Abstract
Connexin43 (Cx43) phosphorylation alters gap junction localization and function. In particular, phosphorylation at serine-368 (S368) has been suggested to alter gap junctional conductance, but previous reports have shown inconsistent results for both timing and functional effects of S368 phosphorylation. The objective of this study was to determine the functional effects of isolated S368 phosphorylation. We evaluated wild type Cx43 (AdCx43) and mutations simulating permanent phosphorylation (Ad368E) or preventing phosphorylation (Ad368A) at S368. Function was assessed by optical mapping of electrical conduction in patterned cultures of neonatal rat ventricular myocytes, under baseline and metabolic stress (MS) conditions. Baseline conduction velocity (CV) was similar for all groups. In the AdCx43 and Ad368E groups, MS moderately decreased CV. Ad368A caused complete conduction block during MS. Triton-X solubility assessment showed no change in Cx43 location during conduction impairment. Western blot analysis showed that Cx43-S368 phosphorylation was present at baseline, and that it decreased during MS. Our data indicate that phosphorylation at S368 does not affect CV under baseline conditions, and that preventing S368 phosphorylation makes Cx43 hypersensitive to MS. These results show the critical role of S368 phosphorylation during stress conditions.
Keywords: Connexin43, phosphorylation, conduction velocity, metabolic stress, serine 368
Introduction
Ventricular tachyarrhythmias from acute ischemia or chronic infarction are the leading cause of death in the developed world.1–3 The underlying arrhythmia mechanism is reentry arising from the ischemic or healed infarct borderzone. Alterations in connexin function have been implicated in conduction slowing associated with these reentrant arrhythmias. Better understanding of factors affecting connexin function could therefore improve tachyarrhythmia therapy.
Cx43 is the principal ventricular gap junctional protein. Most cardiac disease states cause Cx43 lateralization and decreased conductance.4–6 Alterations in Cx43 phosphorylation affect both localization and function.7, 8 Cx43 has 18 different phosphorylation sites in the C-terminal domain that are susceptible to multiple kinases.9–11 Various stressors have been reported to induce phosphorylation or dephosphorylation at many of these sites, but literature reports are often inconsistent regarding the functional effects of Cx43 phosphorylation. Of particular relevance to arrhythmogenesis, S368 phosphorylation has been implicated to alter CV during stress, but the extent, timing and functional effects of S368 phosphorylation are inconsistently reported.10, 12–16 A potential source of variability in the literature has been prior methodology used. Either protein kinase C (PKC)-activation or ischemia has generally been used to induce S368 phosphorylation, but these manipulations affect multiple Cx43 phosphorylation sites in addition to their many other effects on cellular function, making it difficult to attribute the observed effects directly to S368 phosphorylation.10, 17, 18
Since the prior literature would suggest an important but still unresolved role for S368 phosphorylation in connexin function, the main objective of our study was to eliminate as many confounding factors as possible in order to reliably describe the effects of isolated S368 phosphorylation on Cx43 function. We created point mutations at S368 that either simulated continuous phosphorylation (serine to glutamate mutation) or prevented phosphorylation (serine to alanine mutation), and then evaluated electrical conduction as a measure of gap junctional function. To better understand the range of responses with S368 phosphorylation, we performed these measures at baseline and under simplified stress conditions.
Methods
Overall Study Design
We altered Cx43 with either a phosphomimetic mutation by substituting a glutamate (Ad368E) or a nonphosphorylatable mutation by substituting an alanine (Ad368A) at S368. AdGFP and AdCx43 served as controls. All experiments were performed under baseline and MS conditions (no glucose, K = 5.6mM, pH=6.2). First, we evaluated CV using optical mapping methods after exposing micro-patterned NRVM monolayers to the indicated virus. Second, we used whole cell patch clamp methods to assess INa activity in NRVMs. Then we evaluated triton-X extraction in NRVMs to determine Cx43 cellular localization. Finally, we used Western blot analysis to assess endogenous Cx43-S368 phosphorylation patterns. Experiments were carried out in accordance with the United States Public Health Service guidelines for the care and use of laboratory animals.
Adenoviral vectors
The viruses used in our study included AdGFP encoding green fluorescent protein, AdCx43 encoding wild-type rat Cx43, Ad368A with the S368A mutation of Cx43, and Ad368E with the S368E mutation of Cx43. All Cx43-encoding adenoviruses were bicistronic constructs consisting of the CMV immediate/early promoter/enhancer, GFP, an internal ribosomal entry site, and the indicated Cx43 gene or mutation. All Cx43 viruses had the human influenza virus hemagglutinin amino acid 98–106 sequence (HA tag) fused to the C-terminus of Cx43 by site-directed mutagenesis. The S368A and S368E mutations were made using the Qiagen QuikChange lightning kit, and correct mutagenesis was confirmed by sequencing. Viruses were constructed using the Cre-lox system, amplified and quality controlled as previously described.19
NRVMs
Hearts were extracted from P0–P2 Sprague-Dawley rats, trypsinized overnight at 4°C, and then digested with collagenase using a previously published protocol.20 Isolated NRVM were spun down at 2000 rpm for 5 min at 4°C to pellet, then resuspended in maintenance media (DMEM with 5% fetal bovine serum and 1% penicillin/streptomycin solution). Cells were pre-plated for 2 hours at 37°C for fibroblast removal. NRVMs were collected in maintenance media supplemented with 100 µM BrdU. Cells were plated onto 25mm micro-patterned cover slips (2 × 106 cells) or 60mm dishes (3 × 106 cells). After 24 hours, culture medium was exchanged for maintenance media supplemented with 10 µM BrdU. After 48hrs, the BrdU-containing media was removed and cells were cultured in maintenance media.
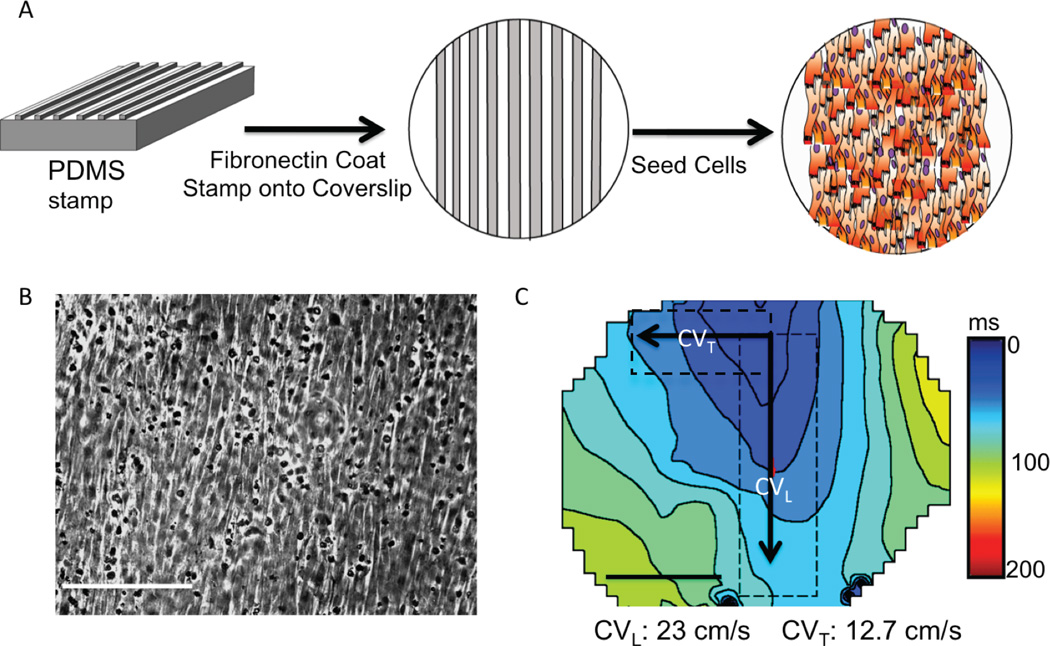
To mimic in vivo myocyte structure and anisotropic conduction, we implemented the patterned NRVM culture system as follows: polydimethylsiloxane stamps were used to coat fibronectin (25 µg/µl) onto 25 mm cover slips in patterns that forced myocyte attachment and growth into linear strands (Figure 1) using a previously reported technique.21
Figure 1. Patterned NRVM monolayers.
(A) NRVMs were plated onto cover slips coated with fibronectin lines (B). Light microscopy shows myocytes growing in a longitudinal direction (white bar=10µm). (C) Patterned NRVMs had anisotropic conduction, faster in the longitudinal (CVL) than the transverse direction (CVT). (n=3)
Cos-7 cells
Cos-7 cells were originally obtained from ATCC. Cells were plated onto 10 cm dishes and incubated at 37°C for 24 hours in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin solution before gene delivery.
Gene transfer
NRVM or Cos-7 cells were infected with the indicated virus at a concentration of 1 × 106 pfu/ml and incubated for 48–72 hours before study.
Metabolic Stress
At baseline or after metabolic stress (MS), cells were placed in normal Tyrode’s solution (mM) NaCl 140, KCl 4.5, CaCl2 1.25, MgCl 0.7, glucose 5.5, HEPES 10, pH 7.4. For MS, we switched the cells to (mM): NaCl 140, KCl 5.6, CaCl2 0.9, MgCl 0.5, HEPES 20, no glucose, pH 6.2 for the duration indicated. At all solution changes, monolayers were flushed 3-times with the following solution then the time began.
Optical mapping
The optical mapping system used a light-emitting diode (LED) (SOLA light engine, Lumencore) as excitation light source. The source light passed through a 510 nm band-pass filter (Chroma Technology Corp) to reach the patterned cell monolayer. Emitted light from the culture passed through a 565 nm dichroic mirror and a 685 nm band-pass filter (Chroma Technology Corp) prior to detection with a CCD camera (PentaMAX, Princeton Instruments). Hardware-binning of 10 × 10 pixels was required to achieve a frame rate of 200 s−1, which was fast enough to capture the propagation of the depolarizing wave across the approximately 17mm by 12mm imaging field. With a 0.5× de-magnifying camera adapter, the pixel-to-pixel distance was 0.44 mm.
Monolayers were stained with 20 µM Di-4-ANEPPS for 5 minutes and placed in a heated chamber. Monolayers were paced at 1.5Hz using a patch pipette with a ~0.2 mm diameter tip that was brought into contact with the cells near the border of the imaging field. Stimulation was at 1.5× threshold pacing voltage for all experiments, except in the Ad368A monolayers where we increased stimulation strength to 4× threshold to verify loss of conduction with MS. After 15 minutes of pacing, a baseline recording was taken. Culture solution was switched to MS and recordings were taken at 15 and 30 minutes of metabolic stress for AdGFP, AdCx43, Ad368A and Ad368E monolayers. Ad368A monolayers were also mapped after 5 minutes MS. In experiments with loss of conduction, we moved the pacing electrode around to several different spots on the edge of the mapping field to verify global conduction block. Monolayers were changed back to normal Tyrode’s after 30 minutes in the MS solution and mapped 15 minutes after media exchange.
Propagating action potentials were acquired as 12-bit tiff stacks and analyzed using custom software (Mathworks) as previously described.22 Velocity vectors, which represent the magnitude and the direction of the conduction velocity at each recording site, were calculated by fitting the activation time to a parabolic surface. The activation time was defined for each recording site as the time of 50% depolarization. The components of the conduction velocity vector were then derived from the gradient of the surface at each site, as described by Bayly et al.23 Isochronal maps that represent the positions of the wave front in 10 ms intervals were generated from activation maps and used to depict the spread of activation. Conduction velocities were averaged across pixels with active signal in 3mm by 10mm regions-of-interest.
Triton-X fractionation and Western blot analysis
Triton-X isolations were performed in non-patterned NRVMs infected with the indicated virus. NRVMs were incubated with normal Tyrode's or MS solution for 30 minutes. Cells were washed 3-times in cold PBS, scraped and spun down at 2000g. Cells were lysed for 1 hour at 4°C in Triton-X isolation buffer (50 mM Tris pH 7.4, 1% Triton X-100, 2 mM EDTA, 2 mM EGTA, 250 mM NaCl, 1 mM NaF, 0.1 mM Na3VO4, complete mini-protease inhibitors). 150 µl of lysate was collected as the “total lysate” fraction and added 1:1 to Triton-X isolation buffer with 8M urea and 2M thiourea. Remaining lysate was spun at 13000g for 20 minutes at 4°C. Resulting supernatant was collected as the “soluble” fraction (cytosolic and free membrane Cx43) and added 1:1 to Triton-X isolation buffer with 8M urea and 2M thiourea. The pellet or triton-X insoluble fraction was resuspended in Triton-X isolation buffer with 4M urea and 1M thiourea.
Western blot analysis was performed using conventional methods. Bradford Reagents were used for protein quantification (Bio-Rad). 5µg of protein samples were loaded with sample XT buffer and reducing agent (Bio-Rad). Samples were heated at 95°C for 5 minutes and run on 4–12% Bis-Tris gels (Criterion-XT, Bio-Rad). Blots were probed overnight for total Cx43 (1:1000, rabbit, Zymed 71-0700), HA-tag (1:1000, peroxidase conjugated, Roche 12013819001), Cx43 phosphorylated at S368 (P-S368) (1:1000, rabbit, Cell Signaling 3511) and N-cadherin (1:1000, mouse, Invitrogen 33-3900). Blots were developed using SuperSignal ECL (Thermo-Scientific). Densitometry was performed using ImageJ. In each blot, the signal of interest was normalized to N-cadherin.
Immunohistochemistry
Micro-patterned NRVM monolayers were washed with cold PBS 3 times then fixed with methanol at −20°C for 15 minutes. Monolayers were then blocked with PBS/1%BSA, 1% normal goat serum, 0.1% triton-X for 30 minutes at room temperature. Monolayers were probed for total Cx43 (above) and HA (1:1000, Rat, Roche 11867423001) overnight at 4°C. Following 3× wash with PBS, Cy-3 anti-rabbit (1:400, Jackson ImmunoResearch) and Alexa 647 anti-rat (1:300, Jackson ImmunoResearch) was placed for 1 hour at room temperature. Monolayers were washed 3× in PBS and mounted with mounting media (Vectashield, Vector Laboratories). Laser scanning confocal microscopy was used to obtain images that were analyzed for signal location.
INa activity
Sodium currents (INa) were recorded by ruptured-patch whole cell voltage clamp at room temperature. Microelectrodes were filled with a solution of (mM) CsF 120, MgCl2 2, HEPES 10, EGTA 11 and brought to a pH of 7.2. Neonatal myocytes were placed in the solution containing (mM) NaCl 50, N-methyl D-glucamine 90, CsCl 5, MgCl2 1, CaCl2 2, NiCl2 1, glucose 10, HEPES 10, pH 7.4 for baseline or in the same solution without glucose and with pH 6.2 for MS. INa was elicited from a holding potential of −80 mV with depolarizing voltage pulses from −60 mV to 45 mV for 16 ms starting at minimum 5 minutes of MS. Ionic current density (pA/pF) was calculated from the ratio of current amplitude to cell capacitance. Steady-state activation curves (Gv curves) were constructed from I/V plots and fitted with a Boltzmann function where V1/2 was the half-activation potential.
Data analysis and statistics
All experiments were randomized for virus treatment. Investigators were blinded to study group identity during data analysis. The number of subjects in each group was prospectively determined from pilot study data. All reported analyses were prospectively defined. Optical mapping data was analyzed using IBM SPSS statistics 20 (IBM Corp). To analyze between-group differences either repeated measures ANOVA was performed with Bonferroni post-hoc testing for specific between group analyses or paired t-tests were used as appropriate. A p-value less than 0.05 after correction for multiple measures was considered significant. All data are presented as mean and standard error.
Results
Validation of successful gene transfer
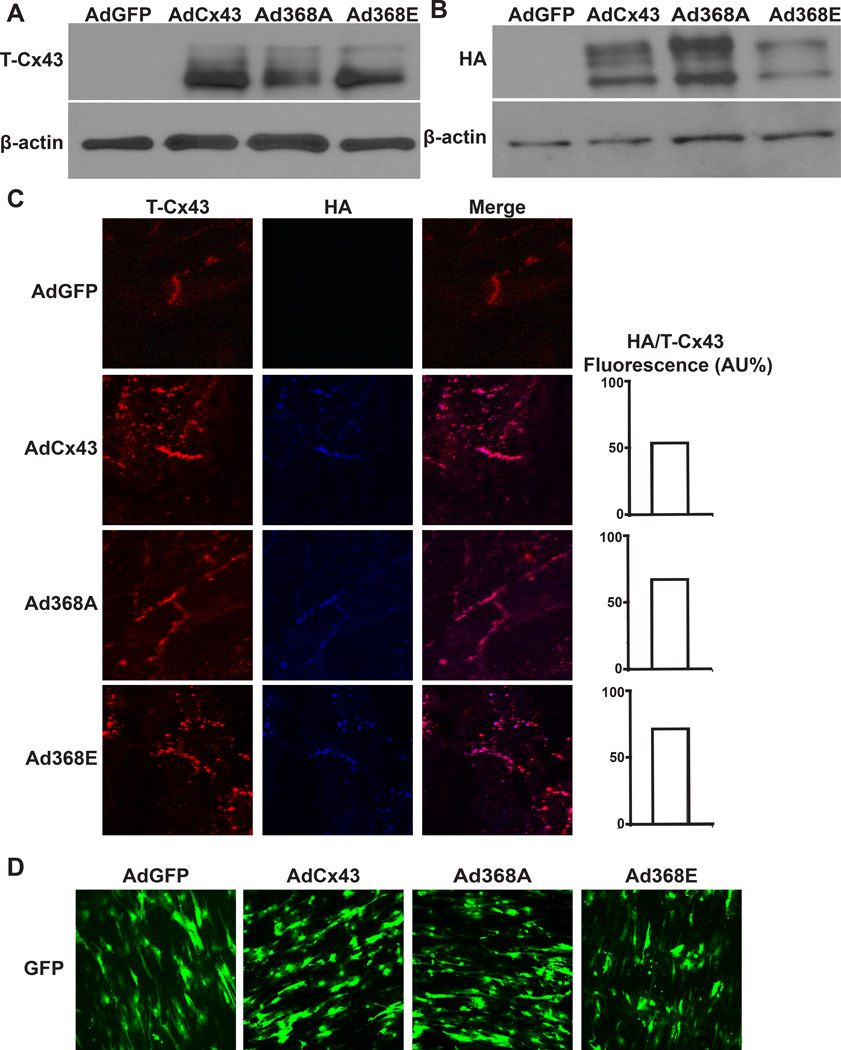
We verified transgene expression using several methods. Validation of transgenic Cx43 expression was performed in Cos-7 cells that do not have endogenous Cx43 expression (Figure 2A). All transgenic connexins were fused to a terminal HA-tag. We observed HA expression (Figure 2B) and confirmed co-localization of the HA-tag with total Cx43 in disc-like structures on NRVMs after adenoviral exposure (Figure 2C). Before all optical mapping studies, we verified transgene expression by visualizing GFP that was expressed in tandem with Cx43 (Figure 2D). We observed 71 ± 4% infection efficiency for all optical mapping experiments, with no significant difference in percent of GFP positive cells between groups.
Figure 2. Transgenic Cx43 Expression.
(A) Total Cx43 (T-Cx43) expression detected in Cos-7 cells infected with indicated virus. (B) NRVMs infected with indicated virus show transgenic Cx43 expression detected through the fused HA-tag. (C) Immunofluorescence staining shows co-localization of transgenic (blue labeled HA tag antibody) and total Cx43 (red labeled Cx43 antibody). The ratio of HA fluorescence to total Cx43 fluorescence was not different between groups, (n=2 each). (D) Dense transgene expression was verified by observing GFP fluorescence from the bicistronic Cx43-GFP constructs prior to experimentation.
S368 phosphorylation does not alter CV at baseline
Since anisotropic conduction is fundamental to cardiac tissue, and connexin expression plays a key role in anisotropic conduction, we used an established NRVM patterned-culture technique (Figure 1).21 With this system, the NRVMs developed elongated shapes and they grew into linear strands. In non-infected NRVMs we observed anisotropic conduction with average longitudinal CV of 23 ± 2 cm/s and transverse CV of 13 ± 1 cm/s. We saw no significant differences in either longitudinal or transverse CV for patterned NRVMs exposed to AdGFP (control NRVMs that still expressed endogenous Cx43), AdCx43 (overexpressing wild-type Cx43 in addition to endogenous Cx43), Ad368A (nonphosphorylatable Cx43-S368A in addition to endogenous Cx43) and Ad368E (phosphomimetic Cx43-S368E in addition to endogenous Cx43) in the baseline, unstressed state (Figure 3A/B).
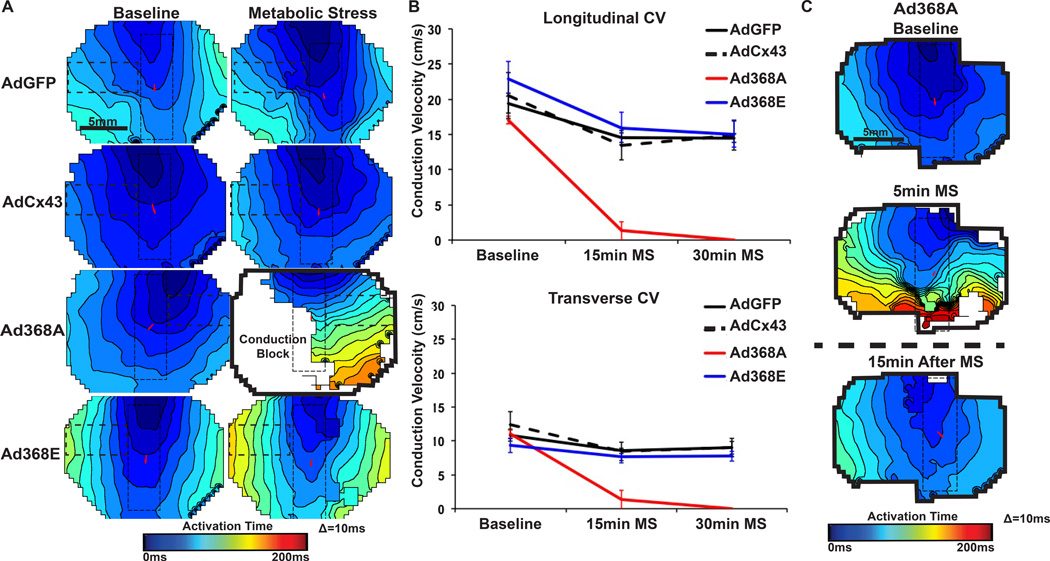
Figure 3. Optical mapping mutant Cx43.
(A) Example isochronal maps of patterned NRVMs at baseline and 15 min MS. Pixels with active signal inside the dashed rectangles were used for CV analysis (horizontal rectangles for transverse CV and vertical rectangles with red arrows for longitudinal CV). The only Ad368A monolayer with conduction at 15 min MS is shown (black outline shows full mapping field). (B) Both longitudinal and transverse CV significantly slowed after 15 and 30 min MS in all groups (p<0.05 for each, within-group comparison to baseline, n=5 each). Ad368A monolayers behaved significantly differently than all other groups during MS († p<0.01 longitudinal, * p<0.05 transverse, n=5). (C) Ad368A monolayers had significantly reduced CV at 5min MS (p<0.01 relative to baseline). CV returned to normal after removal of MS.
Preventing S368 phosphorylation caused conduction block during stress
We exposed NRVMs to MS to assess the effects of S368 phosphorylation status on CV under stress conditions. We adapted a model of MS (no glucose, elevated potassium, low pH) originally reported by Johansen et al.13 The AdGFP, AdCx43 and Ad368E groups displayed similar behavior (Figure 3A/B). Each had modest but significantly decreased CV at 15 and 30 minutes MS (p < 0.05, within-group comparison to baseline CV), but there were no significant between-group differences for AdGFP, AdCx43 and Ad368E at any time point.
Ad368A-expressing monolayers had much more severe reductions in CV. After 5 minutes of MS, all Ad368A-expressing monolayers had significantly impaired conduction (Figure 3C). At 15 minutes of MS, 4 of 5 monolayers had complete conduction block. At 30 minutes of MS, all 5 monolayers had complete conduction block.
Conduction in all monolayers of all groups returned to baseline levels 15 minutes after removal of MS media.
MS uniformly affected cellular excitability across groups
To demonstrate Ad368A-exposed cells maintained excitability during MS, we used field stimulation to induce a simultaneous depolarization across each monolayer. We were able to elicit uniform action potentials across each 368A-expressing monolayer (Figure 4A), demonstrating that the lack of signal propagation was from conduction block and not loss of excitability. Similarly field-stimulated AdGFP-expressing cells showed comparable action potential morphology and duration as the Ad368A results (Figure 4A).
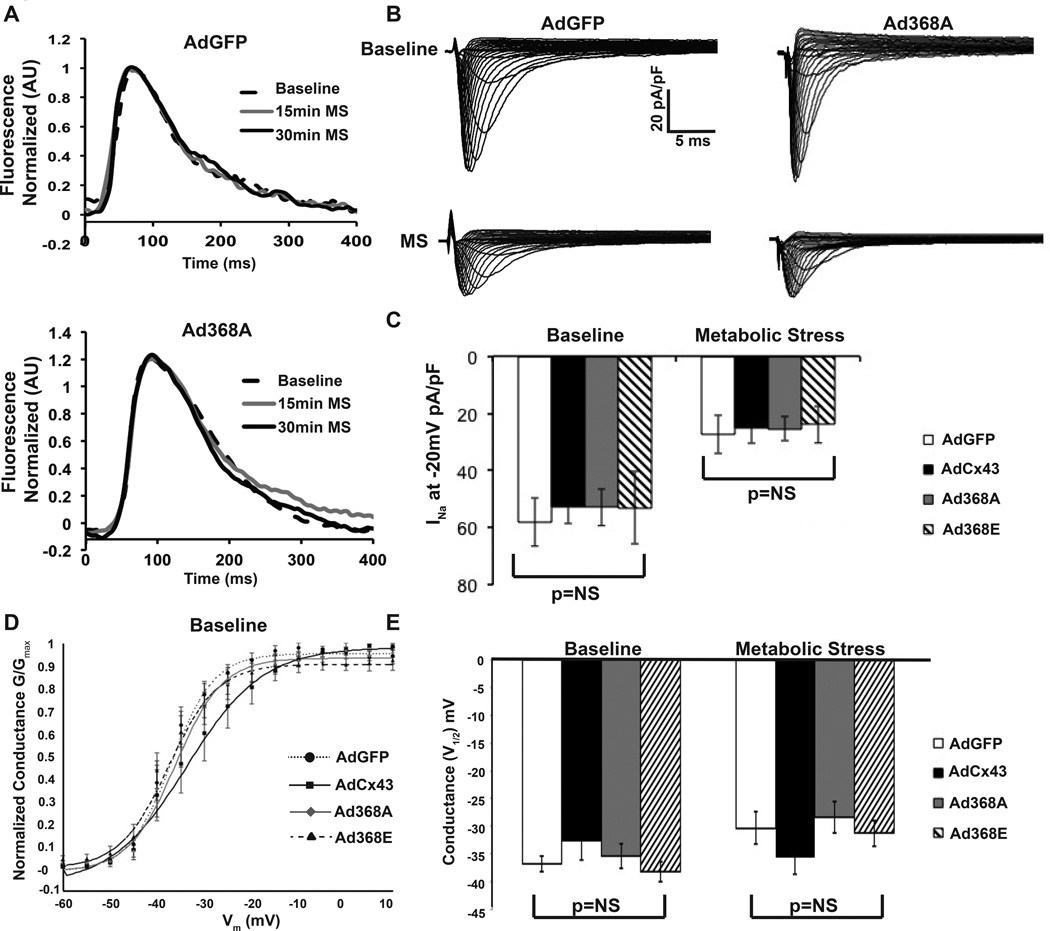
Figure 4. Metabolic stress uniformly reduced cellular excitability.
(A) Field stimulation of Ad368A monolayers during conduction block showed intact excitability (n=3). Similar action potentials were seen with field-stimulated of AdGFP monolayers (n=3). (B) INa elicited in NRVMs under baseline and MS conditions. INa significantly decreased with MS (p<0.01). (C) Summary data of INa at −20mV (AdGFP baseline n=7, MS n=8; AdCx43 n=7 each, Ad368A n=7 each, Ad368E baseline n=7, MS n=6). (D) Baseline sodium voltage dependent activation curve is shown for indicated virus (E) Summary of V(1/2 max) shows no difference among groups at baseline or MS (p=NS). AdGFP baseline n=7, MS n=8; AdCx43 n=7 each, Ad368A n=7 each, Ad368E baseline n=7, MS n=6.
To further assess cellular excitability, we evaluated sodium current density and kinetics at baseline and after 15 min MS. We saw no significant differences between groups in either peak sodium current or voltage dependence of sodium channel activation, either at baseline or with MS (Figure 4B/C). These results suggest that the between-group differences in CV with MS could not be explained by differences in cellular excitability.
Cellular location of Cx43 was unaltered by MS
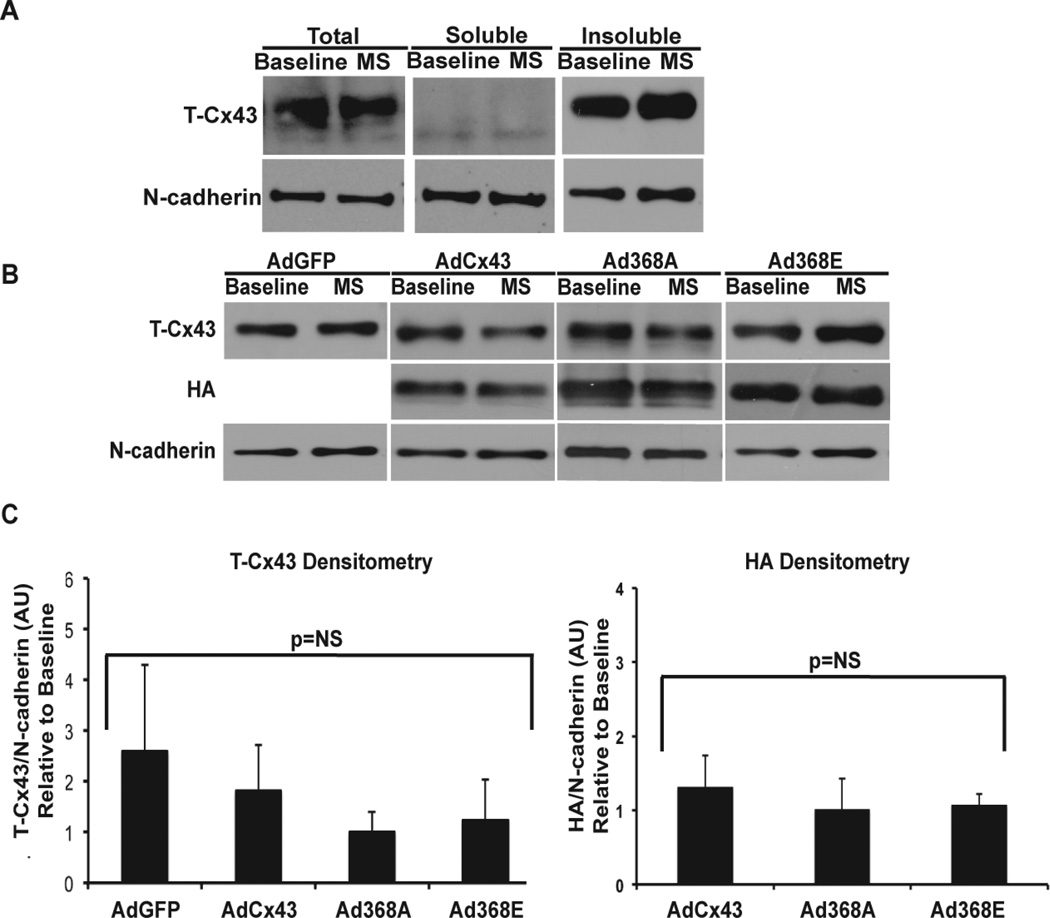
Triton-X isolation has been validated by others to separate junctional/perinexal Cx43 from non-junctional Cx43, where the triton-X insoluble fraction correlated with junctional/perinexal location.16, 24, 25 To further examine the mechanism of conduction block with Ad368A during MS, we assessed the relative amounts of Cx43 in triton-insoluble fractions to determine whether the loss of conduction correlated with connexin migration away from the junctional region during MS. Similar to previous publications,25 we observed all 3 Cx43 bands in the total fraction with a majority of the total in the P1 and P2 bands. We also noted that there were no between-group differences in transgenic Cx43 expression using the HA tag to quantify transgenic connexin expression. After triton-X isolation, we saw separation with the non-phosphorylated band predominately in the triton-soluble fraction and the phosphorylated bands in the triton-insoluble fraction (Figure 5A). The clean separation suggested efficient extraction.
Figure 5. Cx43 localization does not change with MS.
(A) An example of triton-X extraction products showing total (pre-extraction), soluble and insoluble fractions. Consistent extraction into the soluble fraction was achieved as demonstrated by the separation of the P1 and P2 bands generally found in the junction (insoluble fraction) from the P0 band generally not in the junction (soluble fraction). (B) Westerns for T-Cx43, exogenous Cx43 (HA-tag) and N-cadherin. (C) Summary of change in T-Cx43 expression (bottom left) and in exogenously expressed Cx43 (HA, bottom right). Cx43 expression was normalized to N-cadherin, and expressed as fold-change relative to baseline Cx43 expression. A value of 1 indicates equal Cx43 expression in insoluble fractions at baseline and MS. (AdGFP n=5 each; AdCx43 n=5 each, Ad368A n=6 each; Ad368E baseline n=4, MS n=5; p=NS for all groups).
When comparing baseline to MS (Figures 5B and 5C), we found in all of the tested subgroups that normalized levels of total and transgenic Cx43 remained the same in insoluble fractions, suggesting that Cx43 did not migrate from the junctional/perinexal region during MS.
S368 phosphorylation decreased during MS
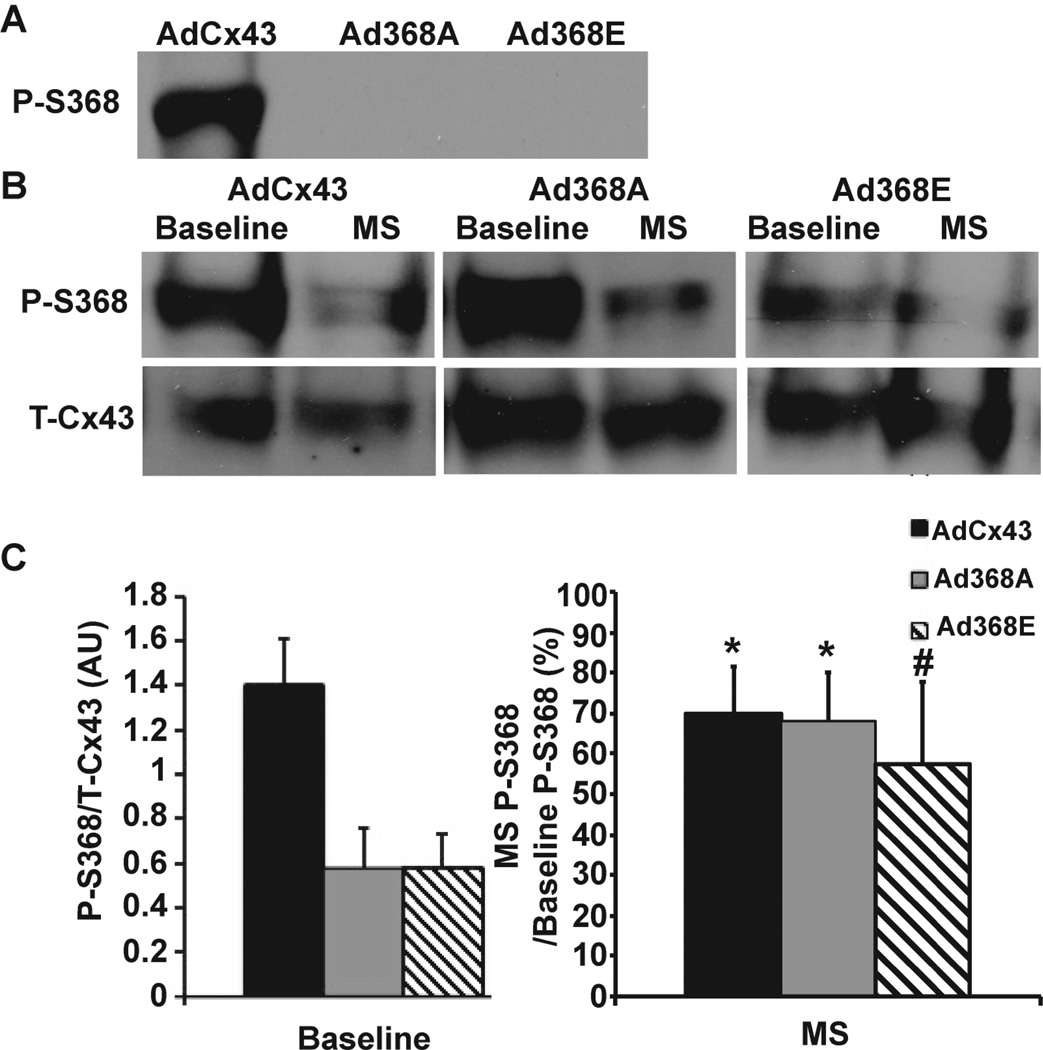
To assess levels of endogenous Cx43-S368 phosphorylation under baseline and MS conditions, we first tested the ability of a phospho-S368 antibody to distinguish between phosphorylation and mutation at S368 in Cos-7 cells. AdCx43-exposed Cos-7 cells had a clear band on Western blot when probed with the phospho-specific antibody indicating that transgenic Cx43 is expressed and phosphorylated at S368 in these cells (Figure 6A).
Figure 6. Phosphorylated S368 under metabolic stress.
(A) Cos-7 cells expressing AdCx43, Ad368A or Ad368E were probed for phosphorylated S368 (P-S368). (B) Westerns for P-S368 and total Cx43 (T-Cx43) under baseline and MS conditions in NRVMs. (C) Summary data showing decreased p-S368 in the Ad368A and Ad368E groups relative to AdCx43. When exposed to MS, all groups had a similar percentage decrease in p-S368 relative to baseline. AdGFP n=5 each; AdCx43 n=5 each, Ad368A n=6 each; Ad368E baseline n=4, MS n=5 *p<0.05, # p = 0.07 for change in p-S368 with MS compared to respective baseline. p = NS for comparison of percent change with MS across groups.
With either Ad368A or Ad368E gene transfer in Cos-7 cells, we saw no band with the phospho-S368 antibody (Figure 6A). Paired with our demonstration of transgenic 368A and 368E expression in these cells (Figure 2A), these results indicated that the S368 phospho-specific antibody did not cross-react with S368 mutations, so it was usable to measure phosphorylation of endogenous Cx43 after transduction with Ad368A or Ad368E.
Having established that we could measure endogenous S368 phosphorylation status without contamination by transgenic 368A or 368E expression, we evaluated S368 phosphorylation in NRVMs under baseline and MS conditions (Figures 6B and 6C). The Ad368A and Ad368E groups had less phospho-S368 than AdCx43 at baseline, which was not surprising since AdCx43-expressing cells could phosphorylate both endogenous and transgenic Cx43, and Ad368A- or Ad368E-expressing cells could only phosphorylate endogenous Cx43. With MS, the amount of phospho-S368 decreased in all groups. The relative reduction in phospho-S368 (expressed as percent reduction relative to baseline) did not differ between the 3 groups.
Discussion
In this study, our objective was to evaluate effects of isolated S368 phosphorylation on intercellular connectivity. We created point mutations at S368 that either simulated continuous phosphorylation or prevented phosphorylation. We then evaluated electrical conduction as a measure of gap junctional function. Since an element of variability in the literature has been caused by complexities in the experimental environments used in prior reports, we performed all experiments under the simplest, most reproducible conditions possible. Since exposure to various substrates or conditions to induce Cx43 phosphorylation has multiple other effects, we used point mutations to simulate phosphorylation conditions. Since in vitro simulated ischemia does not reproduce true ischemia, and either oxygen contamination or oxygen scavenger exposure in vitro add elements of variability, we used the simpler metabolic stress of glucose-free, low pH, slight hyperkalemia solution. Since conduction through intact tissues has multiple potential direct and indirect pathways, we used the more controlled environment of patterned NRVM monolayers. These conditions reproduce enough of the in vivo environment to have relevance (although future studies in vivo are warranted to verify our results), but our conditions maintain sufficient simplicity to reduce the possibility of confounding factors influencing our results.
We found that manipulating S368 phosphorylation had no effect on Cx43 CV under baseline conditions, and mimicking S368 phosphorylation had no effect under any circumstances when compared to wild-type Cx43. Nonphosphorylatable S368 led to conduction block during MS. Conduction block appeared entirely functional because it was readily reversible with removal of MS, and there was no change in overall or compartmental Cx43 expression. Changes in cellular excitability could not explain the conduction block because monolayers could continually be excited by field stimulation and sodium current was similar across all groups. Further analysis of wild type Cx43-S368 phosphorylation showed measurable baseline phosphorylation that was reduced during MS, so the loss of S368 phosphorylation correlated with reduced CV. Overall, our results show that S368 phosphorylation status has no effect on CV under normal conditions; mimicking S368 phosphorylation does not preserve function during MS, but nonphosphorylatable S368 correlates with loss of electrical conduction during stress.
Connexin phosphorylation
Cx43 intercalated disc localization and conductance are reduced in diseased myocardium4–6. Alterations in Cx43 phosphorylation pattern have been implicated in both localization and function.7, 8, 26 The underlying causes driving this decrease in functional Cx43 at the intercalated disk remain unknown. Remo et al. found that substituting phosphomimetic amino acids at S325, S328 and S330 was protective during an ischemic insult.27 Axelsen et al. correlated the time of conduction block during ischemia to dephosphorylation of either S297, S306, or S36810 and Procida et al. found that dephosphorylated S306 had decreased junctional conductance.28 Huang et al. reported S368A knock-in mice had no developmental heart defects and normal life spans.29 They did not study stress effects on S368A in their knock-in mice. Multiple studies have projected a dominant role of S368 phosphorylation in connexin function, but the timing and functional effects of S368 phosphorylation have been inconsistently reported.10, 12, 15, 30
Variability of S368 phosphorylation across studies
Our findings of measurable baseline phosphorylation at S368 that decreased with MS are consistent with prior reports by Axelsen et al. and Johansen et al.10, 13 Axelsen performed a comprehensive mass spectroscopy study of Cx43 phosphorylation in Langendorff-perfused rat hearts under normal and ischemic conditions. At baseline, 12 serines including S368 were phosphorylated. S368 phosphorylation was maintained through 15 minutes of ischemia and then lost between 15 and 30 minutes of ischemia. Johansen simulated ischemia in tissue culture by exposing cultured rat neonatal fibroblasts to 15.6 mM potassium, varying doses of the oxygen scavenger dithionite and no glucose at pH 6.2. They found baseline S368 phosphorylation that decreased in proportion to the severity of simulated ischemia.
At odds with these findings are reports by Hund et al. and Ek-vitorin et al.10, 12, 30 Both evaluated mouse hearts after 30 minutes of global no-flow ischemia. Both saw minimal S368 phosphorylation in controls and 5-fold increases in S368 phosphorylation with ischemia. The reason for the different findings is unclear. Species-specific differences are a possibility. Hund and Ek-vitorin used mice, and our results as well as those of Axelsen and Johansen came from rats. Differences in methods is an unlikely cause. Our MS method was different than the others, but Hund, Ek-vitorin and Axelsen all used the same method of global ischemia with different results. Differences in phospho-specific antibodies is an unlikely cause. Hund and Ek-vitorin used the same antibody that we used but with different results. Johansen used an antibody from a different source and obtained results similar to ours. Axelsen corroborated our and Johansen’s findings with an entirely independent method (mass spectroscopy). Ultimately, the goal of these studies is to understand how S368 phosphorylation is altered by ischemia in humans. The differences in results across various studies suggests that future work should use multiple independent methods to evaluate this question in large mammalian, preferable human tissues.
Effects of S368 phosphorylation on electrical conductance
Previous reports have generally used indirect methods to manipulate S368 phosphorylation, including ischemia, exposure to rotigaptide, direct activation of PKC, or exposure to 12-O-tetradecanoylphorbol-13-acetate (TPA). The lack of specificity for these methods limits interpretation of the results. In addition to inducing Cx43 phosphorylation or dephosphorylation at multiple residues, each of these methods affects numerous other cellular processes.7, 10, 31 Published reports have generally attributed Cx43 functional changes in response to these interventions entirely to S368 phosphorylation state. That line of thought misses the broader possibility that other changes brought about by their interventions may be contributing to the observed functional alterations.
The only available data using direct methods to affect S368 phosphorylation come from a report by Lampe et al.12, 15 They compared transgenic wild type Cx43 to Cx43-S368A in a mouse fibroblasts line that lacked endogenous Cx43 expression. For wild-type Cx43, they observed 2 conductance states for Cx43: 90–120 pS and 55–70 pS, with a predominance of 90–120 pS events. In cells expressing S368A, they saw no difference in relative frequency of 90–120 pS and 55–70 pS events compared to wild type Cx43-expressing fibroblasts. Our finding of similar CV under normal conditions for 368A and wild-type Cx43 expressing NRVMs is consistent with Lampe’s findings of similar single channel conductance in fibroblasts. Data on single channel conductance for Cx43-S368A during MS or ischemia has not yet been reported. Work by Rudy, Kléber and others would suggest that intercellular resistance needs to be very high and thus single channel conductance would need to be reduced to near 0 in order to explain our findings of conduction block with MS in the 368A-expressing NRVMs.32–34
Study limitations
Certain limitations should be considered in extrapolating our data. Although patterned NRVMs have anisotropic conduction, there are numerous differences between cultured neonatal cells and intact, mature myocardium. An additional consideration is that responses to high potassium, low pH, no glucose stress may differ from ischemia or other stress responses, but the available literature supports the validity of our approach. Several published studies have shown that extracellular solution changes in cultured NRVMs are transmitted efficiently to the intracellular environment over the time course used in our experiments. The most directly relevant are a series of studies showing decreased intracellular pH and a variety of physiological responses in NRVMs within 6 minutes of solution change.35–37 The basis for this response is that cardiac myocytes do not have much internal metabolic reserve. Their very extensive network of mitochondria is designed for quick processing of metabolites. The remainder of these cells is mostly contractile machinery with negligible energy stores. Consistent with a stress effect is our observation of reduced sodium current and altered Cx43 phosphorylation in NRVMs during exposure to the MS solution. Future in vivo experimentation could verify our findings during actual ischemia.
Limitations in the scope of this study prevent us from checking phosphorylation at all sites on Cx43. Although mutations specifically targeted a single amino acid, we cannot rule out the possibility that other Cx43 phosphorylation sites were affected indirectly by the status of S368 phosphorylation. The normal function observed for these mutations under baseline conditions suggests that this is unlikely to be the case.
We also cannot comment on the exact composition of the gap junctions. Transgenic connexin co-localized with total Cx43, but the level of resolution is insufficient to determine if these were adjacent homomeric channels, or if they were heteromeric channels composed of both wild type and transgenic Cx43. The functional changes in the 368A group with MS suggests some interaction between wild-type and transgenic Cx43.
Conclusions
Our data show phosphorylation status at S368 does not affect baseline Cx43 function; however, the absence of a phosphate at S368 leads to severely depressed electrical conduction during stress. Coupled with Axelsen’s finding that S368 is dephosphorylated 15–30 minutes after the onset of ischemia, our data suggest a mechanism for conduction-related contributions to phase 1b arrhythmias during ischemia. We are the first to show that a single-phosphorylation site on Cx43 can have a profound effect on CV under stressed conditions, which has implications for arrhythmia susceptibility. S368 has proven to be an essential phosphorylation site in maintaining CV during stress conditions.
Acknowledgements
We would like to thank André Kléber, Angelina Ramierez-Navarro and Drew M. Nassal for technical assistance with patterned NRVMs and Joseph S. Piktel for statistical analysis.
Supported by NIH grants R01HL67148, R01HL93486, T32CM007250, T32HL105338
List of abbreviations
- Cx43
Connexin 43
- CV
Conduction Velocity
- PKC
Protein Kinase C
- MS
Metabolic stress
- S368
serine-368 residue of connexin43
- P-S368
Phosphorylated serine-368
- Ad368E
adenovirus with the connexin43 S368E mutation
- Ad368A
adenovirus with the connexin43 S368A mutation
Footnotes
Disclosures: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts WC. Coronary arteries in fatal acute myocardial infarction. Circulation. 1972;45:215–230. doi: 10.1161/01.cir.45.1.215. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC, Potkin BN, Solus DE, Reddy SG. Mode of death, frequency of healed and acute myocardial infarction, number of major epicardial coronary arteries severely narrowed by atherosclerotic plaque, and heart weight in fatal atherosclerotic coronary artery disease: analysis of 889 patients studied at necropsy. J Am Coll Cardiol. 1990;15:196–203. doi: 10.1016/0735-1097(90)90201-y. [DOI] [PubMed] [Google Scholar]
- 4.Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 5.Huang XD, Sandusky GE, Zipes DP. Heterogeneous loss of connexin43 protein in ischemic dog hearts. J Cardiovasc Electrophysiol. 1999;10:79–91. doi: 10.1111/j.1540-8167.1999.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 7.Macia E, Dolmatova E, Cabo C, Sosinsky AZ, Dun W, Coromilas J, Ciaccio EJ, Boyden PA, Wit AL, Duffy HS. Characterization of gap junction remodeling in epicardial border zone of healing canine infarcts and electrophysiological effects of partial reversal by rotigaptide. Circulation Arrhythmia and electrophysiology. 2011;4:344–351. doi: 10.1161/CIRCEP.110.959312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HH, Baty CJ, Maeda T, Brooks S, Baker LC, Ueyama T, Gursoy E, Saba S, Salama G, London B, Stewart AF. Transcription enhancer factor-1-related factor-transgenic mice develop cardiac conduction defects associated with altered connexin phosphorylation. Circulation. 2004;110:2980–2987. doi: 10.1161/01.CIR.0000146902.84099.26. [DOI] [PubMed] [Google Scholar]
- 9.Huang RY, Laing JG, Kanter EM, Berthoud VM, Bao M, Rohrs HW, Townsend RR, Yamada KA. Identification of CaMKII phosphorylation sites in Connexin43 by high-resolution mass spectrometry. Journal of proteome research. 2011;10:1098–1109. doi: 10.1021/pr1008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjolbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen D, Cruciani V, Sundset R, Ytrehus K, Mikalsen SO. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell Physiol Biochem. 2011;28:103–114. doi: 10.1159/000331719. [DOI] [PubMed] [Google Scholar]
- 14.Liao CK, Cheng HH, Wang SD, Yeih DF, Wang SM. PKCvarepsilon mediates serine phosphorylation of connexin43 induced by lysophosphatidylcholine in neonatal rat cardiomyocytes. Toxicology. 2013;314:11–21. doi: 10.1016/j.tox.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. The Journal of cell biology. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhett JM, Gourdie RG. The perinexus: a new feature of Cx43 gap junction organization. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:619–623. doi: 10.1016/j.hrthm.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 18.Sroka J, Czyz J, Wojewoda M, Madeja Z. The inhibitory effect of diphenyltin on gap junctional intercellular communication in HEK-293 cells is reduced by thioredoxin reductase 1. Toxicol Lett. 2008;183:45–51. doi: 10.1016/j.toxlet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–225. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fast VG, Darrow BJ, Saffitz JE, Kleber AG. Anisotropic activation spread in heart cell monolayers assessed by high-resolution optical mapping. Role of tissue discontinuities. Circ Res. 1996;79:115–127. doi: 10.1161/01.res.79.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Werdich AA, Brzezinski A, Jeyaraj D, Khaled Sabeh M, Ficker E, Wan X, McDermott BM, Jr, Macrae CA, Rosenbaum DS. The zebrafish as a novel animal model to study the molecular mechanisms of mechano-electrical feedback in the heart. Prog Biophys Mol Biol. 2012;110:154–165. doi: 10.1016/j.pbiomolbio.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng. 1998;45:563–571. doi: 10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. The Journal of cell biology. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Procida K, Jorgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, Nielsen MS, Braunstein TH. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm. 2009;6:1632–1638. doi: 10.1016/j.hrthm.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang GY, Xie LJ, Linask KL, Zhang C, Zhao XQ, Yang Y, Zhou GM, Wu YJ, Marquez-Rosado L, McElhinney DB, Goldmuntz E, Liu C, Lampe PD, Chatterjee B, Lo CW. Evaluating the role of connexin43 in congenital heart disease: Screening for mutations in patients with outflow tract anomalies and the analysis of knock-in mouse models. J Cardiovasc Dis Res. 2011;2:206–212. doi: 10.4103/0975-3583.89804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hund TJ, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Protein kinase Cepsilon mediates salutary effects on electrical coupling induced by ischemic preconditioning. Heart Rhythm. 2007;4:1183–1193. doi: 10.1016/j.hrthm.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhein S, Hagen A, Jozwiak J, Dietze A, Garbade J, Barten M, Kostelka M, Mohr FW. Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:221–234. doi: 10.1007/s00210-009-0473-1. [DOI] [PubMed] [Google Scholar]
- 32.Rudy Y, Quan WL. A model study of the effects of the discrete cellular structure on electrical propagation in cardiac tissue. Circ Res. 1987;61:815–823. doi: 10.1161/01.res.61.6.815. [DOI] [PubMed] [Google Scholar]
- 33.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998;83:781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 34.Kleber AG, Saffitz JE. Role of the intercalated disc in cardiac propagation and arrhythmogenesis. Frontiers in physiology. 2014;5:404. doi: 10.3389/fphys.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasutake M, Haworth RS, King A, Avkiran M. Thrombin activates the sarcolemmal Na(+)-H+ exchanger. Evidence for a receptor-mediated mechanism involving protein kinase C. Circ Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]
- 36.Gunasegaram S, Haworth RS, Hearse DJ, Avkiran M. Regulation of sarcolemmal Na(+)/H(+) exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT(1) versus AT(2) receptors. Circ Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- 37.Haworth RS, McCann C, Snabaitis AK, Roberts NA, Avkiran M. Stimulation of the plasma membrane Na+/H+ exchanger NHE1 by sustained intracellular acidosis. Evidence for a novel mechanism mediated by the ERK pathway. J Biol Chem. 2003;278:31676–31684. doi: 10.1074/jbc.M304400200. [DOI] [PubMed] [Google Scholar]