification of its chemical structure and combination of anandamide with inhibitors of its enzymatic degradation, and both were reported to increase Δ9-THC-like behavioral effects. Methanandamide, for example, retains agonist activity at cannabinoid receptors, is more resistant to metabolism than anandamide (Abadji et al. 1994; Lang et al. 1999), and produces more reliable substitution than anandamide for the discriminative stimulus effects of Δ9-THC in rodents (Burkey and Nation 1997; Järbe et al. 2001). When combined with an inhibitor of fatty acid amide hydrolase (e.g., URB 597), anandamide can substitute for the discriminative stimulus effects of Δ9-THC (Solinas et al. 2007), further suggesting that behavioral effects vary as a function of anandamide metabolism.
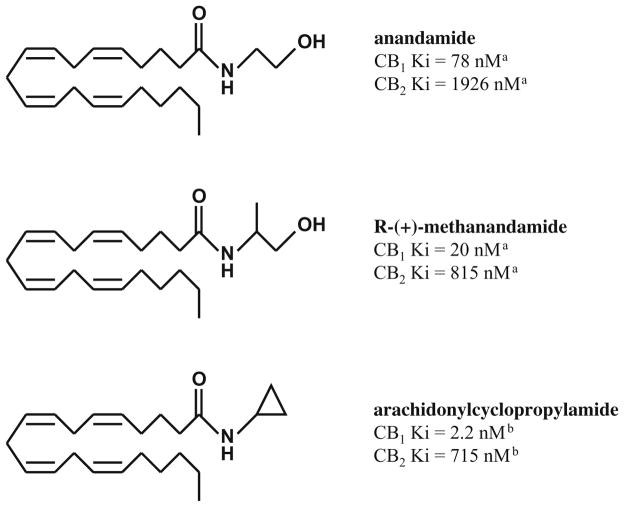
The goal of the current study was to examine the effects of anandamide in rhesus monkeys discriminating Δ9-THC; drugs were administered i.v. to increase delivery to the brain. The mechanism of action of anandamide was compared with two analogs of anandamide, methanandamide, and arachidonylcyclopropylamide (ACPA). Both analogs bind to cannabinoid receptors (Fig. 1), are agonists (Abadji et al. 1994; Hillard et al. 1999), and substitute for Δ9-THC in rhesus monkeys (McMahon 2006). Dose–response curves for Δ9-THC, ACPA, methanandamide, and anandamide were determined in the absence and presence of at least one dose of rimonabant. Quantitative analysis of antagonism (i.e., Schild analysis and single-dose apparent affinity estimates) was used to compare the receptor mechanism(s) of action of each agonist. Using this approach, a previous study demonstrated that rimonabant had the same potency for antagonizing the discriminative stimulus effects of Δ9-THC and two other cannabinoid agonists (CP 55940 and WIN 55212-2), thereby suggesting that the same receptors mediated the discriminative stimulus of Δ9-THC, CP 55940, and WIN 55212-2 (McMahon 2006).
Fig. 1.
Chemical structure and cannabinoid receptor binding affinities of anandamide, methanandamide, and arachidonylcyclopropylamide. a Khanolkar et al. (1996), b Hillard et al. (1999)
Materials and methods
Subjects
Three male and two female adult rhesus monkeys (Macaca mulatta) were housed individually on a 14-h light/10-h dark schedule, were maintained at 95% free-feeding weight (range 6.6–9.2 kg) with a diet consisting of primate chow (High Protein Monkey Diet, Harlan Teklad, Madison, WI, USA), fresh fruit, and peanuts, and were provided water in the home cage. Monkeys received cannabinoids and non-cannabinoids in a previous study (McMahon 2006). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Catheter insertion
Monkeys were anesthetized with ketamine (10 mg/kg i.m.) and isoflurane (1.5–3.0% inhaled via facemask) and received chronic indwelling catheters (heparin coated polyurethane, od=1.68 mm, id=1.02 mm, Instech Solomon, Plymouth Meeting, PA, USA). Each catheter was inserted and advanced 5 cm into a subclavian or femoral vein. Suture silk (coated vicryl, Ethicon Inc., Somerville, NJ, USA) was used to anchor the catheter to the vessel and to ligate the section of the vessel proximal to the catheter insertion. The other end of the catheter passed s.c. to the mid-scapular region of the back and was attached to a vascular access port (Mida-cbas-c50, Instech Solomon).
Apparatus
Monkeys were seated in chairs (Model R001, Primate Products, Miami, FL, USA) that provided restraint and were placed in ventilated, sound-attenuating chambers equipped with two levers and lights. Feet were placed in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator. An interface (MedAssociates, St. Albans, VT, USA) connected the chambers to a computer, which controlled and recorded experimental events with Med-PC software.
Drug discrimination procedure
Monkeys discriminated Δ9-THC (0.1 mg/kg i.v.) from vehicle (consisting of 1:1:18 parts of absolute ethanol, Emulphor-620, and saline, respectively) while responding under a fixed ratio 5 (FR5) schedule of stimulus-shock termination in a multiple-cycle procedure (McMahon et al. 2005). Each cycle began with a 15-min timeout, during which responses had no programmed consequence; a 5-min response period followed, during which illumination of red lights (one positioned above each lever) signaled that an electric stimulus was to be delivered every 40 s (i.e., limited hold). Five consecutive responses on the correct lever extinguished the red lights and postponed the schedule for 30 s (i.e., timeout); responding on the incorrect lever reset the response requirement on the correct lever. Determination of correct levers varied among monkeys and remained the same for that monkey for the duration of the study.
Training sessions were conducted non-systematically by administering Δ9-THC or vehicle 15 min before sessions; vehicle or sham (dull pressure applied to the skin overlying the vascular access port) was administered non-systematically at the beginning of cycles. Training with Δ9-THC consisted of two cycles, and training with vehicle consisted of 2–6 cycles; completion of the FR on the correct lever was required for a reinforcer during each training cycle. Monkeys had previously satisfied the criteria for testing, i.e., at least 80% of the total responses occurred on the correct lever and fewer than five responses (one FR) occurred on the incorrect lever before completion of the first FR on the correct lever for all cycles during five consecutive or six of seven training sessions. Tests were conducted at least 3 days apart and only after performance for consecutive training sessions, including both vehicle and Δ9-THC training sessions, satisfied the testing criteria. The type of training session preceding test sessions varied non-systematically.
During test sessions, five consecutive responses on either lever postponed the shock schedule, and monkeys received various doses of Δ9-THC, anandamide, methanandamide, and ACPA administered intravenously, alone and in combination with rimonabant. Rimonabant or vehicle was administered 15 min before sessions. A dose of Δ9-THC or vehicle was administered 15 min before the 5-min period of responding. Due to a relatively short duration of action (see “Results”), a dose of anandamide, methanandamide, or ACPA was administered 5 min before the 5-min period of responding. Agonist dose–effect curves were established by studying ineffective doses (i.e., doses producing responses predominantly on the vehicle lever) up to doses producing greater than 80% of responses on the Δ9-THC lever. Δ9-THC and ACPA dose–effect curves were determined in the presence of three doses (0.32–3.2 mg/kg i.v.) of rimonabant. Due to relatively low potency and limited supply, anandamide and methanandamide were studied in the presence of one (0.32 mg/kg) and two (0.32 and 1 mg/kg) doses of rimonabant, respectively. To establish the duration of discriminative stimulus effects, the training dose of Δ9-THC was administered at the beginning of and at 2-h increments prior to separate sessions each consisting of six cycles. Anandamide (3.2 mg/kg), methanandamide (1 mg/kg), and ACPA (0.32 mg/kg) were administered 5 min prior to the 5-min period of responding of the first cycle; these tests consisted of three additional consecutive, 20-min cycles (i.e., anandamide and its analogs were studied up to 70 min).
Drugs
Rimonabant and Δ9-THC (100 mg/ml in absolute ethanol) were obtained from The Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD, USA). ACPA and (R)-methanandamide were obtained from Tocris (Ellisville, MO, USA). Anandamide was synthesized according to a protocol developed by Giuffrida and Piomelli (1998a), and the identity of the final product was confirmed with gas chromatography and mass spectrometry as described (Giuffrida and Piomelli 1998b; Hardison et al. 2006).
Drugs were dissolved in a 1:1:18 mixture of absolute ethanol, Emulphor-620 (Rhone-Poulenc Inc., Princeton, NJ, USA), and physiologic saline, respectively. Drugs were administered i.v. in a volume of 0.1–3 ml/kg. All doses were expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
Data analyses
Each agonist was studied in four monkeys. Δ9-THC, ACPA, and methanandamide were studied in the same four monkeys. Three of those monkeys were used for studies with anandamide; anandamide was studied in a fourth monkey that did not receive the other cannabinoids. Discrimination data were expressed as a percentage of responses on the Δ9-THC lever out of total responses on both the Δ9-THC and vehicle levers. Rate of responding on both levers (i.e., Δ9-THC and vehicle) was calculated as responses per second excluding responses during timeouts. Rate of responding during a test was expressed as the percentage of the control response rate for individual animals, defined as the average response rate for all cycles during the immediately previous five vehicle training sessions excluding sessions during which the test criteria were not satisfied. Discrimination and rate data were averaged among subjects (±SEM) and plotted as a function of dose or time. Effects of agonists on response rate were examined with analyses of variance for repeated measures; Dunnett’s test was used to examine significant differences relative to the vehicle control (p<0.05).
To establish the potency of agonists in producing Δ9-THC-lever responding, the individual dose–response data for each agonist were analyzed simultaneously using GraphPad Prism version 4.03 for Windows (San Diego, CA, USA). Doses included in the analyses included those producing 25–75% of responses on the Δ9-THC lever, including not more than one dose producing less than 25% of responses on the Δ9-THC lever and not more than one dose producing greater than 75% of responses on the Δ9-THC lever. Other doses were excluded from the analyses. If the slopes of agonist dose–response curves were not significantly different as determined by an F-ratio test, then a common, best-fitting slope was used for further analyses (Kenakin 1997). Doses corresponding to the 50% level of effect (ED50 value), potency ratios, and their 95% confidence limits were calculated by parallel line analyses of data for individual subjects (Tallarida 2000). Potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1.
Schild plots were constructed by expressing the logarithm of the dose ratio −1 as a function of the negative logarithm of the molar dose of rimonabant for individual monkeys (Arunlakshana and Schild 1959). Straight lines were simultaneously fit to individual Schild plots with the equation log(dose ratio – 1) = –log(molar dose of rimonabant)× slope + intercept. Schild plots for rimonabant in combination with Δ9-THC and ACPA were compared using two mathematical models: a simpler model (i.e., slope constrained to unity or −1) and a more complex model that allowed slopes for each Schild plot to vary. The two models were compared with an F-ratio test. If the calculated F value was not significant, then the pA2 value was calculated with both the constrained and unconstrained slope. For rimonabant in combination with anandamide and methanandamide, a single-dose apparent affinity estimate was calculated for individual monkeys with the following equation: pKB = –log[B/dose ratio – 1], with B expressed in moles per kilogram of body weight.
Results
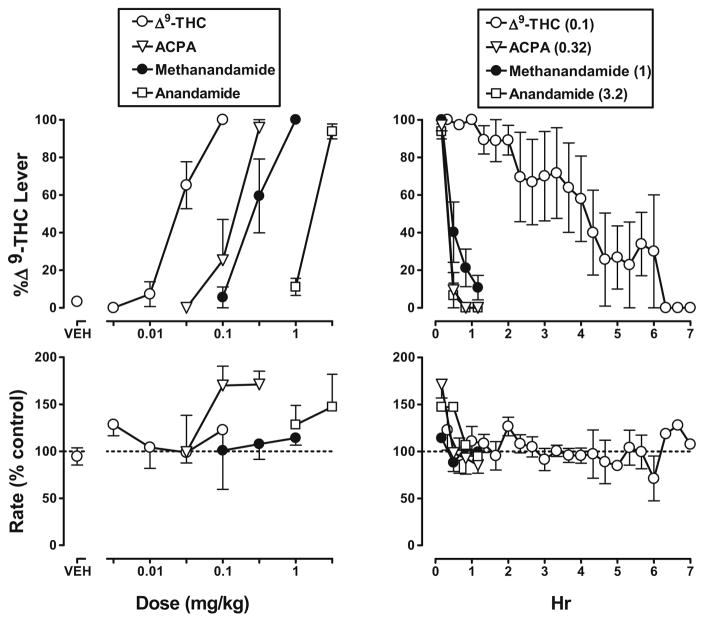
In rhesus monkeys discriminating Δ9-THC (0.1 mg/kg i.v.), the training drug dose dependently increased mean (±SEM) responding on the Δ9-THC lever to 100% at the training dose (0.1 mg/kg) (Fig. 2, top left, open circles). ACPA, methanandamide, and anandamide administered intravenously dose dependently increased responding on the Δ9-THC lever (Fig. 2, top left, triangles, closed circles, and squares, respectively); maximum responding was a mean (±SEM) of 96 (±4)%, 100%, and 96 (±4)% at doses of 0.32 mg/kg of ACPA, 1 mg/kg of methanandamide, and 3.2 mg/kg of anandamide, respectively. Following administration of vehicle, mean (±SEM) responding on the Δ9-THC lever was 3 (±2)% (Fig. 2, top, VEH). The dose–effect curves for Δ9-THC, ACPA, methanandamide, and anandamide did not deviate from parallelism, and their ED50 values (95% confidence limits) were 0.024 (0.018–0.030), 0.14 (0.071–0.28), 0.28 (0.17–0.47) mg/kg, and 1.7 (1.4–2.0) mg/kg, respectively. Δ9-THC was significantly more potent than ACPA and methanandamide, which were equipotent; anandamide was significantly less potent than the other cannabinoid agonists.
Fig. 2.
Discriminative stimulus effects of Δ9-THC, ACPA, methanandamide, and anandamide in rhesus monkeys expressed as a function of dose (left) and time (right). Left abscissae: dose in milligram per kilogram body weight or vehicle (VEH). Right abscissae: time in hours. Top ordinates: mean (±SEM) percentage of responses on the Δ9-THC lever. Bottom ordinates: mean (±SEM) response rate expressed as a percentage of control (VEH training days) rate [Rate (% control)]. Values in parentheses in the right legend are doses in milligram per kilogram body weight and correspond to data shown in right panels. The horizontal dashed line (100%) shows control response rate
For five vehicle training sessions immediately preceding a test, mean rate of responding for individual monkeys was 0.79, 0.86, 1.02, 1.57, and 1.71 responses per second. ACPA (0.1 and 0.32 mg/kg) significantly increased response rate (p<0.05; Fig. 2, bottom left, triangles), whereas Δ9-THC, methanandamide, and anandamide did not significantly modify response rate (p>0.20).
For discriminative stimulus effects, the duration of action of Δ9-THC was greater than the duration of action of anandamide, methanandamide, and ACPA. For example, the training dose (0.1 mg/kg i.v.) of Δ9-THC produced 89–100% of responses on the Δ9-THC lever for up to 2 h and greater than 57% responding on the drug lever for up to 4 h; responding was predominantly on the vehicle lever at 7 h (Fig. 2, top right, open circles). ACPA, methanandamide, and anandamide had a relatively short duration of action. At the smallest doses that produced full discriminative effects at 10 min (i.e., 0.32 mg/kg of ACPA, 1 mg/kg of methanandamide, and 3.2 mg/kg of anandamide), responding on the Δ9-THC lever was 20% or less at 50 min for each agonist (Fig. 2, top right, triangles, closed circles, and squares, respectively). The significant increase in response rate at 10 min following administration of 0.32 mg/kg of ACPA was no longer evident at 30 min (Fig. 2, bottom right, triangles).
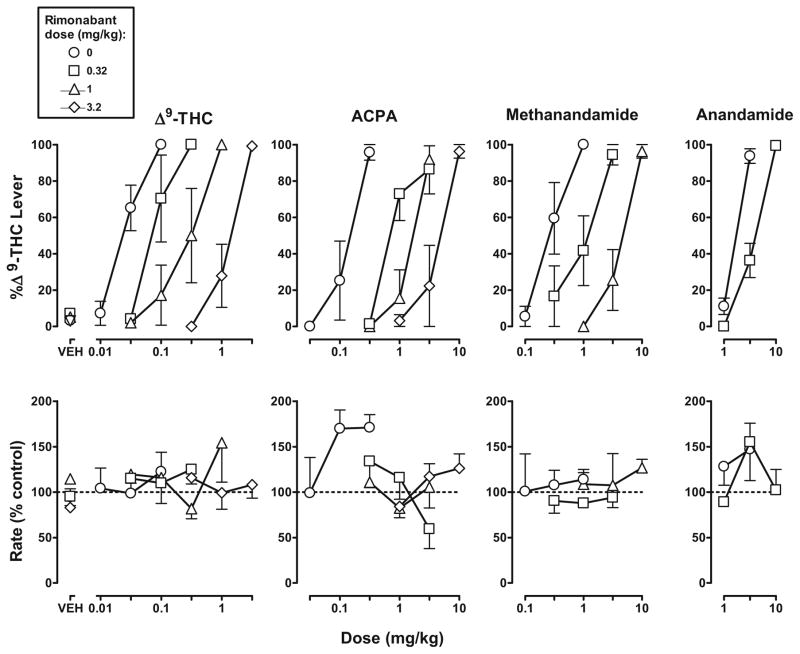
Rimonabant (0.32–3.2 mg/kg i.v.) produced a maximum average of 7 (±5)%of responses on the Δ9-THC lever (Fig. 3, top left, VEH). Rimonabant dose dependently attenuated the discriminative stimulus effects of each agonist (Fig. 3, top). For example, doses of 0.32, 1, and 3.2 mg/kg of rimonabant increased the ED50 value of Δ9-THC 3.6-, 14-, and 69-fold, respectively. The same doses of rimonabant increased the ED50 value of ACPA 6.2-, 14-, and 43-fold, respectively. Doses of 0.32 and 1 mg/kg of rimonabant increased the ED50 value of methanandamide 3.9- and 19-fold, respectively. A dose of 0.32 mg/kg of rimonabant increased the ED50 value of anandamide 2.3-fold. Response rate was not significantly altered following administration of rimonabant alone or in combination with any dose of Δ9-THC, ACPA, or methanandamide (Fig. 3, bottom).
Fig. 3.
Discriminative stimulus effects of Δ9-THC, ACPA, methanandamide, and anandamide: antagonism by rimonabant. Abscissae: dose in milligram per kilogram body weight or vehicle (VEH). Ordinates: mean (±SEM) percentage of responses on the Δ9-THC lever (top) and mean (±SEM) response rate expressed as a percentage of control (VEH training days) rate [Rate (% control)]. The horizontal dashed line (100%) shows control response rate. The control dose–effect curves for Δ9-THC, ACPA, methanandamide, and anandamide are re-plotted from Fig. 2
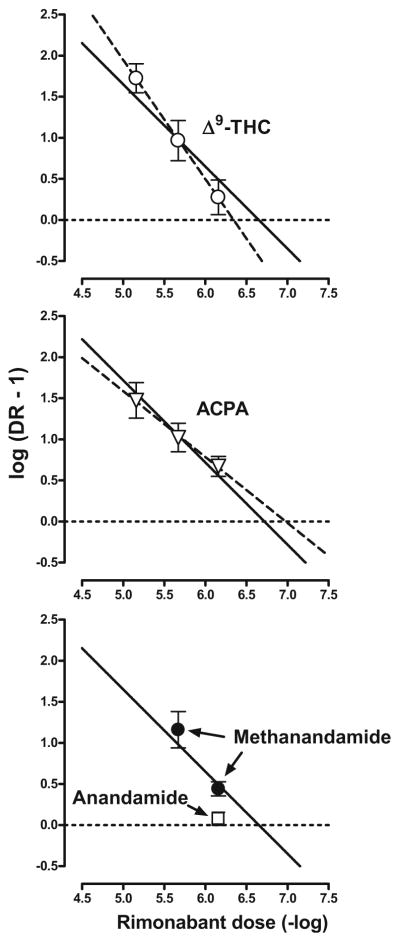
Figure 4 shows the Schild plots for antagonism of Δ9-THC (top, open circles) and ACPA (middle, triangles) by rimonabant. The coefficient of determination (r2) was 0.99 in the presence of both Δ9-THC and ACPA; the unconstrained slopes (95% confidence limits) were −1.45 (−2.08, −0.814) and −0.803 (−1.33, −0.281), respectively (Fig. 4, dashed lines; Table 1). The apparent pA2 values (95% confidence limits) of rimonabant calculated from the unconstrained slopes were 6.35 (5.96, 7.05) in the presence of Δ9-THC and 6.98 (6.30, 9.58) in the presence of ACPA. The slopes of the Schild plots for rimonabant in combination with Δ9-THC or ACPA were not significantly different from unity (i.e., −1) or from each other (p>0.20 in both cases). The apparent pA2 values (95% confidence limits) calculated from the constrained slopes were 6.65 (6.38, 6.93) in the presence of Δ9-THC and 6.72 (6.51, 6.93) in the presence of ACPA. Figure 4 (bottom) shows mean (±SEM) log(DR-1) values for doses of rimonabant studied in combination with methanandamide (closed circles) and anandamide (square). For 0.32 and 1 mg/kg of rimonabant in combination with methanandamide, the single-dose apparent affinity estimates (i.e., pKB values; 95% confidence limits) were 6.60 (6.33, 6.87) and 6.83 (5.89, 7.77), respectively. The apparent pKB value (95% confidence limits) for 0.32 mg/kg of rimonabant in combination with anandamide was 6.24 (5.98, 6.49).
Fig. 4.
Schild plots for Δ9-THC (top) and ACPA (middle) and log (DR-1) values for methanandamide and anandamide (bottom) constructed from the mean data shown in Fig. 3. Abscissae: negative logarithm of the dose in moles per kilogram. Ordinates: mean (±SEM) logarithm of the dose ratio −1. Schild plots were constructed from the unconstrained slopes (dashed lines) and by constraining the slopes to −1 (solid lines). The solid line in the bottom panel is the constrained Schild plot calculated in the presence of Δ9-THC
Table 1.
Slopes of Schild plots and apparent pA2 values (with 95% confidence limits) for rimonabant determined in the presence of Δ9-THC and ACPA and apparent pKB values (95% confidence limits) for rimonabant determined in the presence of methanandamide and anandamide
| Slope (95% confidence limits) | pA2 (unconstrained slope) | pA2 (slope constrained to −1) | Single-dose apparent affinity; pKB | |
|---|---|---|---|---|
| Δ9-THC | −1.45 (−2.08, −0.814) | 6.35 (5.96, 7.05) | 6.65 (6.38, 6.93) | |
| ACPA | −0.803 (−1.33, −0.281) | 6.98 (6.30, 9.58) | 6.72 (6.51, 6.93) | |
| Methanandamide (0.32 mg/kg rimonabant) | 6.60 (6.33, 6.87) | |||
| Methanandamide (1 mg/kg rimonabant) | 6.83 (5.89, 7.77) | |||
| Anandamide (0.32 mg/kg rimonabant) | 6.24 (5.98, 6.49) |
Apparent pA2 values were calculated from both constrained (i.e., to unity or −1) and unconstrained slopes.
Discussion
Anandamide and two of its chemical analogs (ACPA and methanandamide) substituted for a Δ9-THC discriminative stimulus in rhesus monkeys. The cannabinoid CB1 antagonist rimonabant surmountably antagonized each agonist, the Schild plots for antagonism of Δ9-THC and ACPA were not different from unity, and rimonabant had the same potency for antagonizing each agonist as evidenced by similar pA2 values (Δ9-THC and ACPA) and pKB values (methanandamide and anandamide). Therefore, Δ9-THC, anandamide, and these particular anandamide analogs appear to act through the same receptors (CB1) to produce their discriminative stimulus effects in primates.
Although anandamide and Δ9-THC are cannabinoid agonists (Howlett et al. 2002), they do not always share behavioral effects as evidenced by failure of anandamide to substitute for the discriminative stimulus effects of Δ9-THC in rhesus monkeys (Wiley et al. 1997) and rats (Burkey and Nation 1997; Wiley et al. 1995; Järbe et al. 2001). The results of these previous studies seem to indicate that anandamide and Δ9-THC differ in their mechanism of action in vivo. Alternatively, the failure of anandamide to substitute for Δ9-THC might reflect conversion of anandamide to behaviorally active metabolites that act at Δ9-THC-insensitive (non-CB1) receptors (e.g., Wiley et al. 2006). The metabolically stable cannabinoid agonist methanandamide (Abadji et al. 1994; Lang et al. 1999) substituted for Δ9-THC (Järbe et al. 2001; McMahon 2006; but see McMahon et al. 2008), and inhibitors of anandamide metabolism (URB 597) can increase the Δ9-THC-like discriminative stimulus effects of anandamide (Solinas et al. 2007). Moreover, Δ9-THC shared discriminative stimulus effects with methanandamide in rats discriminating methanandamide (Järbe et al. 2001). Metabolism appears to play a role in the extent to which anandamide shares behavioral effects with Δ9-THC.
In rhesus monkeys discriminating Δ9-THC, anandamide and two analogs (methanandamide and ACPA) administered intravenously fully substituted for Δ9-THC at doses that did not alter rate of responding. The relative potency of anandamide and its analogs were similar to their relative binding affinity at cannabinoid receptors (see Fig. 1). Although Δ9-THC and anandamide exhibit comparable binding affinity at CB1 receptors (Palmer et al. 2002), the potency of Δ9-THC was greater than anandamide. That rank order potency in vivo differed from cannabinoid binding affinity in vitro likely reflected pharmacokinetic factors as well as the well-established role for agonist efficacy in determining relative potency in vivo (Kenakin 1997). That anandamide and its analogs substituted for Δ9-THC provides strong evidence that these drugs are qualitatively similar and share a mechanism of action in vivo.
Anandamide, methanandamide, and ACPA had a much shorter duration of action than Δ9-THC, consistent with differences in rate of metabolism. Δ9-THC is metabolized primarily in the liver (Grotenhermen 2003), and its relatively slow rate of metabolism in rhesus monkeys has been documented (Perlin et al. 1985). Comparatively less is known about the metabolism of anandamide and its analogs in animals, although the mechanism involves degradation in brain and peripheral tissues by fatty acid amide hydrolase (Giuffrida et al. 2001). Fatty acid amide hydrolase degrades methanandamide more slowly than anandamide in vitro (Lang et al. 1999), perhaps resulting in the slightly longer duration of discriminative stimulus effects for methanandamide as compared to anandamide in monkeys. ACPA appears to be degraded by fatty acid amide hydrolase (Jarrahian et al. 2000), and its rate of metabolism relative to anandamide has not been reported; the current results indicate that ACPA and anandamide have a similar rate of metabolism in primates.
Through quantitative analysis of interactions of an antagonist and various agonists at their receptors, it is possible to compare the mechanism by which agonists produce their effects. In vivo Schild analysis, for example, examines the relationship between the dose of an antagonist and magnitude of antagonism (Arunlakshana and Schild 1959). This function is described by a Schild plot. A slope that is not different from unity is consistent with a simple, competitive, and reversible interaction, and the apparent affinity (pA2) of the antagonist can be estimated. For agonists acting at the same receptors, the apparent pA2 of an antagonist should be the same irrespective of the agonist. The slope of the Schild plot for rimonabant in combination with Δ9-THC and ACPA was not different from unity, consistent with a simple, competitive, and reversible interaction. If rimonabant-insensitive receptors had mediated the effects of the agonists, then the slopes of Schild plots would have been expected to differ from unity. The apparent pA2 (6.65) of rimonabant in combination with Δ9-THC was not different from the apparent pA2 (6.72) of rimonabant in combination with ACPA. A single-dose apparent affinity estimate, which assumed the relationship between dose of antagonist and magnitude of antagonism was not different from unity, provided an estimate of the potency of rimonabant in the presence of methanandamide and anandamide. For methanandamide, the apparent affinity estimates (pKB) for two doses of rimonabant were 6.60 and 6.83. Using a different approach (i.e., inhibition curves), a previous study demonstrated that rimonabant had the same potency as an antagonist of Δ9-THC and methanandamide in rats (Järbe et al. 2006). In the current study, the pKB value for a single dose of rimonabant in combination with anandamide was 6.24. The results of the current study provide strong evidence that Δ9-THC, ACPA, methanandamide, and anandamide act at the same receptors to produce their discriminative stimulus effects.
For an antagonist that has differential binding affinity at multiple receptor subtypes, a difference in apparent pA2 value is consistent with differential involvement of those receptor subtypes. When determined in the presence of Δ9-THC, the pA2 value of rimonabant in the current study was different from that calculated in a previous study (McMahon 2006). In this case, differences in apparent pA2 values were not likely due to differential involvement of cannabinoid receptor subtypes but likely to differences in the interval between administration of rimonabant and Δ9-THC. In the cumulative dosing procedure used in the previous study (McMahon 2006), the interval (approximately 80 min) between administration of rimonabant and behaviorally active doses of Δ9-THC was longer than the interval (15 min) used in the current study. The potency (i.e., apparent affinity) of rimonabant appears to have increased as a function of decreasing the pretreatment interval. For other pharmacologic classes (e.g., opioids), the apparent affinity varies as a function of the interval between antagonist and agonist administration, whereas the nature of the drug interaction does not necessarily vary (Gerak and France 2007). Collectively, these studies underscore the importance of using the same parameters of antagonist administration (i.e., pretreatment time and route) when conducting in vivo Schild analysis to compare the mechanism of action of agonists.
One noteworthy result of the present study was that discriminative stimulus effects of anandamide and its analogs could be studied in the absence of any change in overall rate of operant responding. Anandamide did not fully substitute for Δ9-THC in previous studies when studied up to doses that disrupted operant responding (Burkey and Nation 1997; Wiley et al. 1995, 1997; Järbe et al. 2001). When anandamide substituted for Δ9-THC, response rate was decreased (Solinas et al. 2007). The training dose is one factor that can determine the relative potency of drugs to produce discriminative stimulus effects and changes in rate of responding. The training dose of Δ9-THC in the current study might have been relatively small and particularly sensitive to cannabinoid agonists, although training dose cannot be readily compared across studies because different species and routes of administration were used. The rate and pattern of behavior maintained by various schedules of reinforcement are other factors that determine the effects of drugs on rate of responding. As compared to the schedule and reinforcer (food) used in most other drug discrimination procedures, the particular schedule and reinforcer (stimulus-shock termination) used in the current study might have been more resistant to the rate-decreasing effects of cannabinoids.
The present study demonstrated that the shared discriminative stimulus effects of Δ9-THC, anandamide, and two structural analogs of anandamide (ACPA and methanandamide) could be surmountably antagonized by rimonabant. The apparent affinity of rimonabant calculated from Schild analysis and single-dose apparent affinity estimates suggested that the same, likely CB1, receptors mediated the discriminative stimulus effects of each cannabinoid agonist. The similar discriminative stimulus effects of anandamide and Δ9-THC following i.v. administration are to some extent consistent with the capacity of these same drugs to exert positive reinforcing effects when administered by the same route (Tanda et al. 2000; Justinova et al. 2005). The extent to which discriminative stimulus effects of plant- and brain-derived cannabinoids vary as a function of species (rat versus monkey) is not clear, whereas route of administration and metabolism appear to be critical factors. The qualitatively similar effects of Δ9-THC and anandamide in primates suggest that at least some endogenous cannabinoids exert marijuana-like effects in vivo.
Acknowledgments
Supported by the U.S. Public Health Service Grant DA19222. The author thanks Drs. A. Giuffrida and M. Morgese for assistance with synthesis and GC/MS analysis of anandamide, Drs. L. Gerak and W. Koek for assistance with data analysis, and V. Carlton, L. Hargrove, and J. Usrey for technical assistance.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of Δ9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Darmani NA. Δ9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol Biochem Behav. 2001;69:239–249. doi: 10.1016/s0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Gerak LR, France CP. Time-dependent decreases in apparent pA2 values for naltrexone studied in combination with morphine in rhesus monkeys. Psychopharmacology. 2007;193:315–321. doi: 10.1007/s00213-007-0787-y. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Piomelli D. Purification and high-resolution analysis of anandamide and other fatty acylethanolamides. In: Laychock SG, Rubin RP, editors. Lipid second messengers. CRC; Boca Raton FL: 1998a. pp. 113–133. [Google Scholar]
- Giuffrida A, Piomelli D. Isotope dilution GC/MS determination of anandamide and other fatty acylethanolamides in rat blood plasma. FEBS Lett. 1998b;422:373–376. doi: 10.1016/s0014-5793(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of Δ9-THC and (R)-methanandamide in rats. Psychopharmacology. 2006;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Jarrahian A, Manna S, Edgemond WS, Campbell WB, Hillard CJ. Structure-activity relationships among N-arachidonylethanolamine (anandamide) head group analogues for the anandamide transporter. J Neurochem. 2000;74:2597–25606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R (+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic analysis of drug-receptor interaction. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- Khanolkar AD, Abadji V, Lin S, Hill WA, Taha G, Abouzid K, Meng Z, Fan P, Makriyannis A. Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. J Med Chem. 1996;39:4515–4519. doi: 10.1021/jm960152y. [DOI] [PubMed] [Google Scholar]
- Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. Substrate specificity and stereoselectivity of rat brain microsomal anandamide amidohydrolase. J Med Chem. 1999;42:896–902. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–414. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Δ9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology. 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of Δ9-THC in rhesus monkeys. Behav Pharmacol. 2005;16:363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Δ9-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology. 2008;198:487–495. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chem Phys Lipids. 2002;121:3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- Perlin E, Smith CG, Nichols AI, Almirez R, Flora KP, Cradock JC, Peck CC. Disposition and bioavailability of various formulations of tetrahydrocannabinol in the rhesus monkey. J Pharm Sci. 1985;74:171–174. doi: 10.1002/jps.2600740213. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces Δ9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman and Hall/CRC; Boca Raton: 2000. [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Δ9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide’s in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]